1. Introduction
Characterizing antimicrobial metal and metal oxide nanoparticles using state-of-the-art instrumental methods is a pivotal component in their research and development, particularly in the realm of medical applications. This comprehensive protocol involves a series of fundamental steps, beginning with identification and progressing to the quantification of various attributes, including morphological, physical, chemical, biological, and protective properties of these nanoparticles. The wealth of information garnered through this thorough characterization is indispensable for gaining insights into their behavior within biological systems. It provides valuable insights into their interactions with living organisms and microorganisms, offering crucial data on their antimicrobial potency, potential toxicity, efficacy, and stability under diverse conditions. In essence, this meticulous characterization process serves as the foundation for advancing our understanding of antimicrobial nanoparticles and their potential contributions to the realm of medical coatings.
Monitoring the antimicrobial effects of coatings with metal and metal oxide nanoparticles heavily relies on their chemical and morphological properties. Not only do the chemistry, concentration, and quantity affect the particular antimicrobial potential, but the size and morphology, such as sphere, needle-like, or star-shaped structures, can also make a substantial difference. Small nanoparticles in low concentrations often exhibit stronger antimicrobial effects than those in higher concentrations. Additionally, the agglomeration of nanoparticles in some samples leads to changes in their antimicrobial effects. To accurately monitor antimicrobial effects, it is crucial to conduct a thorough characterization of coatings containing metal and metal oxide nanoparticles, linking antimicrobial properties to a sound physical, chemical, and morphological foundation.
Furthermore, nanoparticles can accumulate in the human or animal body and the environment, leading to toxic, genotoxic, and other undesirable effects. Preserving human health and other living organisms necessitates the meticulous formulation of coatings with nanoparticles using current state-of-the-art methodologies. Therefore, this manuscript underscores the importance of proper instrumental characterization of antimicrobial coatings with nanoparticles. Analytical procedures must be sensitive, robust, and accurate enough to detect minute samples and distinguish them within the complex matrices of polymeric-coated materials. Prior to validation, including the determination of limits of detection and limits of quantification, robustness and optimization, and the measurement uncertainty of the particular methodology, a comprehensive understanding of the antimicrobial and toxic mechanisms of antimicrobial nanoparticles (NPs) is essential.
Antimicrobial coatings serve multifaceted purposes, encompassing protection, aesthetics, decorativeness, and more. They are meticulously engineered with the overarching goal of inhibiting bacterial proliferation, surface colonization, and the formation of biofilms (refer to Figure 1).
Figure 1. Model of antimicrobial coating on medical items and dentistry applications with impregnated metallic nanoparticles that have antibacterial, antiviral, and antifungal activity. Moreover, with particular formulation, hollow nanoparticles may also be prepared for enhanced activation and preservation of active compounds.
Antimicrobial coatings have gained extensive traction in the realms of medicine and dentistry, largely owing to their notable attributes such as powerful antibacterial properties, antimicrobial capabilities, and resilience against water, alongside various other protective features. However, despite their broad usage and diverse applications, effectively monitoring their potential adverse impacts remains a challenging task. While the development of legal frameworks is currently underway, their efficacy will ultimately hinge on the precision of the testing procedures implemented.
The ongoing investigation emphasizes that among all the materials enumerated in
Table 1 [1][2][3], silver nanoparticles (AgNPs) are leading the way in commercialization. This trend persists particularly in the fields of medicine and dentistry, where AgNPs find extensive use due to their robust antibacterial properties
[4]. Significantly, within the industry, the segment associated with AgNPs stands as one of the fastest-growing product sectors
[5].
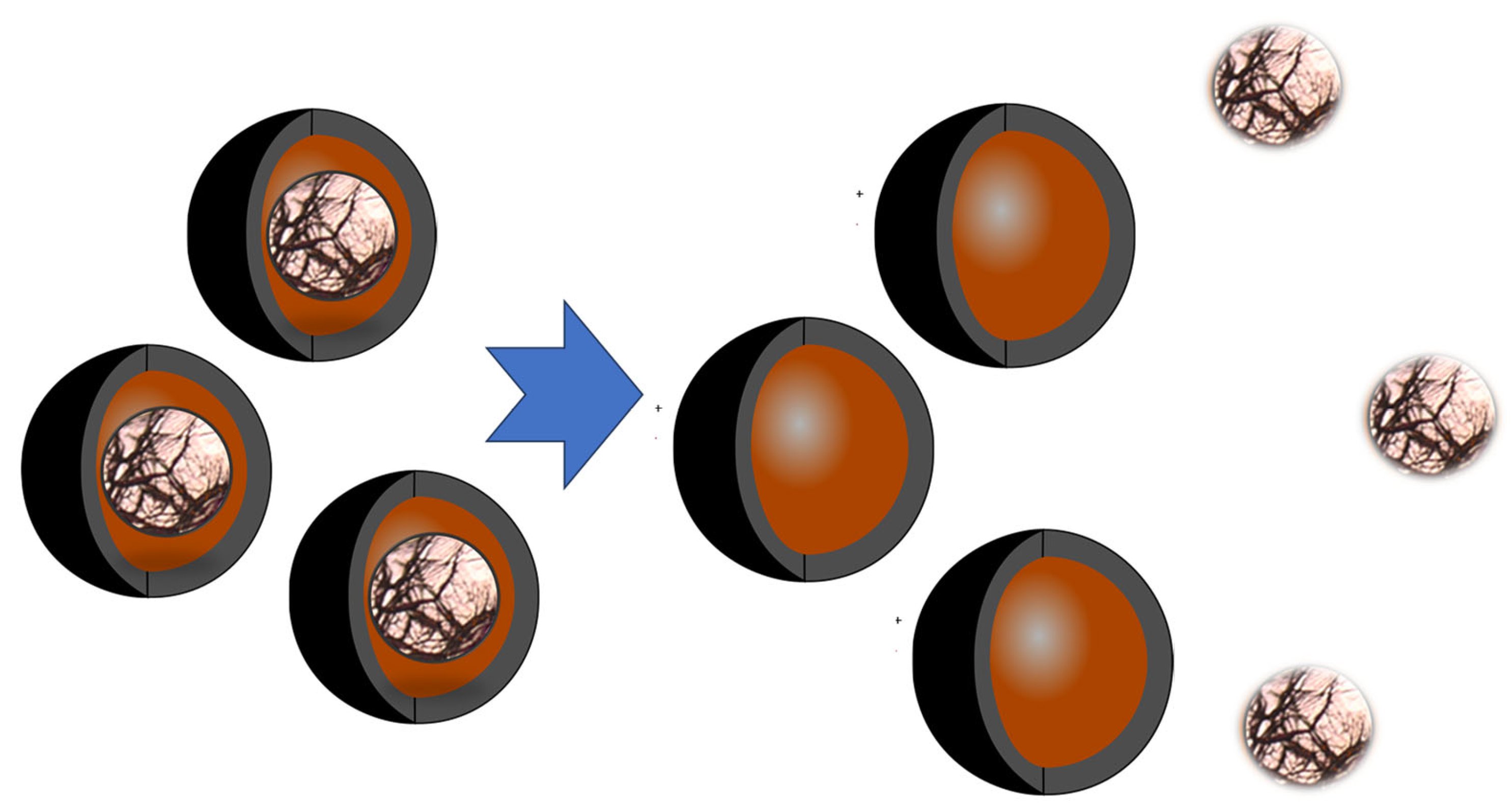
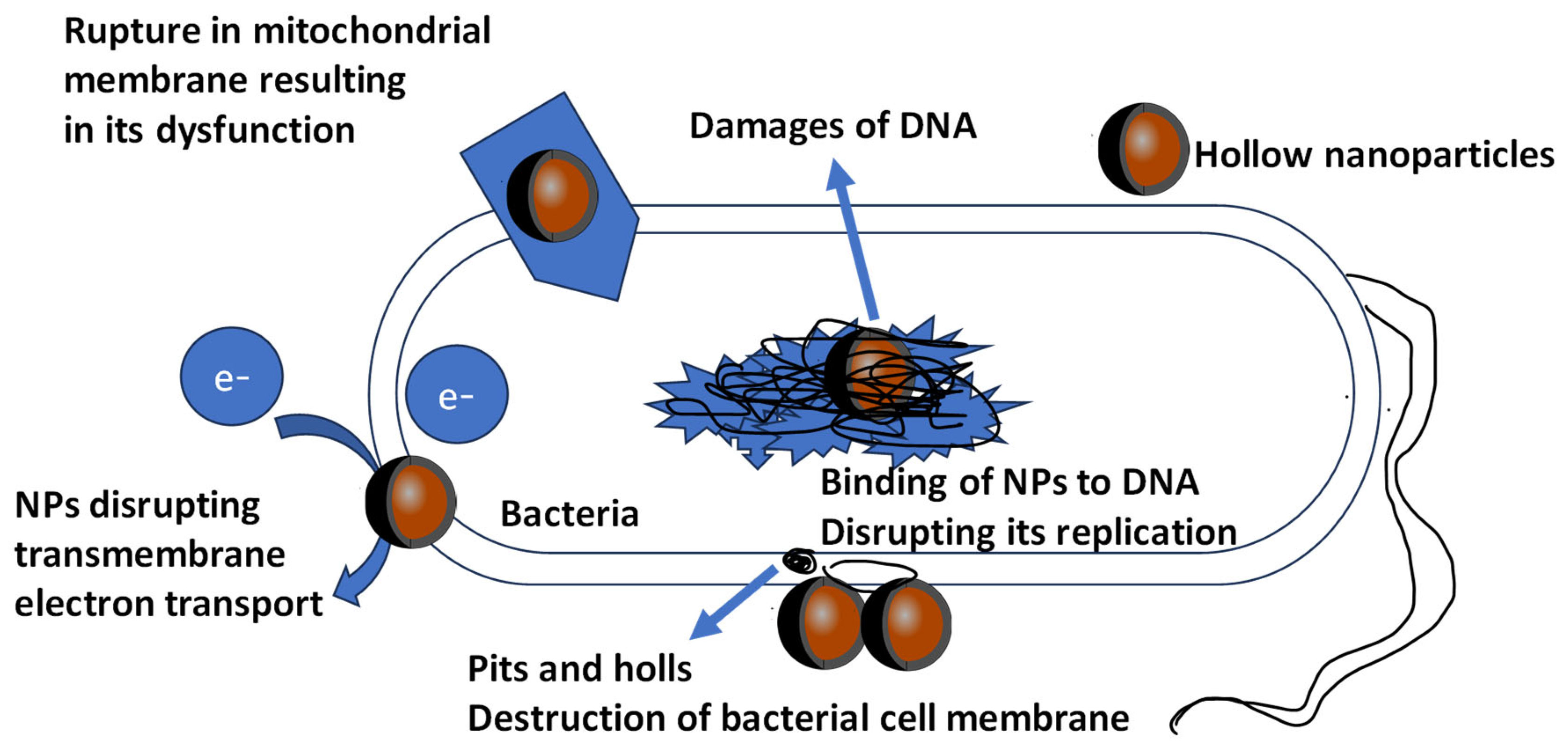
Table 1. Nanoparticles in antimicrobial coatings. The antimicrobial activity of the nanoparticles depends strongly not only on the chemical composition of the particular component but also on the size of the nanoparticle. Not all compounds have the good activity. Those with the highest potential are small particles that can easily interact with the microorganism cell (Figure 2 and Figure 3).
Figure 2. Schematic presentation of the release of active compounds from the hollow nanoparticles impregnated inside the antimicrobial coating.
Figure 3. Graphical presentation of toxic and antimicrobial effects of metallic NPs against microorganism cell. NPs have antibacterial effect due to formation of pits or pores in bacterial cell wall or membrane, leading to cell death. In addition, damage occurs to the DNA of the microorganisms, and other mechanisms are still being investigated.
The significance of nanoparticles (NPs) is further highlighted by their capacity to modify the physical and chemical characteristics of coatings. This alteration encompasses the enhancement of stain and water resistance, improvement in the absorption capabilities of materials, and adjustments in wettability based on surface energy and roughness. These versatile properties cater to a wide spectrum of applications, extending across both medical and non-medical domains
[5]. Notably, silver nanoparticles (AgNPs) have demonstrated their effectiveness through substantiated antimicrobial and antibacterial effects, as delineated in
Table 1.
Considering that a substantial portion of medical materials often encounters prolonged and direct contact with microorganisms, including those highly perilous to human health, it becomes crucial to examine a specific group of potentially harmful substances: metallic and metal oxide antimicrobial nanoparticles (NPs) (as illustrated in Table 1). While recent research primarily focuses on their beneficial properties, it is essential to address other aspects as well. Therefore, the principal objective of this manuscript is to underscore the intricate task of selecting appropriate characterization methods that facilitate the precise and accurate determination of nanoparticles present in antimicrobial coatings.
2. Silver Nanoparticles in Antimicrobial Coatings
Silver nanoparticles (AgNPs) offer distinct advantages over molecular antimicrobials, mainly due to their facile integration into polymers, facilitating the creation of functional antimicrobial coatings. This attribute is particularly notable because of the controlled release capabilities of AgNPs, which enable them to maintain their antimicrobial potency over prolonged periods. Furthermore, specific encapsulation can even extend the release duration. Once encapsulated, the active compounds remain preserved until they come into contact with microorganisms on human skin, wounds, the oral cavity, or any other targeted area. Following this initial contact, the nanoparticles are released. Moreover, if these nanoparticles are loaded with drugs or other active agents, their potential medical applications could significantly broaden. Figure 2 illustrates the release of active compounds from the hollow nanoparticles embedded within the antimicrobial coating.
Several researchers have delved into the antimicrobial efficacy of materials coated with AgNPs. When comparing the effectiveness of silver/polyamide 6 systems with nanoparticles at the nanometer scale to those at the micrometer scale, it was observed that nanocomposites with lower silver content exhibited superior efficacy against Escherichia coli compared to microcomposites with significantly higher silver content. Notably, even after 100 days of immersion in water, polyamide 6 infused with 2 wt. % AgNPs remained effective against E. coli.
The effectiveness of these materials is contingent on surface roughness, as rough surfaces provide a larger area for the release of silver ions, resulting in higher antimicrobial activity than smoother surfaces. Additionally, factors influencing silver ion release rates, such as the degree of polymer crystallinity, type of filler, matrix hydrophobicity, and particle size, can impact antimicrobial activity.
A coating based on AgNPs demonstrates effectiveness against a broad spectrum of bacteria and fungi, with a relatively higher efficacy against Gram-negative compared to Gram-positive bacteria. Despite significant progress in the use of silver nanostructures for medicine applications, further research is required to elucidate the key factors influencing not only the benefits, but also the disadvantages and toxicity of such coatings.
3. Titanium Oxide Nanoparticles in Antimicrobial Coatings
Titanium dioxide (TiO2) is widely recognized for its application as a photocatalytic disinfecting agent in surface coatings. The development of TiO2 coatings has demonstrated the capability to deactivate Escherichia coli in vitro when exposed to UV light.
In the field of nanoparticle-based antimicrobial research, substantial evidence has accumulated, confirming the antimicrobial properties of titanium dioxide nanoparticles (TiO
2 NPs), despite ongoing discussions concerning their interaction with UV light. These antimicrobial characteristics make TiO
2 NPs effective in combating bacterial infections. It is crucial to acknowledge that a significant portion of the findings supporting their antimicrobial effectiveness has been derived from in vitro studies often conducted in the presence of various additional nanomaterials. Various methodologies have been employed to assess these properties, contingent on the specific objectives of the research. Researchers have utilized a variety of techniques, including the determination of minimum inhibitory concentrations, quantification of cell counts, and the application of disk and well diffusion assays. Notably, cell count assessment has emerged as the predominant and most prevalent criterion for evaluating the antimicrobial activity of TiO
2 NPs
[6].
Biodegradable poly(butylene adipate-co-terephthalate) (PBAT) nanocomposite films incorporating titanium dioxide (TiO
2) nanoparticles were characterized through Fourier-transform infrared spectroscopy, X-ray diffraction analysis, scanning electron microscopy, and transmission electron microscopy. Interestingly, the PBAT/TiO
2 nanocomposite films exhibited remarkable antimicrobial activity against both Gram-positive and Gram-negative food-borne pathogenic bacteria, particularly
Escherichia coli and
Staphylococcus aureus. These results underscore the significance of filler–polymer interactions and highlight the role of TiO
2 as a reinforcing component in these nanocomposites, ultimately contributing to their enhanced antimicrobial properties
[7].
4. Zinc Oxide and Magnesium Oxide in Antimicrobial Coatings
More recently, the antimicrobial attributes of nano-sized zinc oxide (ZnO) and magnesium oxide (MgO) have come to the forefront. In contrast to nano-silver, ZnO and MgO nanoparticles are anticipated to provide more cost-effective coating solutions. Unlike nanocomposites loaded with TiO2, which necessitate UV light, materials containing nano-ZnO-based photocatalysts can sterilize in indoor lighting. Both ZnO and MgO showcase antibacterial properties that amplify with diminishing particle size.
The potential impact of metallic nanoparticles on the horizontal transfer of resistance genes has become a subject of investigation, attributable to their toxicity to bacterial cells and the mechanisms of metal-induced co-selection. An examination of the toxicity of ZnO nanoparticles on the
E. coli DH5α laboratory strain was conducted, alongside an assessment of the influence of ZnO nanoparticles on the transfer of resistance genes. The study revealed that even at concentrations as low as 10 mg L
−1, ZnO nanoparticles brought about a 14.6% reduction in the survival rate of
E. coli cells. Intriguingly, these nanoparticles also resulted in an almost 1.8-fold increase in the frequency of transformation of resistance genes. Furthermore, an evaluation of the environmental impact in the soil environment, where ZnO nanoparticles were present at a concentration of 1000 mg kg
−1, showed a significant impact on the overall abundance of bacteria, leading to a decrease in the copy number of the rRNA gene. These collective findings underscore the role of metallic nanoparticles in facilitating the dissemination of both antibiotic and metal resistance genes
[8].
Furthermore, the implications of this research extend broadly to environmental concerns, suggesting that the inevitable pollution of environments with nanoparticles may exacerbate the spread of antibiotic resistance, potentially exerting a significant influence on public health. These findings emphasize the need for heightened awareness and proactive measures in managing nanoparticle pollution to mitigate its potential impact on the dissemination of resistance genes and, consequently, public health
[8].
5. Chromium Oxide NPs in Antimicrobial Coatings
Chromium (Cr) has long been recognized as a hazard to the environment and its associated biota, particularly when its levels surpass permissible thresholds. Extensively utilized across various industries, the toxicological implications of Cr have undergone comprehensive scrutiny. However, the emerging applications of nano-sized chromium (nano-Cr) or chromium oxide nanoparticles (Cr2O3-NPs) have not received as much attention in the existing literature, particularly concerning their phytotoxic effects.
A recent study sought to bridge this research gap by investigating the morpho-physiological impacts of both macro- and nano-sized Cr on Hordeum vulgare L. plants. The findings indicated that the escalated accumulation and translocation of Cr, regardless of its form (macro or nano), disrupted cellular metabolism, resulting in the inhibition of germination, growth, and interference with photosynthesis in the plants. Importantly, the phytotoxicity was more pronounced when exposed to Cr nanoparticles compared to their macro-sized counterparts. This heightened phytotoxicity in the presence of Cr nanoparticles likely stemmed from the increased bioavailability of Cr ions, a phenomenon supported by observations related to total Cr content, mobility, and the factor toxicity index.