
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sophie Laurent | -- | 4870 | 2023-11-02 10:11:27 | | | |
| 2 | Catherine Yang | Meta information modification | 4870 | 2023-11-03 02:05:59 | | |
Video Upload Options
MRI contrast agents are required in clinic to detect some pathologies, such as cancers. Nevertheless, at the moment, only small extracellular and non-specific gadolinium complexes are available for the clinicians. Moreover, safety issues have recently appeared concerning the use of gadolinium complexes, so that alternatives are urgently needed. Manganese-based MRI contrast agents could be one of these alternatives and more and more studies are available in the literature. This work aims at synthesizing all those researches, to highlight all the efforts already made by the scientific community to obtain highly efficient agents, but also evidence the weaknesses of the developed systems.
1. Non-Specific Contrast Agents
Similarly to the Gd-complexes, the efficacy of molecular Mn-based contrast agents is based on the presence of at least one exchanging water molecule in the inner coordination sphere of the metal characterized by a fast exchange rate. With the typical coordination number of Mn(II) complexes in aqueous solution being six, seven, or sometimes eight, this innersphere water molecule is assured if the ligand possesses five or six coordination bonds with the metal. Nevertheless, the thermodynamic stability and the kinetic inertness of Mn complexes is generally lower than that of Gd-complexes because of the lower charge of Mn ions and the lack of ligand-field stabilization energy (high spin d5 electron configuration). Moreover, the possible oxidation of Mn2+ to Mn3+, which is often related to the thermodynamic stability of the Mn(II)-complex, also has to be avoided as it will lead to a loss of efficacy because of the loss of one unpaired electron and of a less favorable electronic relaxation.
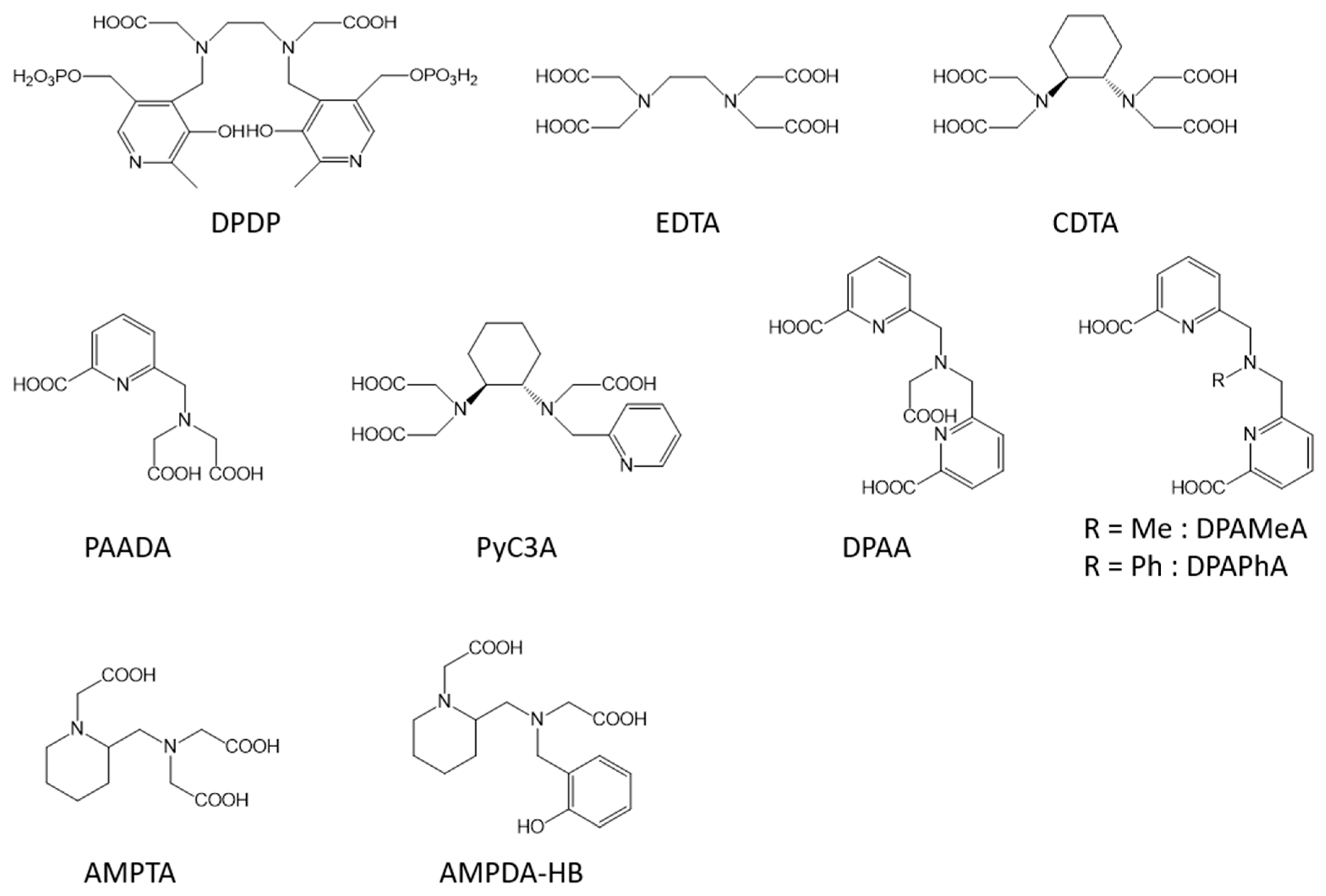
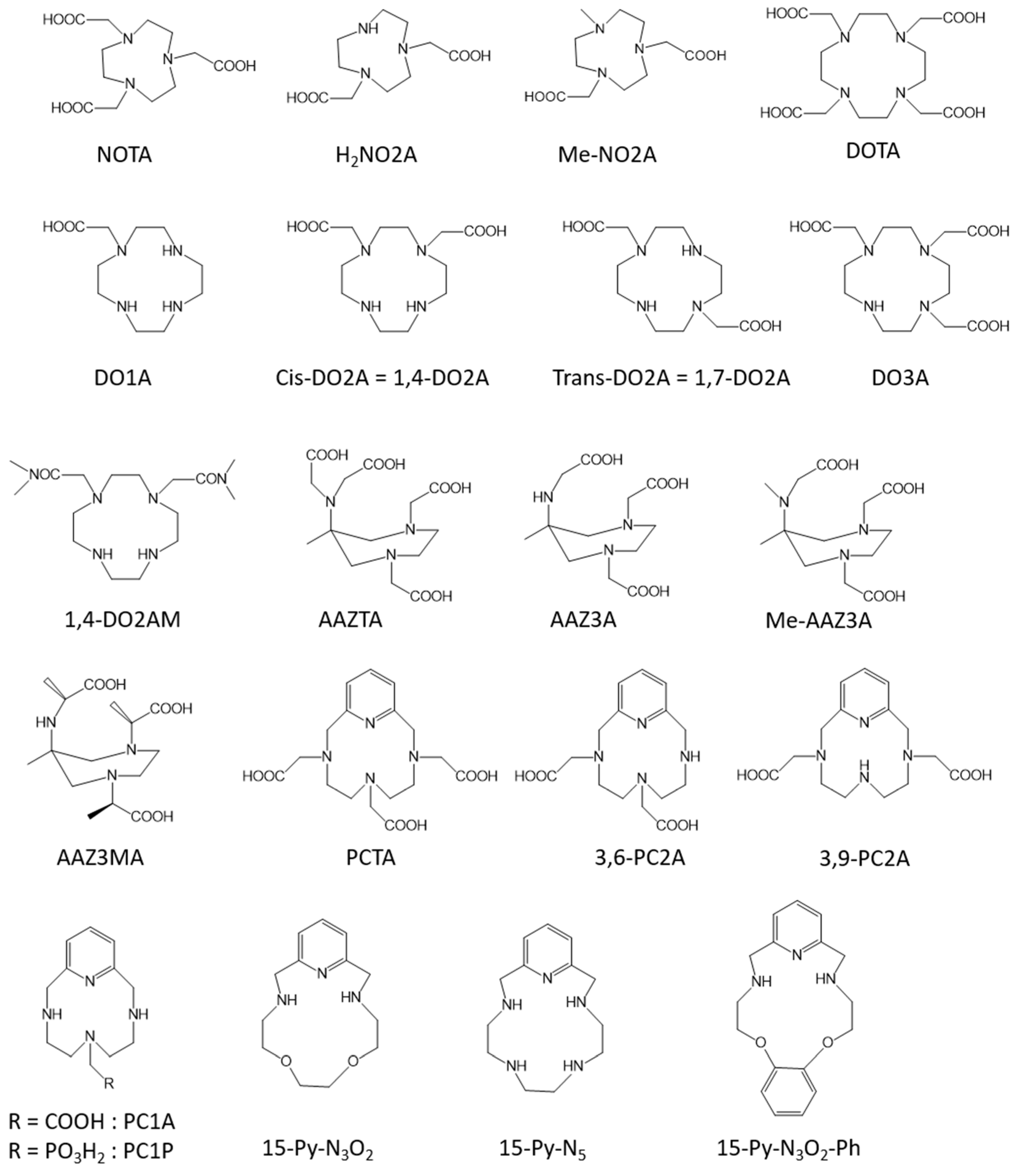
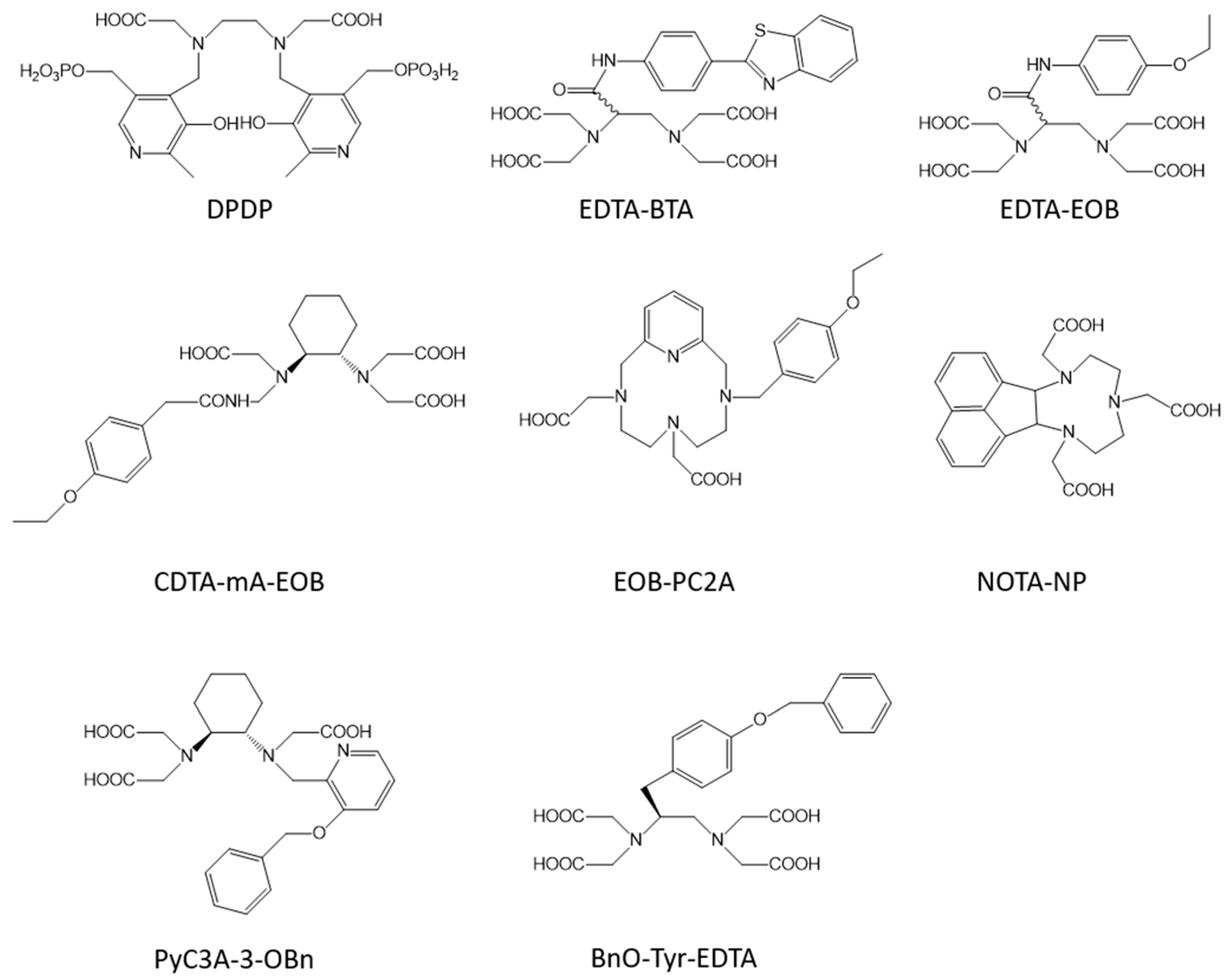
2. Liver Targeted Contrast Agents
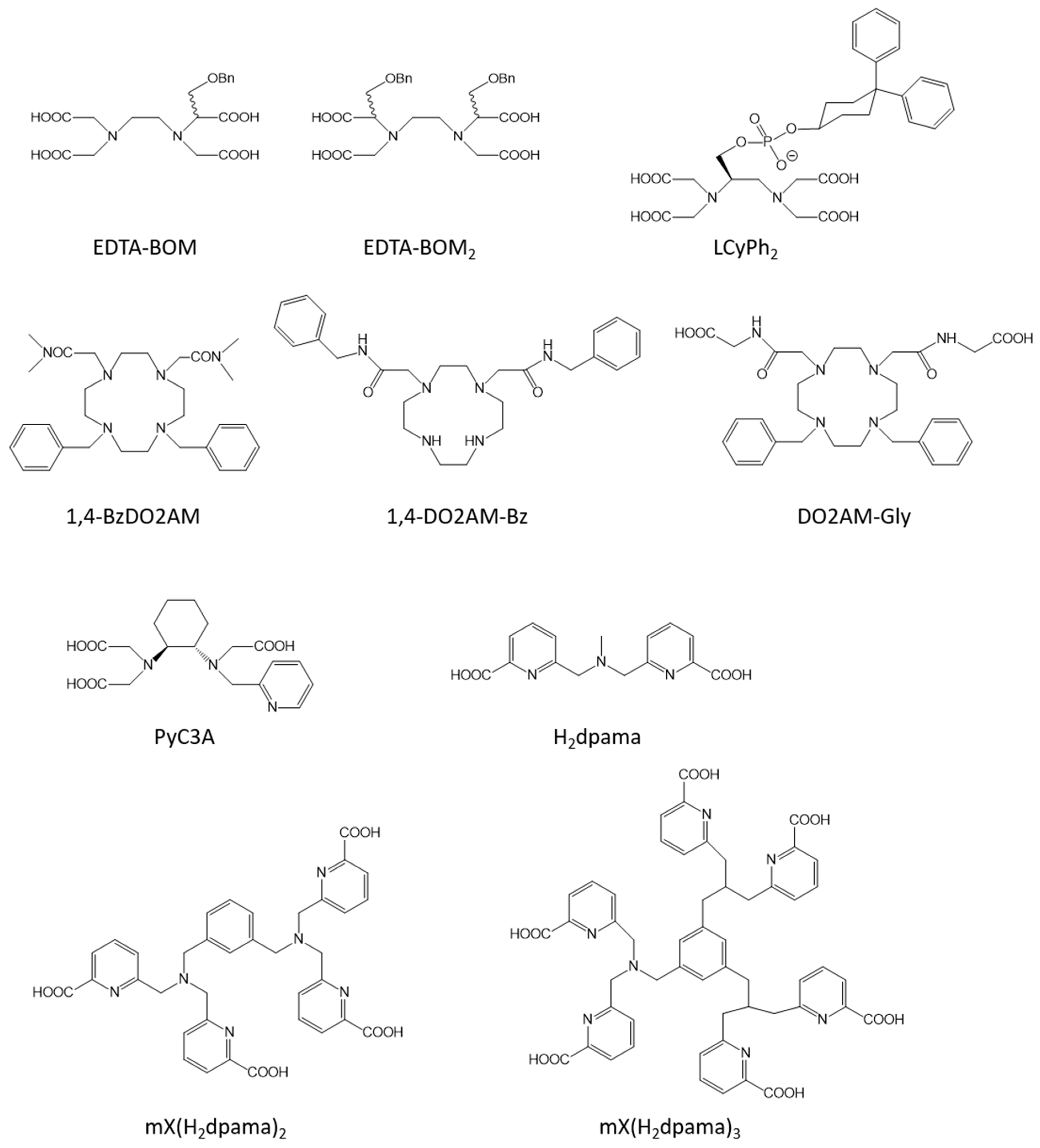
3. Blood Pool Agents
4. Responsive Contrast Agents
5. Multimodal Contrast Agents
| q | r1 in Water or Buffer (s−1 mM−1) | r1 in the Presence of HSA (s−1 mM−1) | Application Area | Tested In Vitro and/or In Vivo | |
|---|---|---|---|---|---|
| Mn-EDTA | 1 | 2.9 (0.47 T, 35 °C, [2]) | extracellular | no | |
| Mn-CDTA | 1 | 3.0 (0.47 T, 40 °C, [69]) | extracellular | no | |
| Mn-PyC3A | 1 | 2.1 (1.4 T, 37 °C, [3]) | 3.5 (1.4 T, 37 °C, [3]) | extracellular/blood pool | yes [5][6] |
| Mn-DPAA | 1 | 2.7 (0.47 T, 37 °C, [70]) | extracellular | no | |
| Mn-DPAMeA | 2 | 5.1 (0.47 T, 37 °C, [70]) | extracellular | no | |
| Mn-DPAPhA | 2 | 4.2 (0.47 T, 37 °C, [70]) | extracellular | no | |
| Mn-PAADA | 2 | 3.3 (0.47 T, 37 °C, [71]) | extracellular | no | |
| Mn-AMPTA | 1 | 2.6 (0.47 T, 37 °C, [72]) | extracellular | no | |
| Mn-AMPDA-HB | 1 | 2.7 (0.47 T, 37 °C, [72]) | extracellular | no | |
| Mn-MeNO2A | 1 | 2.2 (0.47 T, 37 °C, [15]) | extracellular | no | |
| Mn-DO3A | 0 | 1.3 (0.47 T, 37 °C, [18]) | extracellular | no | |
| Mn-1,7-DO2A | 0 | 1.3 (0.47 T, 37 °C, [18]) | extracellular | no | |
| Mn-1,4-DO2A | 1 | 1.7 (0.47 T, 37 °C, [18]) | extracellular | no | |
| Mn-1,4-DO2AM | 1 | 2.0 (0.47 T, 37 °C, [20]) | extracellular | no | |
| Mn-AAZTA | 0 | 1.6 (0.47 T, 25 °C, [23]) | extracellular | no | |
| Mn-AAZ3A | 1 | 2.5 (0.47 T, 25 °C, [23]) | extracellular | no | |
| Mn-MeAAZ3A | 1 | 2.0 (0.47 T, 25 °C, [23]) | extracellular | no | |
| Mn-AAZ3MA | 1 | 1.9 (0.47 T, 25 °C, [23]) | extracellular | no | |
| Mn-3,6-PC2A | 1 | 2.7 (0.47 T, 25 °C, [73]) | extracellular | no | |
| Mn-3,9-PC2A | 1 | 2.9 (0.47 T, 25 °C, [73]) | extracellular | no | |
| Mn-15-pyN5 | 2 | 3.1 (0.47 T, 37 °C, [25]) | extracellular | no | |
| Mn-15-pyN3O2 | 2 | 3.6 (0.47 T, 37 °C, [25]) | extracellular | no | |
| Mn-EDTA-BTA | 1 | 3.5 (1.5 T, 24 °C, [33]) | 15.1 (1.5 T, 24 °C, [33]) | liver | yes [32] |
| Mn-EDTA-EOB | 1 | 2.3 (1.5 T, 24 °C, [33]) | 6.3 (1.5 T, 24 °C, [33]) | liver | yes [33] |
| Mn-EOB-PC2A | 1 | 2.8 (1.5 T, 25 °C, [35]) | 5.9 (1.5 T, 25 °C, [35]) | liver | yes [35] |
| Mn-NOTA-NP | 1 | 3.6 (3 T, 25 °C, [36]) | 9.0 (3 T, 25 °C, [36]) | liver | yes [36] |
| Mn-PyC3A-3-Obn | 1 | 2.6 (1.4 T, 37 °C, [37]) | 9.0 (1.4 T, 37 °C, [37]) | liver | yes [37] |
| Mn-BnO-TyEDTA | 1 | 4.3 (0.47 T, 32 °C, [38]) | 15.8 (0.47 T, 32 °C, [38]) | liver | yes [38][39] |
| Mn-EDTA-BOM | 1 | 3.6 (0.47 T, 25 °C, [41]) | 55.3 (0.47 T, 25 °C, [41]) | blood pool | no |
| Mn-LCyPh2 | 1 | 5.8 (0.47 T, 37 °C, [42]) | 48.0 (0.47 T, 37 °C, [42]) | blood pool | yes [42] |
| Mn-1,4-BzDO2AM | 1 | 3.8 (0.47 T, 25 °C, [45]) | 18.5 (0.47 T, 25 °C, [45]) | blood pool | no |
| Mn-1,4-DO2AM-Bz | 1 | 3.5 (0.47 T, 25 °C, [45]) | 27.4 (0.47 T, 25 °C, [45]) | blood pool | no |
| Mn-DO2AM-Gly | 1 | 4.5 (1 T, 25 °C, [46]) | 14.0 (1 T, 25 °C, [46]) | blood pool | yes [46] |
| Mn-dpama | 2 | 4.2 (0.47 T, 37 °C, [48]) | 12.2 (0.47 T, 37 °C, [48]) | blood pool | no |
| mX(Mn-dpama)2 | 2 | 6.1 (0.47 T, 37 °C, [48]) | 39.0 (0.47 T, 37 °C, [48]) | blood pool | no |
| mX(Mn-dpama)3 | 2 | 8.3 (0.47 T, 37 °C, [48]) | 45.2 (0.47 T, 37 °C, [48]) | blood pool | no |
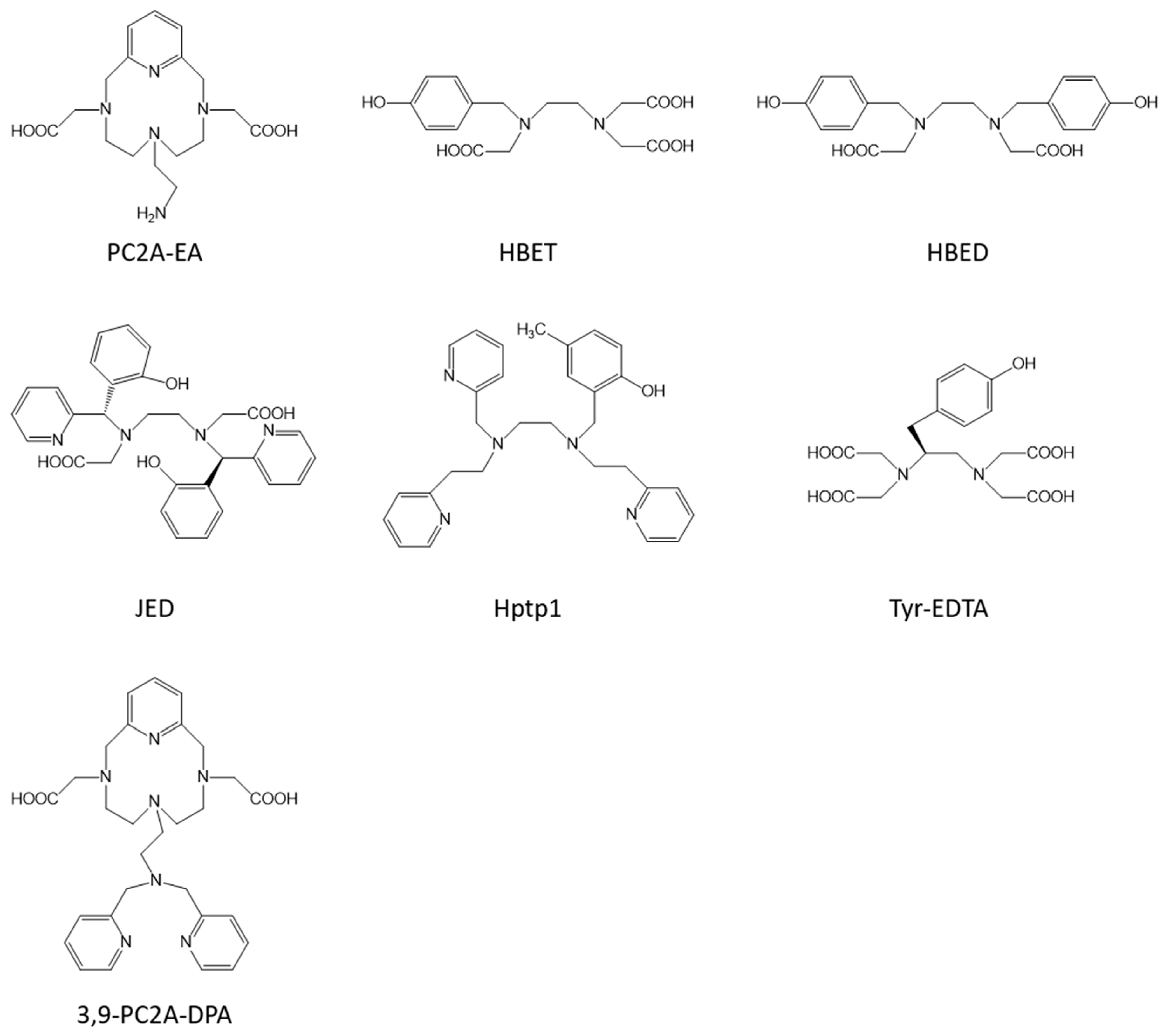
| Mn-PC2A-EA | 1 | 3.5/2.1 (0.47 T, 25 °C, [55]) | pH responsive | no | |
| MnII/III-HBET | 1 | 1.0/2.8 (1.4 T, 37 °C, [57]) | redox responsive | no | |
| MnII/III-JED | 1 | 0.5/3.3 (1.4 T, 37 °C, [59]) | redox responsive | no | |
| Mn-Hptp1 | 1/2 | 4.7/5.3 (3 T, 25 °C, [61]) | redox responsive | no | |
| Mn-Tyr-EDTA | 1 | 3.3/8.5 (0.47 T, 32 °C, [62]) | 8.0 (0.47 T, 32 °C, [62]) | redox responsive | yes [62] |
| Mn-3,9-PC2A-DPA | 1 | 3.2 (1.4 T, 37 °C, [63]) | 12.1 (1.4 T, 37 °C, [63]) | Zn responsive | yes [63] |
6. In Vitro/In Vivo Studies and Toxicity Issues
References
- Wolf, G.L.; Burnett, K.R.; Goldstein, E.J.; Joseph, P.M. Magnetic Resonance Annual 1985; Kressel, H., Ed.; Raven: New York, NY, USA, 1985; p. 231.
- Koenig, S.H.; Baglin, C.; Brown, R.D., III; Brewer, C.F. Magnetic field dependence of solvent proton relaxation induced by Gd3+ and Mn2+ complexes. Magn. Reson. Med. 1984, 1, 496–501.
- Gale, E.M.; Atanasova, I.P.; Blasi, F.; Ay, I.; Caravan, P. A Manganese Alternative to Gadolinium for MRI Contrast. J. Am. Chem. Soc. 2015, 137, 15548–15557.
- Gale, E.M.; Wey, H.Y.; Ramsay, I.A.; Yen, Y.F.; Sosnovik, D.E.; Caravan, P. A manganese-based alternative to gadolinium: Contrast-enhanced MR angiography, excretion, pharmacokinetics, and metabolism. Radiology 2018, 286, 865–872.
- Erstad, D.J.; Ramsay, I.A.; Jordan, V.C.; Sojoodi, M.; Fuchs, B.C.; Tanabe, K.K.; Caravan, P.; Gale, E.M. Tumor Contrast Enhancement and Whole-Body Elimination of the Manganese-Based Magnetic Resonance Imaging Contrast Agent Mn-PyC3A. Investig. Radiol. 2019, 54, 697–703.
- Zhou, I.Y.; Ramsay, I.A.; Ay, I.; Pantazopoulos, P.; Rotile, N.J.; Wong, A.; Caravan, P.; Gale, E.M. Positron Emission Tomography–Magnetic Resonance Imaging Pharmacokinetics, In Vivo Biodistribution, and Whole-Body Elimination of Mn-PyC3A. Investig. Radiol. 2021, 56, 261–270.
- Bonner, B.P.; Yurista, S.R.; Coll-Font, J.; Chen, S.; Eder, R.A.; Foster, A.N.; Nguyen, K.D.; Caravan, P.; Gale, E.M.; Nguyen, C. Contrast-Enhanced Cardiac Magnetic Resonance Imaging with a Manganese-Based Alternative to Gadolinium for Tissue Characterization of Acute Myocardial Infarction. J. Am. Heart Assoc. 2023, 12, e026923.
- Cieslik, P.; Comba, P.; Dittmar, B.; Ndiaye, D.; Toth, E.; Velmurugan, G.; Wadepohl, H. Exceptional Manganese (II) Stability and Manganese(II)/Zinc(II) Selectivity with Rigid Polydentate Ligands. Angew. Chem. Int. Ed. 2022, 61, e202115580.
- Anbu, S.; Hoffmann, S.H.L.; Carniato, F.; Kenning, L.; Price, T.W.; Prior, T.J.; Botta, M.; Martins, A.F.; Stasiuk, G.J. A single-pot template reaction towards a manganese-based T1 contrast agent. Angew. Chem. Int. Ed. 2021, 60, 10736–10744.
- Drahos, B.; Kubicek, V.; Bonnet, C.S.; Hermann, P.; Lukes, I.; Toth, E. Dissociation Kinetics of Mn2+ complexes of NOTA and DOTA. Dalton Trans. 2011, 40, 1945–1951.
- Uzal-Varela, R.; Rodriguez-Rodriguez, A.; Martinez-Calvo, M.; Carniato, F.; Lalli, D.; Esteban-Gomez, D.; Brandariz, I.; Pérez-Lourido, P.; Botta, M.; Platas-Iglesias, C. Mn2+ complexes containing sulfonamide groups with pH-responsive relaxivity. Inorg. Chem. 2020, 59, 14306–14317.
- Uzal-Varela, R.; Valencia, L.; Lalli, D.; Maneiro, M.; Esteban-Gomez, D.; Platas-Iglesias, C.; Botta, M.; Rodriguez-Rodriguez, A. Understanding the effect of the electron spin relaxation on the relaxivities of Mn(II) complexes with triazacyclononane derivatives. Inorg. Chem. 2021, 60, 15055–15068.
- Balogh, E.; He, Z.; Hsieh, W.; Liu, S.; Tóth, E. Dinuclear Complexes Formed with the Triazacyclononane Derivative ENOTA4−: High-Pressure 17O NMR Evidence of an Associative Water Exchange on . Inorg. Chem. 2007, 46, 238–250.
- Ducommun, Y.; Newman, K.E.; Merbach, A.E. High-pressure oxygen-17 NMR evidence for a gradual mechanistic changeover from Ia to Id for water exchange on divalent octahedral metal ions going from manganese(II) to nickel(II). Inorg. Chem. 1980, 19, 3696–3703.
- Patinec, V.; Rolla, G.A.; Botta, M.; Tripier, R.; Esteban-Gomez, D.; Platas-Iglesias, C. Hyperfine Coupling Constants on Inner-Sphere Water Molecules of a Triazacyclononane-based Mn(II) Complex and Related Systems Relevant as MRI Contrast Agents. Inorg. Chem. 2013, 52, 11173–11184.
- de Sá, A.; Bonnet, C.S.; Geraldes, C.F.G.C.; Tóth, E.; Ferreira, P.M.T.; André, J.P. Thermodynamic stability and relaxation studies of small, triaza-macrocyclic Mn(ii) chelates. Dalton Trans. 2013, 42, 4522–4532.
- Pujales-Paradela, R.; Carniato, F.; Esteban-Gomez, D.; Botta, M.; Platas-Iglesias, C. Controlling water exchange rates in potential Mn2+-based MRI agents derived from NO2A2−. Dalton Trans. 2019, 48, 3962–3972.
- Rolla, G.A.; Platas-Iglesias, C.; Botta, M.; Tei, L.; Helm, L. 1H and 17O NMR Relaxometric and Computational Study on Macrocyclic Mn(II) Complexes. Inorg. Chem. 2013, 52, 3268–3279.
- Garda, Z.; Forgacs, A.; Do, Q.N.; Kalman, F.K.; Timari, S.; Baranyai, Z.; Tei, L.; Toth, I.; Kovacs, Z.; Tircso, G. Physico-chemical properties of MnII complexes formed with cis- and trans-DO2A: Thermodynamic, electrochemical and kinetic studies. J. Inorg. Biochem. 2016, 163, 206–213.
- Forgacs, A.; Tei, L.; Baranyai, Z.; Toth, I.; Zekany, L.; Botta, M. A Bisamide Derivative of –Solution Thermodynamic, Kinetic, and NMR Relaxometric Studies. Eur. J. Inorg. Chem. 2016, 2016, 1165–1174.
- Garda, Z.; Molnár, E.; Kálmán, F.K.; Botár, R.; Nagy, V.; Baranyai, Z.; Brücher, E.; Kovács, Z.; Tóth, I.; Tircsó, G. Effect of the Nature of Donor Atoms on the Thermodynamic, Kinetic and Relaxation Properties of Mn(II) Complexes Formed with Some Trisubstituted 12-Membered Macrocyclic Ligands. Front. Chem. 2018, 6, 232.
- Kálmán, F.K.; Tircsó, G. Kinetic inertness of the Mn2+ complexes formed with AAZTA and some open-chain EDTA derivatives. Inorg. Chem. 2012, 51, 10065–10067.
- Tei, L.; Gugliotta, G.; Fekete, M.; Kalman, F.K.; Botta, M. Mn(II) complexes of novel hexadentate AAZTA-like chelators: A solution thermodynamics and relaxometric study. Dalton Trans. 2011, 40, 2025–2032.
- Mo, Y.; Huang, C.; Liu, C.; Duan, Z.; Liu, J.; Wu, D. Recent Research Progress of 19F Magnetic Resonance Imaging Probes: Principle, Design, and Their Application. Macromol. Rapid Commun. 2023, 44, 2200744.
- Drahos, B.; Kotek, J.; Hermann, P.; Lukes, I.; Toth, E. Mn2+ Complexes with Pyridine-Containing 15-Membered Macrocycles: Thermodynamic, Kinetic, Crystallographic, and 1H/17O Relaxation Studies. Inorg. Chem. 2010, 49, 3224–3238.
- Pota, K.; Molnar, E.; Kalman, F.K.; Freire, D.M.; Tircso, G.; Green, K.N. Manganese Complex of a Rigidified 15-Membered Macrocycle: A Comprehensive Study. Inorg. Chem. 2020, 59, 11366–11376.
- Prazakova, M.; Ndiaye, D.; Toth, E.; Drahos, B. A seven-coordinate Mn(II) complex with a pyridine-based 15-membered macrocyclic ligand containing one acetate pendant arm: Structure, stability and relaxation properties. Dalton Trans. 2023, 52, 7936–7947.
- Nagendraraj, T.; Kumaran, S.S.; Mayilmurugan, R. Mn(II) complexes of phenylenediamine based macrocyclic ligands as T1-MRI contrast agents. J. Inorg. Biochem. 2022, 228, 111684–111692.
- Uzal-Varela, R.; Perez-Fernandez, F.; Valencia, L.; Rodriguez-Rodriguez, A.; Platas-Iglesias, C.; Caravan, P.; Esteban-Gomez, D. Thermodynamic stability of Mn(II) complexes with aminocarboxylate ligands analyzed using structural descriptors. Inorg. Chem. 2022, 61, 14173–14186.
- Rocklage, S.M.; Cacheris, W.P.; Quay, S.C.; Hahn, F.E.; Raymond, K.N. Manganese(II) N,N′-dipyridoxylethylenediamine-N,N′-diacetate 5,5′-bis(phosphate). Synthesis and characterization of a paramagnetic chelate for magnetic resonance imaging enhancement. Inorg. Chem. 1989, 28, 477–485.
- Elizondo, G.; Fretz, C.J.; Stark, D.D.; Rocklage, S.M.; Quay, S.C.; Worah, D.; Tsang, D.D.; Chen, M.C.; Ferrucci, J.T. Preclinical Evaluation of MnDPDP: New Paramagnetic Hepatobiliary Contrast Agent for MR Imaging. Radiology 1991, 178, 73–78.
- Islam, M.K.; Kim, S.; Kim, H.K.; Park, S.; Lee, G.H.; Kang, H.J.; Jung, J.C.; Park, J.S.; Kim, T.J.; Chang, Y. Manganese Complex of Ethylenediaminetetra acetic acid (EDTA)-Benzothiazole Aniline (BTA) Conjugate as a Potential Liver-Targeting MRI Contrast Agent. J. Med. Chem. 2017, 60, 2993–3001.
- Islam, M.K.; Kim, S.; Kim, H.K.; Kim, Y.H.; Lee, Y.M.; Choi, G.; Baek, A.R.; Sung, B.K.; Kim, M.; Cho, A.E.; et al. Synthesis and Evaluation of Manganese (II)-based Ethylenediaminetetraacetic Acid-Ethoxybenzyl Conjugate as a Highly Stable Hepatobiliary Magnetic Resonance Imaging Contrast Agent. Bioconjug. Chem. 2018, 29, 3614.
- McRae, S.W.; Cleary, M.; DeRoche, D.; Martinez, F.M.; Xia, Y.; Caravan, P.; Gale, E.M.; Ronald, J.A.; Scholl, T.J. Development of a Suite of Gadolinium-Free OATP1-Targeted Paramagnetic Probes for Liver MRI. J. Med. Chem. 2023, 66, 6567–6576.
- Hall, R.C.; Qin, J.; Laney, V.; Ayat, N.; Lu, Z.R. Manganese(II) EOB-Pyclen Diacetate for Liver-Specific MRI. ACS Appl. Bio Mater. 2022, 5, 451–458.
- Islam, M.K.; Baek, M.K.; Yang, A.R.; Kim, B.W.; Hwang, S.; Nam, D.W.; Lee, S.W.; Chang, G.H. Manganese (II) Complex of 1,4,7-Triazacyclononane-1,4,7- Triacetic Acid (NOTA) as a Hepatobiliary MRI Contrast Agent. Pharmaceuticals 2023, 16, 602.
- Wang, J.; Wang, H.; Ramsay, I.A.; Erstad, D.J.; Fuchs, B.C.; Tanabe, K.K.; Caravan, P.; Gale, E.M. Manganese-Based Contrast Agents for Magnetic Resonance Imaging of Liver Tumors: Structure Activity Relationships and Lead Candidate Evaluation. J. Med. Chem. 2018, 61, 8811–8824.
- Chen, K.; Li, P.; Zhu, C.; Xia, Z.; Xia, Q.; Zhong, L.; Xiao, B.; Cheng, T.; Wu, C.; Shen, C.; et al. Mn(II) Complex of Lipophilic Group-Modified Ethylenediaminetetraacetic Acid (EDTA) as a New Hepatobiliary MRI Contrast Agent. J. Med. Chem. 2021, 64, 9182–9192.
- Xue, Y.; Xiao, B.; Xia, Z.; Dai, L.; Xia, Q.; Zhong, L.; Zhu, C.; Zhu, J. A New OATP-mediated hepatobliliary-specific Mn(II)-based MRI contrast agent for hepatocellular carcinoma in mice: A comparison with Gd-EOB-DTPA. J. Magn. Reson. Imaging 2023, 58, 926–933.
- Ning, Y.; Zhou, I.Y.; Rotile, N.J.; Pantazopoulos, P.; Wang, H.; Barrett, S.C.; Sojoodi, M.; Tanabe, K.K.; Caravan, P. Dual Hydrazine-Equipped Turn-On Manganese-Based Probes for Magnetic Resonance Imaging of Liver Fibrogenesis. J. Am. Chem. Soc. 2022, 144, 16553–16558.
- Aime, S.; Anelli, P.L.; Botta, M.; Brocchetta, M.; Canton, S.; Fedeli, F.; Gianolio, E.; Terreno, E. Relaxometric evaluation of novel manganese(III) complexes for application as contrast agents in magnetic resonance imaging. J. Biol. Inorg. Chem. 2002, 7, 58–67.
- Troughton, J.S.; Greenfield, M.T.; Greenwood, J.M.; Dumas, S.; Wiethoff, A.J.; Wang, J.; Spiller, M.; McMurry, T.J.; Caravan, P. Synthesis and Evaluation of a High Relaxivity Manganese(II)-Based MRI Contrast Agent. Inorg. Chem. 2004, 43, 6313–6323.
- Baroni, S.; Serra, S.C.; Mingo, A.F.; Lux, G.; Giovenzana, G.B.; Lattuada, L. Synthesis and Relaxometric Characterization of a New Mn(II)-EDTA-Deoxycholic Acid Conjugate Complex as a Potential MRI Blood Pool Agent. Chem. Sel. 2016, 1, 1607–1612.
- Kalman, F.K.; Nagy, V.; Varadi, B.; Garda, Z.; Molnar, E.; Trencsenyi, G.; Kiss, J.; Même, S.; Meme, W.; Toth, E.; et al. Mn(II)-based MRI contrats agent candidate for vascular imaging. J. Med. Chem. 2020, 63, 6057.
- Forgacs, A.; Tei, L.; Baranyai, Z.; Esteban-Gomez, D.; Platas-Iglesias, C.; Botta, M. Optimising the relaxivities of Mn2+ complexes by targeting human serum albumin (HSA). Dalton Trans. 2017, 46, 8494–8504.
- Leone, L.; Anemone, A.; Carella, A.; Botto, E.; Longo, D.L.; Tei, L. A neutral and stable macrocyclic Mn(II) complex for MRI tumor visualization. ChemMedChem 2022, 17, e202200508.
- Zhou, Z.; Bai, R.; Wang, Z.; Bryant, H.; Lang, L.; Merkle, H.; Munasinghe, J.; Tang, L.; Tang, W.; Tian, R.; et al. An albumin-binding T1-T2 dual-modal MRI contrast agents for improved sensitivity and accuracy in tumor imaging. Bioconjug. Chem. 2019, 30, 1821–1829.
- Forgacs, A.; Regueiro-Figueroa, M.; Barriada, J.L.; Esteban-Gomez, D.; de Blas, A.; Rodriguez-Blas, T.; Botta, M.; Platas-Iglesis, C. Mono-, Bi-, and Trinuclear Bis-Hydrated Mn2+ Complexes as Potential MRI Contrast Agents. Inorg. Chem. 2015, 54, 9576.
- Su, H.; Wu, C.; Zhu, J.; Miao, T.; Wang, D.; Xia, C.; Zhao, X.; Gong, Q.; Song, B.; Ai, H. Rigid Mn(II) chelate as efficient MRI contrast agent for vascular imaging. Dalton Trans. 2012, 41, 14480.
- Rolla, G.; De Biasio, V.; Giovenzana, G.B.; Botta, M.; Tei, L. Supramolecular assemblies based on amphiphilic Mn2+-complexes as high relaxivity MRI probes. Dalton Trans. 2018, 47, 10660–10670.
- Mulas, G.; Rolla, G.A.; Geraldes, C.F.G.C.; Starmans, L.W.E.; Botta, M.; Terreno, E.; Tei, L. Mn(II)-Based Lipidic Nanovesicles as High-Efficiency MRI Probes. ACS Appl. Bio Mater. 2020, 3, 2401–2409.
- Liu, X.; Fu, S.; Xia, C.; Li, M.; Cai, Z.; Wu, C.; Lu, F.; Zhu, J.; Song, B.; Gong, Q.; et al. PEGylated amphiphilic polymeric manganese(II) complexes as magnetic resonance angiographic agents. J. Mater. Chem. B 2022, 10, 2204–2214.
- Chen, K.; Cai, Z.; Cao, Y.; Jiang, L.; Jiang, Y.; Gu, H.; Fu, S.; Xia, C.; Lui, S.; Gong, Q.; et al. Kinetically inert manganese (II)-based hybrid micellar complexes for magnetic resonance imaging of lymph node metastasis. Regen. Biomater. 2023, 10, rbad053.
- Swietach, P.; Vaughan-Jones, R.D.; Harris, A.L.; Hulikova, A. The chemistry, physiology, and pathology of pH in cancer. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130099.
- Botar, R.; Molnar, E.; Trencsenyi, G.; Kiss, J.; Kalman, F.K.; Tircso, G. Stable and inert Mn(II)-based and pH responsive contrast agents. J. Am. Chem. Soc. 2020, 142, 1662.
- Shen, X.; Pan, Y.; Liang, G. Development of macrocyclic Mn(II)-Bispyridine complexes as pH responsive magnetic resonance imaging contrast agents. Eur. J. Inorg. Chem. 2023, 26, e202200786.
- Loving, G.S.; Mukherjee, S.; Caravan, P. Redox-activated manganese-based MR contrast agent. J. Am. Chem. Soc. 2013, 135, 4620–4623.
- Gale, M.E.; Mukherjee, S.; Liu, C.; Loving, G.S.; Caravan, P. Structure-redox-relaxivity relationships for redox responsive manganese-based magnetic resonance imaging probes. Inorg. Chem. 2014, 53, 10748–10761.
- Gale, E.M.; Jones, C.M.; Ramsay, I.; Farrar, C.T.; Caravan, P. A Janus Chelator Enables Biochemically Responsive MRI Contrast with Exceptional Dynamic Range. J. Am. Chem. Soc. 2016, 138, 15861–15864.
- Yu, M.; Beyers, R.J.; Gorden, J.D.; Cross, J.N.; Goldsmith, C.R. A Magnetic Resonance Imaging Contrast Agent Capable of DetectingHydrogen Peroxide. Inorg. Chem. 2012, 51, 9153–9155.
- Yu, M.; Ambrose, S.L.; Whaley, Z.L.; Fan, S.; Gorden, J.D.; Beyers, R.J.; Schwartz, D.D.; Goldsmith, C.R. A Mononuclear Manganese(II) Complex Demonstrates a Strategy ToSimultaneously Image and Treat Oxidative Stress. J. Am. Chem. Soc. 2014, 136, 12836–12839.
- Li, Y.; Xia, Q.; Zhu, C.; Cao, W.; Xia, Z.; Liu, X.; Xiao, B.; Chen, K.; Liu, Y.; Zhong, L.; et al. An activatable Mn(II) MRI probe for detecting peroxidase activity in vitro and in vivo. J. Inorg. Biochem. 2022, 236, 111979.
- Botar, R.; Molnar, E.; Garda, Z.; Madarasi, E.; Trencsenyi, G.; Kiss, J.; Kalman, F.K.; Tircso, G. Synthesis and characterization of a stable and inert MnII-based ZnII responsive MRI probe for molecular imaging of glucose stimulated zinc secretion (GSZS). Inorg. Chem. Front. 2022, 9, 577–583.
- Vitor, T.; Martins, K.M.; Ionescu, T.M.; Cunha, M.L.; Baroni, R.H.; Garcia, M.R.T.; Wagner, J.; Campos Neto, G.d.C.; Nogueira, S.A.; Guerra, E.G.; et al. PET/MRI: A novel hybrid imaging technique. Major clinical indications and preliminary experience in Brazil. Einstein 2017, 15, 115–118.
- Vanasschen, C.; Monar, E.; Tircso, G.; Kalman, F.K.; Toth, E.; Brandt, M.; Coenen, H.H.; Neumaier, B. Novel CDTA-based, Bifunctional Chelators for Stable and Inert MnII Complexation: Synthesis and Physicochemical Characterization. Inorg. Chem. 2017, 56, 7746–7760.
- Csupasz, T.; Szücs, D.; Kalman, F.K.; Holloczki, O.; Fekete, A.; Szikra, D.; Toth, E.; Toth, I.; Tircso, G. A new oxygen containing pyclen-type ligand as a manganese(II) binder for MRI and 52Mn PET applications: Equilibrium, kinetic, relaxometric, structural and radiochemical studies. Molecules 2022, 27, 371.
- Sathiyajith, C.; Hallett, A.J.; Edwards, P.G. Synthesis, photophysical characterization, relaxometric studies and molecular docking studies of gadolinium-free contrast agents for dual modal imaging. Results Chem. 2022, 4, 100307.
- Feng, Z.; Zhu, T.; Wang, L.; Yuan, T.; Jiang, Y.; Tian, X.; Tian, Y.; Zhang, Q. Coordination-Regulated Terpyridine–Mn(II) Complexes for Photodynamic Therapy Guided by Multiphoton Fluorescence/Magnetic Resonance Imaging. Inorg. Chem. 2022, 61, 12652–12661.
- Borodin, O.Y.; Sannikov, M.Y.; Belyanin, M.L.; Filimonov, V.D.; Usov, V.Y.; Rybakov, Y.L.; Gukasov, V.M.; Shimanovskii, N.L. Relaxivity of Paramagnetic complexes of Manganese and Gadolinium. Pharm. Chem. J. 2019, 53, 635–637.
- Forgács, A.; Pujales-Paradela, R.; Regueiro-Figueroa, M.; Valencia, L.; Esteban, D.; Botta, M.; Platas-Iglesias, C. Platas-Iglesias. Developing the family of picolinate ligands for Mn2+ complexation. Dalton Trans. 2017, 46, 1546–1558.
- Pujales-Paradela, R.; Carniato, F.; Uzal-Varela, R.; Brandariz, I.; Iglesias, E.; Platas-Iglesias, C.; Botta, M.; Esteban-Gómez, D. A pentadentate member of the picolinate family for Mn(ii) complexation and an amphiphilic derivative. Dalton Trans. 2019, 48, 696–710.
- Martinelli, J.; Callegari, E.; Baranyai, Z.; Fraccarollo, A.; Cossi, M.; Tei, L. Semi-Rigid (Aminomethyl) Piperidine-Based Pentadentate Ligands for Mn(II) Complexation. Molecules 2021, 26, 5993.
- Garda, Z.; Molnar, E.; Hamon, N.; Barriada, J.L.; Esteban-Gomez, D.; Varadi, B.; Nagy, V.; Pota, K.; Kalman, F.K.; Toth, I.; et al. Complexation of Mn(II) by Rigid Pyclen Diacetates: Equilibrium, Kinetic, Relaxometric, Density Functional Theory, and Superoxide Dismutase Activity Studies. Inorg. Chem. 2021, 60, 1133–1148.




