
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mauro Maniscalco | -- | 3263 | 2023-11-02 09:42:10 | | | |
| 2 | Peter Tang | Meta information modification | 3263 | 2023-11-02 10:16:36 | | |
Video Upload Options
Nitric oxide (NO) is a short-lived gas molecule which has been studied for its role as a signaling molecule in the vasculature and later, in a broader view, as a cellular messenger in many other biological processes such as immunity and inflammation, cell survival, apoptosis, and aging. Fractional exhaled nitric oxide (FeNO) is a convenient, easy-to-obtain, and non-invasive method for assessing active, mainly Th2-driven, airway inflammation, which is sensitive to treatment with standard anti-inflammatory therapy. Consequently, FeNO serves as a valued tool to aid the diagnosis and monitoring of several asthma phenotypes. FeNO has been evaluated in several other respiratory and/or immunological conditions, including allergic rhinitis, chronic rhinosinusitis with/without nasal polyps, atopic dermatitis, eosinophilic esophagitis, and food allergy.
1. Introduction
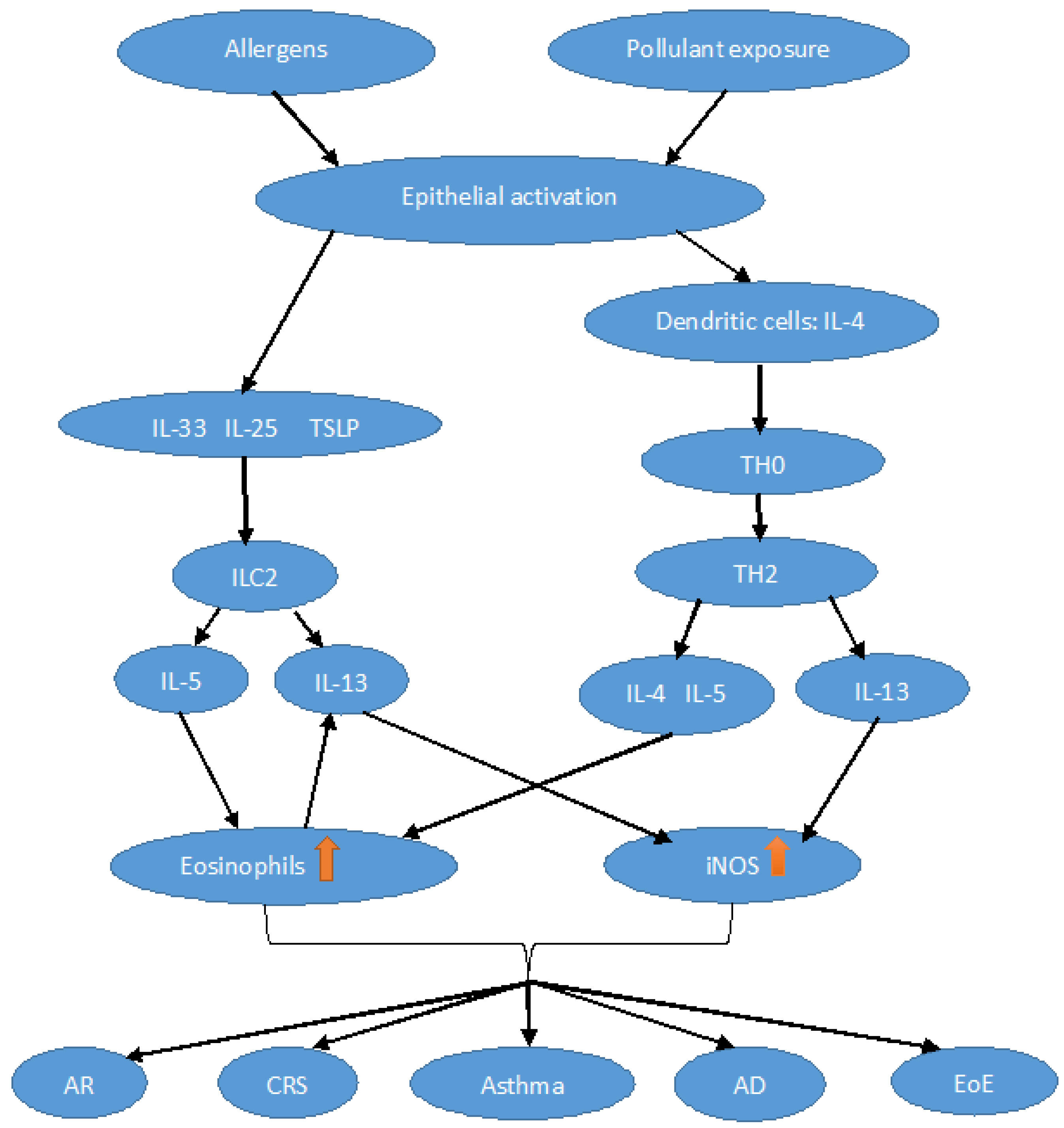
2. Physiopathological Aspects
3. Exhaled NO in Type 2 Lower Airway Diseases
3.1. FeNO in T2 High Asthma
3.2. FeNO in Cough Variant Asthma
3.3. FeNO in COPD
4. Exhaled NO in Type 2 Upper Airway Diseases
4.1. FeNO in Allergic Rhinitis
4.2. FeNO in Chronic Rhino-Sinusitis
5. FeNO in Eosinophilic Esophagitis
6. Exhaled NO in Other Type 2 Diseases
6.1. Atopic Dermatitis
6.2. Food Allergy
References
- Kiss, H.; Orlos, Z.; Gellert, A.; Megyesfalvi, Z.; Mikaczo, A.; Sarkozi, A.; Vasko, A.; Miklos, Z.; Horvath, I. Exhaled Biomarkers for Point-of-Care Diagnosis: Recent Advances and New Challenges in Breathomics. Micromachines 2023, 14, 391.
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142.
- Escamilla-Gil, J.M.; Fernandez-Nieto, M.; Acevedo, N. Understanding the Cellular Sources of the Fractional Exhaled Nitric Oxide (FeNO) and Its Role as a Biomarker of Type 2 Inflammation in Asthma. Biomed. Res. Int. 2022, 2022, 5753524.
- American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 2005, 171, 912–930.
- Medrek, S.K.; Parulekar, A.D.; Hanania, N.A. Predictive Biomarkers for Asthma Therapy. Curr. Allergy Asthma Rep. 2017, 17, 69.
- Mormile, M.; Mormile, I.; Fuschillo, S.; Rossi, F.W.; Lamagna, L.; Ambrosino, P.; de Paulis, A.; Maniscalco, M. Eosinophilic Airway Diseases: From Pathophysiological Mechanisms to Clinical Practice. Int. J. Mol. Sci. 2023, 24, 7254.
- Alving, K.; Malinovschi, A. Basic aspects of exhaled nitric oxide. Eur. Respir. Monogr. 2010, 49, 1–31.
- Bahadoran, Z.; Carlstrom, M.; Mirmiran, P.; Ghasemi, A. Nitric oxide: To be or not to be an endocrine hormone? Acta. Physiol. 2020, 229, e13443.
- Hoiland, R.L.; Caldwell, H.G.; Howe, C.A.; Nowak-Fluck, D.; Stacey, B.S.; Bailey, D.M.; Paton, J.F.R.; Green, D.J.; Sekhon, M.S.; Macleod, D.B.; et al. Nitric oxide is fundamental to neurovascular coupling in humans. J. Physiol. 2020, 598, 4927–4939.
- Horvath, I.; Sandor, N.T.; Ruttner, Z.; McLaughlin, A.C. Role of nitric oxide in regulating cerebrocortical oxygen consumption and blood flow during hypercapnia. J. Cereb. Blood Flow Metab. 1994, 14, 503–509.
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479.
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837.
- Marcos, M.C.; Cisneros Serrano, C. What is the added value of FeNO as T2 biomarker? Front. Allergy 2022, 3, 957106.
- Spector, B.M.; Shusterman, D.J.; Zhao, K. Nasal nitric oxide flux from the paranasal sinuses. Curr. Opin. Allergy Clin. Immunol. 2023, 23, 22–28.
- Van der Veen, R.C. Nitric oxide and T helper cell immunity. Int. Immunopharmacol. 2001, 1, 1491–1500.
- Marcuccio, G.; Ambrosino, P.; Merola, C.; Manzo, F.; Motta, A.; Rea, G.; Cantone, E.; Maniscalco, M. Clinical Applications of Nasal Nitric Oxide in Allergic Rhinitis: A Review of the Literature. J. Clin. Med. 2023, 12, 81.
- Brindicci, C.; Kharitonov, S.A.; Ito, M.; Elliott, M.W.; Hogg, J.C.; Barnes, P.J.; Ito, K. Nitric oxide synthase isoenzyme expression and activity in peripheral lung tissue of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 181, 21–30.
- Ricciardolo, F.L.; Caramori, G.; Ito, K.; Capelli, A.; Brun, P.; Abatangelo, G.; Papi, A.; Chung, K.F.; Adcock, I.; Barnes, P.J.; et al. Nitrosative stress in the bronchial mucosa of severe chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2005, 116, 1028–1035.
- Maarsingh, H.; Leusink, J.; Bos, I.S.; Zaagsma, J.; Meurs, H. Arginase strongly impairs neuronal nitric oxide-mediated airway smooth muscle relaxation in allergic asthma. Respir. Res. 2006, 7, 6.
- Adler, B.L.; Friedman, A.J. Nitric oxide therapy for dermatologic disease. Future. Sci. OA 2015, 1, FSO37.
- Bradley, S.A.; Steinert, J.R. Nitric Oxide-Mediated Posttranslational Modifications: Impacts at the Synapse. Oxidative Med. Cell. Longev. 2016, 2016, 5681036.
- Nakamura, T.; Lipton, S.A. Emerging roles of S-nitrosylation in protein misfolding and neurodegenerative diseases. Antioxid. Redox Signal. 2008, 10, 87–101.
- Tripathi, M.K.; Kartawy, M.; Amal, H. The role of nitric oxide in brain disorders: Autism spectrum disorder and other psychiatric, neurological, and neurodegenerative disorders. Redox Biol. 2020, 34, 101567.
- Haun, F.; Nakamura, T.; Shiu, A.D.; Cho, D.H.; Tsunemi, T.; Holland, E.A.; La Spada, A.R.; Lipton, S.A. S-nitrosylation of dynamin-related protein 1 mediates mutant huntingtin-induced mitochondrial fragmentation and neuronal injury in Huntington’s disease. Antioxid. Redox Signal. 2013, 19, 1173–1184.
- Barcellos, L.F.; Begovich, A.B.; Reynolds, R.L.; Caillier, S.J.; Brassat, D.; Schmidt, S.; Grams, S.E.; Walker, K.; Steiner, L.L.; Cree, B.A.; et al. Linkage and association with the NOS2A locus on chromosome 17q11 in multiple sclerosis. Ann. Neurol. 2004, 55, 793–800.
- Kanwar, J.R.; Kanwar, R.K.; Krissansen, G.W. Simultaneous neuroprotection and blockade of inflammation reverses autoimmune encephalomyelitis. Brain 2004, 127, 1313–1331.
- Smith, K.J.; Lassmann, H. The role of nitric oxide in multiple sclerosis. Lancet Neurol. 2002, 1, 232–241.
- Yao, D.; Gu, Z.; Nakamura, T.; Shi, Z.Q.; Ma, Y.; Gaston, B.; Palmer, L.A.; Rockenstein, E.M.; Zhang, Z.; Masliah, E.; et al. Nitrosative stress linked to sporadic Parkinson’s disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc. Natl. Acad. Sci. USA 2004, 101, 10810–10814.
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189.
- Antosova, M.; Mokra, D.; Pepucha, L.; Plevkova, J.; Buday, T.; Sterusky, M.; Bencova, A. Physiology of nitric oxide in the respiratory system. Physiol. Res. 2017, 66, S159–S172.
- Yatera, K.; Mukae, H. Possible pathogenic roles of nitric oxide in asthma. Respir. Investig. 2019, 57, 295–297.
- Akata, K.; Yatera, K.; Wang, K.Y.; Naito, K.; Ogoshi, T.; Noguchi, S.; Kido, T.; Toyohira, Y.; Shimokawa, H.; Yanagihara, N.; et al. Decreased Bronchial Eosinophilic Inflammation and Mucus Hypersecretion in Asthmatic Mice Lacking All Nitric Oxide Synthase Isoforms. Lung 2016, 194, 121–124.
- Wenzel, S.; Castro, M.; Corren, J.; Maspero, J.; Wang, L.; Zhang, B.; Pirozzi, G.; Sutherland, E.R.; Evans, R.R.; Joish, V.N.; et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: A randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016, 388, 31–44.
- Corren, J.; Lemanske, R.F.; Hanania, N.A.; Korenblat, P.E.; Parsey, M.V.; Arron, J.R.; Harris, J.M.; Scheerens, H.; Wu, L.C.; Su, Z.; et al. Lebrikizumab treatment in adults with asthma. N. Engl. J. Med. 2011, 365, 1088–1098.
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet 2012, 380, 651–659.
- Chibana, K.; Trudeau, J.B.; Mustovich, A.T.; Hu, H.; Zhao, J.; Balzar, S.; Chu, H.W.; Wenzel, S.E. IL-13 induced increases in nitrite levels are primarily driven by increases in inducible nitric oxide synthase as compared with effects on arginases in human primary bronchial epithelial cells. Clin. Exp. Allergy 2008, 38, 936–946.
- Suresh, V.; Mih, J.D.; George, S.C. Measurement of IL-13-induced iNOS-derived gas phase nitric oxide in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2007, 37, 97–104.
- Justiz Vaillant, A.A.; Modi, P.; Jan, A. Atopy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Ahmad, J.G.; Marino, M.J.; Luong, A.U. Unified Airway Disease: Future Directions. Otolaryngol. Clin. N. Am. 2023, 56, 181–195.
- Maspero, J.; Adir, Y.; Al-Ahmad, M.; Celis-Preciado, C.A.; Colodenco, F.D.; Giavina-Bianchi, P.; Lababidi, H.; Ledanois, O.; Mahoub, B.; Perng, D.W.; et al. Type 2 inflammation in asthma and other airway diseases. ERJ Open Res. 2022, 8, 00576–2021.
- Popovic-Grle, S.; Stajduhar, A.; Lampalo, M.; Rnjak, D. Biomarkers in Different Asthma Phenotypes. Genes 2021, 12, 801.
- Kharitonov, S.A.; Yates, D.; Robbins, R.A.; Logan-Sinclair, R.; Shinebourne, E.A.; Barnes, P.J. Increased nitric oxide in exhaled air of asthmatic patients. Lancet 1994, 343, 133–135.
- Kharitonov, S.A.; Chung, K.F.; Evans, D.; O’Connor, B.J.; Barnes, P.J. Increased exhaled nitric oxide in asthma is mainly derived from the lower respiratory tract. Am. J. Respir. Crit. Care Med. 1996, 153, 1773–1780.
- Jatakanon, A.; Lim, S.; Kharitonov, S.A.; Chung, K.F.; Barnes, P.J. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax 1998, 53, 91–95.
- Chen, J.; Lin, W.; Gu, H.; Ying, K.; Li, T.; Shao, H. Study on the Relationship Between Bronchoalveolar Lavage Fluid Cell Count, Th1/Th2 Cytokines and Pulmonary Function in Patients with Cough Variant Asthma. J. Asthma Allergy 2022, 15, 1713–1720.
- Lai, K.; Shen, H.; Zhou, X.; Qiu, Z.; Cai, S.; Huang, K.; Wang, Q.; Wang, C.; Lin, J.; Hao, C.; et al. Clinical Practice Guidelines for Diagnosis and Management of Cough-Chinese Thoracic Society (CTS) Asthma Consortium. J. Thorac. Dis. 2018, 10, 6314–6351.
- Kim, C.K.; Kim, J.T.; Kang, H.; Yoo, Y.; Koh, Y.Y. Sputum eosinophilia in cough-variant asthma as a predictor of the subsequent development of classic asthma. Clin. Exp. Allergy 2003, 33, 1409–1414.
- Lougheed, M.D.; Turcotte, S.E.; Fisher, T. Cough variant asthma: Lessons learned from deep inspirations. Lung 2012, 190, 17–22.
- Chen, F.-j.; Huang, X.-y.; Lin, G.-p.; Liu, Y.-l.; Xie, C.-m. Validity of fractional exhaled nitric oxide and small airway function indices in diagnosis of cough-variant asthma. J. Asthma 2018, 55, 750–755.
- Paredi, P.; Kharitonov, S.A.; Meah, S.; Barnes, P.J.; Usmani, O.S. A novel approach to partition central and peripheral airway nitric oxide. Chest 2014, 145, 113–119.
- Bai, H.; Shi, C.; Yu, S.; Wen, S.; Sha, B.; Xu, X.; Yu, L. A comparative study on the value of lower airway exhaled nitric oxide combined with small airway parameters for diagnosing cough-variant asthma. Ther. Adv. Respir. Dis. 2023, 17, 17534666231181259.
- Wang, Y.; Zhao, L.; Chen, F.; Guo, Y.; Ma, H.; Han, B.; Yi, J.; Kong, X. Diagnostic Value of Fractional Exhaled Nitric Oxide and Small Airway Function in Differentiating Cough-Variant Asthma from Typical Asthma. Can. Respir. J. 2021, 2021, 9954411.
- Zhu, H.; Zhang, R.; Hao, C.; Yu, X.; Tian, Z.; Yuan, Y. Fractional Exhaled Nitric Oxide (FeNO) Combined with Pulmonary Function Parameters Shows Increased Sensitivity and Specificity for the Diagnosis of Cough Variant Asthma in Children. Med. Sci. Monit. 2019, 25, 3832–3838.
- Chen, L.C.; Zeng, G.S.; Wu, L.L.; Zi, M.; Fang, Z.K.; Fan, H.Z.; Yu, H.P. Diagnostic value of FeNO and MMEF for predicting cough variant asthma in chronic cough patients with or without allergic rhinitis. J. Asthma 2021, 58, 326–333.
- Shebl, E.; Abdel-moety, H. Assessment of the role of fractional exhaled nitric oxide as a predictor of airway eosinophilia and corticosteroid responsiveness in patients with chronic cough. Egypt. J. Bronchol. 2020, 14, 15.
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27.
- Fernandes, L.; Rane, S.; Mandrekar, S.; Mesquita, A.M. Eosinophilic Airway Inflammation in Patients with Stable Biomass Smoke-versus Tobacco Smoke-Associated Chronic Obstructive Pulmonary Disease. J. Health Pollut. 2019, 9, 191209.
- Singh, D.; Kolsum, U.; Brightling, C.E.; Locantore, N.; Agusti, A.; Tal-Singer, R.; ECLIPSE Investigators. Eosinophilic inflammation in COPD: Prevalence and clinical characteristics. Eur. Respir. J. 2014, 44, 1697–1700.
- Ansarin, K.; Chatkin, J.M.; Ferreira, I.M.; Gutierrez, C.A.; Zamel, N.; Chapman, K.R. Exhaled nitric oxide in chronic obstructive pulmonary disease: Relationship to pulmonary function. Eur. Respir. J. 2001, 17, 934–938.
- Chen, F.J.; Huang, X.Y.; Liu, Y.L.; Lin, G.P.; Xie, C.M. Importance of fractional exhaled nitric oxide in the differentiation of asthma-COPD overlap syndrome, asthma, and COPD. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2385–2390.
- Malerba, M.; Radaeli, A.; Olivini, A.; Damiani, G.; Ragnoli, B.; Montuschi, P.; Ricciardolo, F.L. Exhaled nitric oxide as a biomarker in COPD and related comorbidities. Biomed. Res. Int. 2014, 2014, 271918.
- Zietkowski, Z.; Kucharewicz, I.; Bodzenta-Lukaszyk, A. The influence of inhaled corticosteroids on exhaled nitric oxide in stable chronic obstructive pulmonary disease. Respir. Med. 2005, 99, 816–824.
- Agusti, A.G.; Villaverde, J.M.; Togores, B.; Bosch, M. Serial measurements of exhaled nitric oxide during exacerbations of chronic obstructive pulmonary disease. Eur. Respir. J. 1999, 14, 523–528.
- Alcazar-Navarrete, B.; Ruiz Rodriguez, O.; Conde Baena, P.; Romero Palacios, P.J.; Agusti, A. Persistently elevated exhaled nitric oxide fraction is associated with increased risk of exacerbation in COPD. Eur. Respir. J. 2018, 51, 1701457.
- Maziak, W.; Loukides, S.; Culpitt, S.; Sullivan, P.; Kharitonov, S.A.; Barnes, P.J. Exhaled nitric oxide in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 157, 998–1002.
- De Laurentiis, G.; Maniscalco, M.; Cianciulli, F.; Stanziola, A.; Marsico, S.; Lundberg, J.O.; Weitzberg, E.; Sofia, M. Exhaled nitric oxide monitoring in COPD using a portable analyzer. Pulm. Pharmacol. Ther. 2008, 21, 689–693.
- Schumann, D.M.; Papakonstantinou, E.; Kostikas, K.; Grize, L.; Tamm, M.; Stolz, D. Variability of fractional exhaled nitric oxide is associated with the risk and aetiology of COPD exacerbations. Respirology 2023, 28, 445–454.
- Mormile, I.; Granata, F.; Detoraki, A.; Pacella, D.; Della Casa, F.; De Rosa, F.; Romano, A.; de Paulis, A.; Rossi, F.W. Predictive Response to Immunotherapy Score: A Useful Tool for Identifying Eligible Patients for Allergen Immunotherapy. Biomedicines 2022, 10, 971.
- Di Spigna, G.; Ladogana, P.; Covelli, B.; Ricciardone, M.; Salzano, S.; Spalletti Cernia, D.; Mormile, I.; Varriale, G.; Catapano, O.; Spadaro, G.; et al. Component resolved diagnosis by recombinant allergens in patients with allergies to inhalants. J. Biol. Regul. Homeost. Agents 2020, 34, 1729–1737.
- Brindisi, G.; De Vittori, V.; De Nola, R.; Di Mauro, A.; De Castro, G.; Baldassarre, M.E.; Cicinelli, E.; Cinicola, B.; Duse, M.; Zicari, A.M. The Role of Nasal Nitric Oxide and Anterior Active Rhinomanometry in the Diagnosis of Allergic Rhinitis and Asthma: A Message for Pediatric Clinical Practice. J. Asthma Allergy 2021, 14, 265–274.
- Striz, I.; Golebski, K.; Strizova, Z.; Loukides, S.; Bakakos, P.; Hanania, N.A.; Jesenak, M.; Diamant, Z. New insights into the pathophysiology and therapeutic targets of asthma and comorbid chronic rhinosinusitis with or without nasal polyposis. Clin. Sci. 2023, 137, 727–753.
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464.
- Bachert, C.; Bhattacharyya, N.; Desrosiers, M.; Khan, A.H. Burden of Disease in Chronic Rhinosinusitis with Nasal Polyps. J. Asthma Allergy 2021, 14, 127–134.
- Laidlaw, T.M.; Mullol, J.; Woessner, K.M.; Amin, N.; Mannent, L.P. Chronic Rhinosinusitis with Nasal Polyps and Asthma. J. Allergy Clin. Immunol. Pract. 2021, 9, 1133–1141.
- Harrison, T.W.; Chanez, P.; Menzella, F.; Canonica, G.W.; Louis, R.; Cosio, B.G.; Lugogo, N.L.; Mohan, A.; Burden, A.; McDermott, L.; et al. Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab (ANDHI): A randomised, controlled, phase 3b trial. Lancet Respir. Med. 2021, 9, 260–274.
- Paoletti, G.; Melone, G.; Guida, G.; Pirola, F.; Malvezzi, L.; Pelaia, C.; Mariani, A.; Racca, F.; Malipiero, G.; Ferri, S.; et al. Extended nitric oxide analysis in patients with chronic rhinosinusitis with nasal polyps, with or without associated asthma. J. Breath Res. 2020, 15, 016007.
- Straumann, A.; Katzka, D.A. Diagnosis and Treatment of Eosinophilic Esophagitis. Gastroenterology 2018, 154, 346–359.
- Steinbach, E.C.; Hernandez, M.; Dellon, E.S. Eosinophilic Esophagitis and the Eosinophilic Gastrointestinal Diseases: Approach to Diagnosis and Management. J. Allergy Clin. Immunol. Pract. 2018, 6, 1483–1495.
- Leung, J.; Nguyen-Traxler, A.; Lee, E.M.; Yip, J.S.; Weinstock, J.V.; Chan, W.W.; Ngo, P.; Weinstein, B.J.; Bonis, P.A. Assessment of fractionated exhaled nitric oxide as a biomarker for the treatment of eosinophilic esophagitis. Allergy Asthma Proc. 2012, 33, 519–524.
- Furuta, G.T.; Liacouras, C.A.; Collins, M.H.; Gupta, S.K.; Justinich, C.; Putnam, P.E.; Bonis, P.; Hassall, E.; Straumann, A.; Rothenberg, M.E.; et al. Eosinophilic esophagitis in children and adults: A systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007, 133, 1342–1363.
- Fernandez-Becker, N.Q.; Raja, S.; Scarpignato, C.; Lynch, K.L.; Ahuja, N.K.; Horsley-Silva, J.L. Eosinophilic esophagitis: Updates on key unanswered questions. Ann. N. Y. Acad. Sci. 2020, 1481, 30–42.
- Salava, A.; Rieppo, R.; Lauerma, A.; Salo, V. Age-dependent Distribution of Atopic Dermatitis in Primary Care: A Nationwide Population-based Study from Finland. Acta Derm. Venereol. 2022, 102, adv00738.
- Tsakok, T.; Woolf, R.; Smith, C.H.; Weidinger, S.; Flohr, C. Atopic dermatitis: The skin barrier and beyond. Br. J. Dermatol. 2019, 180, 464–474.
- Czubaj-Kowal, M.; Nowicki, G.J.; Kurzawa, R.; Polak, M.; Slusarska, B. Factors Influencing the Concentration of Exhaled Nitric Oxide (FeNO) in School Children Aged 8–9-Years-Old in Krakow, with High FeNO Values ≥ 20 ppb. Medicina 2022, 58, 146.
- Kumar, R.; Gupta, N. Exhaled nitric oxide atopy, and spirometry in asthma and rhinitis patients in India. Adv. Respir. Med. 2017, 85, 186–192.
- Akdis, C.A.; Arkwright, P.D.; Bruggen, M.C.; Busse, W.; Gadina, M.; Guttman-Yassky, E.; Kabashima, K.; Mitamura, Y.; Vian, L.; Wu, J.; et al. Type 2 immunity in the skin and lungs. Allergy 2020, 75, 1582–1605.
- Hanusch, B.; Sinningen, K.; Brinkmann, F.; Dillenhofer, S.; Frank, M.; Jockel, K.H.; Koerner-Rettberg, C.; Holtmann, M.; Legenbauer, T.; Langrock, C.; et al. Characterization of the L-Arginine/Nitric Oxide Pathway and Oxidative Stress in Pediatric Patients with Atopic Diseases. Int. J. Mol. Sci. 2022, 23, 2136.
- Sicherer, S.H.; Sampson, H.A. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J. Allergy Clin. Immunol. 2014, 133, 291–307.
- Bock, S.A.; Munoz-Furlong, A.; Sampson, H.A. Fatalities due to anaphylactic reactions to foods. J. Allergy Clin. Immunol. 2001, 107, 191–193.
- Hourihane, J.O. Peanut allergy. Pediatr. Clin. North Am. 2011, 58, 445–458.
- Hughes, J.L.; Brown, T.; Edgar, J.D.; Shields, M.D. Peanut allergy and allergic airways inflammation. Pediatr. Allergy Immunol. 2010, 21, 1107–1113.
- Preece, K.; Bhatia, R.; Belcher, J.; Patchett, K.; McElduff, P.; Collison, A.; Mattes, J. The fraction of exhaled nitric oxide improves prediction of clinical allergic reaction to peanut challenge in children. Clin. Exp. Allergy 2014, 44, 371–380.
- Percival, E.; Bhatia, R.; Preece, K.; McElduff, P.; McEvoy, M.; Collison, A.; Mattes, J. Reproducibility of serum IgE, Ara h2 skin prick testing and fraction of exhaled nitric oxide for predicting clinical peanut allergy in children. Allergy Asthma Clin. Immunol. 2016, 12, 35.
- Percival, E.; Bhatia, R.; Preece, K.; McEvoy, M.; Collison, A.; Mattes, J. Change in exhaled nitric oxide during peanut challenge is related to severity of reaction. Allergy Asthma Clin. Immunol. 2020, 16, 64.
- Patelis, A.; Alving, K.; Middelveld, R.; James, A.; Ono, J.; Ohta, S.; Izuhara, K.; Borres, M.P.; Forsberg, B.; Janson, C.; et al. IgE sensitization to food allergens and airborne allergens in relation to biomarkers of type 2 inflammation in asthma. Clin. Exp. Allergy 2018, 48, 1147–1154.




