Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pasquale Buonanno | -- | 2552 | 2023-11-01 22:31:40 | | | |
| 2 | Rita Xu | -9 word(s) | 2543 | 2023-11-02 02:40:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Buonanno, P.; Marra, A.; Iacovazzo, C.; Vargas, M.; Nappi, S.; Squillacioti, F.; De Siena, A.U.; Servillo, G. The PATIENT Approach. Encyclopedia. Available online: https://encyclopedia.pub/entry/51056 (accessed on 14 January 2026).
Buonanno P, Marra A, Iacovazzo C, Vargas M, Nappi S, Squillacioti F, et al. The PATIENT Approach. Encyclopedia. Available at: https://encyclopedia.pub/entry/51056. Accessed January 14, 2026.
Buonanno, Pasquale, Annachiara Marra, Carmine Iacovazzo, Maria Vargas, Serena Nappi, Francesco Squillacioti, Andrea Uriel De Siena, Giuseppe Servillo. "The PATIENT Approach" Encyclopedia, https://encyclopedia.pub/entry/51056 (accessed January 14, 2026).
Buonanno, P., Marra, A., Iacovazzo, C., Vargas, M., Nappi, S., Squillacioti, F., De Siena, A.U., & Servillo, G. (2023, November 01). The PATIENT Approach. In Encyclopedia. https://encyclopedia.pub/entry/51056
Buonanno, Pasquale, et al. "The PATIENT Approach." Encyclopedia. Web. 01 November, 2023.
Copy Citation
PATIENT (P: patient’s perception; A: assessment; T: tailored approach; I: iterative evaluation; E: education; N: non-pharmacological approach; T: team), a bundle which can help to summarize all the steps to follow in the management of chronic pain.
chronic pain
management
assessment
education
1. Introduction
Chronic pain affects about 30% of the adult population and it represents a crucial global health problem with a dramatic impact on socioeconomic systems [1]. Patients affected by chronic pain can experience a decline in quality of life, physical function, productivity, mental health, and social interaction [2]. Even though many recommendations have been published and several pharmacological and minimally invasive treatments are available, chronic pain continues to be overlooked and millions of people are still under-managed [3].
Pain management is one of the most difficult challenges for physicians as many factors can influence the way every patient experiences pain and reports his symptoms; moreover, different mechanisms can be involved in pain pathophysiology and they have to be carefully considered during a patient’s assessment and before choosing the adequate treatment among all the pharmacological and minimally invasive options so far available [4]. In addition, it is fundamental to periodically evaluate symptoms (as pain could change its characteristics over time), and to establish treatment efficacy in order to tailor the therapy to the patient’s needs and responses to it. Given the complexity of pain etiology and management, a multidisciplinary approach is mandatory and the healthcare team should have a different composition according to pain etiology and the patient’s characteristics.
The PATIENT bundle includes Patient’s perception, Assessment, Tailored approach, Iterative evaluation, Education, No pharmacological approach, and Team (Figure 1).
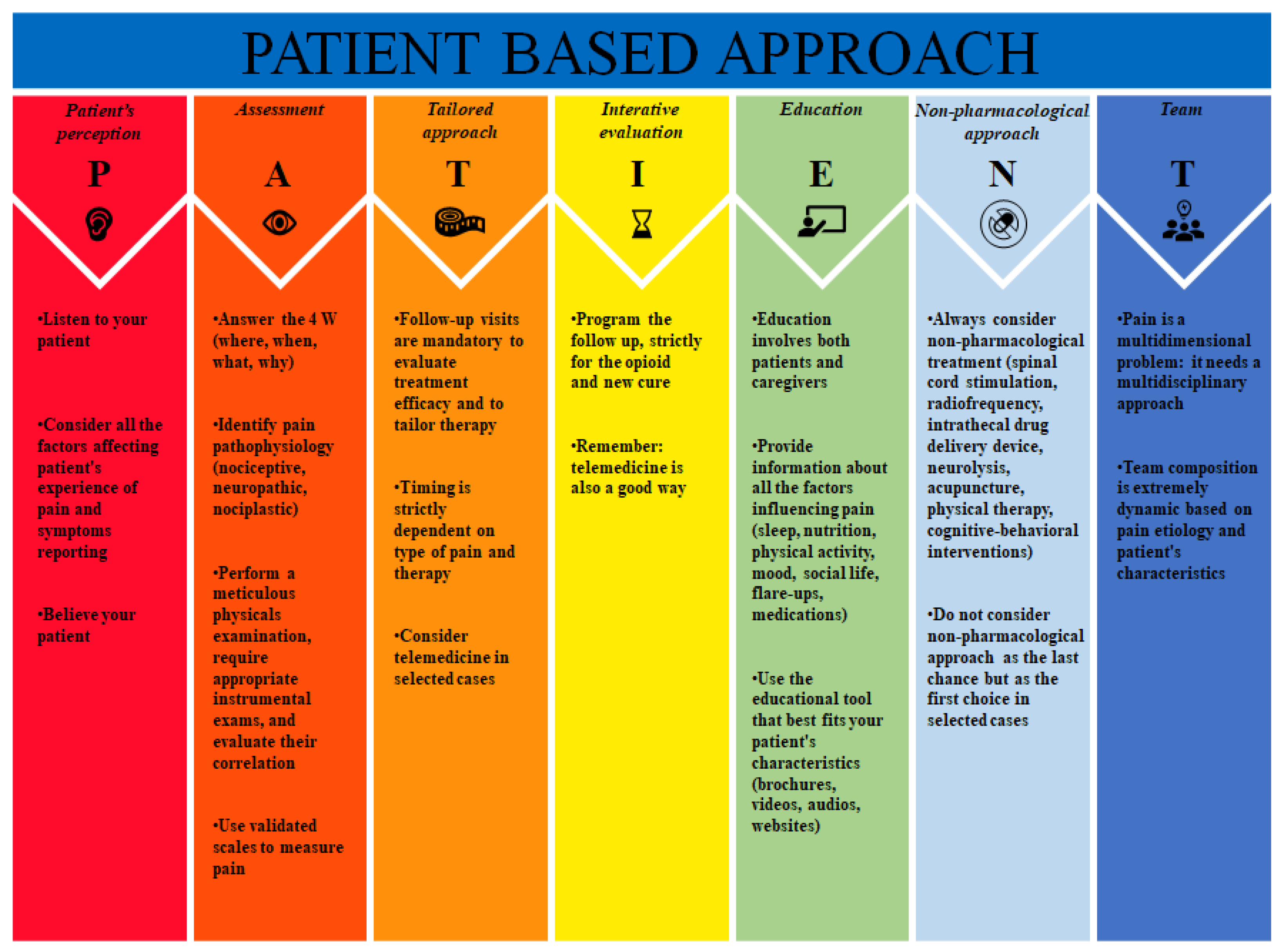
Figure 1. PATIENT bundle.
2. PATIENT Approach
2.1. Patient’s Perception
Pain is considered the fifth vital sign and the primary symptom that induces people to ask for medical assistance [5]. Many factors can alter nociception and influence the pain experience, so the same stimulus can be perceived in different ways by different subjects.
Advanced age is associated with an increased number of pain sites, higher pain-related disability, and increased pain threshold for low-intensity stimuli, which are differently processed in the insula and somatosensory cortex [6][7]. Older people experience a loss of exteroceptive function mediated by C-fibers, so they are at higher risk of burns, injuries, and bruises. Lautenbacher et al. introduced the concept of “presbyalgos” to underline the reduction in pain sensitivity with age; the pain threshold, for some types of stimuli such as pressure or electricity, seems to not be influenced by age because deep tissue nociceptors and the direct activation of A-fibers are not impaired. Regarding pain tolerance (i.e., the maximum stimulus a subject can bear), although correlated with pain threshold (i.e., the minimum intensity of a stimulus experienced as painful), it showed no change with age; this could be explained by a faster rise in the perception of pain intensity as the energy of the stimulus increases [7].
2.2. Assessment
The assessment of pain is a critical step to provide adequate pain management and the lack of a standardized approach is one of the most problematic barriers to establish a tailored treatment [8].
Pain can be classified according to the underlying pathophysiological mechanism: in fact, it can arise from the stimulation of nociceptors in response to inflammation or damage (nociceptive pain), or from an injury or a disease of the peripheral or central nervous system (neuropathic pain). Recently, a definition of nociplastic pain has been introduced, defined as “pain that arises from altered nociception despite no clear evidence of actual or threatened tissue damage” [9].
During a patient’s assessment, a general medical history often reveals important co-factors, such as job, socioeconomic status, prior trauma, mood disorders, social life, as well as previous and current exposures to psychological and physical stimuli. It is recommended to report age, body weight, comorbidities, genetic status, and previous exposure to analgesic medication.
The second step is to investigate the four W of pain assessment:
- -
-
Where: the localization of pain;
- -
-
When: the moment the pain started and the periodicity and frequency of its worsening;
- -
-
What: what are the sensations (e.g., burning, stabbing, itching);
- -
-
Why: possible causes of the pain that the patient can identify (e.g., trauma, surgery, job).
Physical examination in chronic pain relies on the different parts of the body involved in pain symptomatology and implies static and dynamic tests. For instance, in the case of low back pain, it is crucial to study the physio-pathological curves of the spine, the pelvic asymmetry, trigger points due to paravertebral muscle contractions, and pain induced by digital palpation of the spine [4][10][11]. Dynamic tests for low back pain comprehend Lasegue and Wasserman maneuvers, different tests for the elicitation of sacroiliac joint pain (e.g., distraction test, the posterior slipping, the FABER maneuver, the compression test, and the Gaenslen’s test), and gait evaluation.
2.3. Tailored Approach
Pain treatments include pharmacological, physical, behavioral, minimally invasive, and surgical interventions. Pharmacological therapy should take into account patient’s organ impairment, especially kidney and liver failure which are the primary sites of drugs’ metabolism and excretion; this factor generally implies a reduction in the dose of analgesics or a contraindication in their use [12]. It is important to mention that adrenal insufficiency and hypothyroidism can increase the effects of opioids and other analgesic drugs [13].
Physical and behavioral approaches could add a fundamental contribution in relieving pain, as many studies have underlined [14]. Minimally invasive and surgical interventions are generally reserved to refractory cases even if their advantages and drawbacks should be carefully balanced and compared to the adverse effects and efficacy of long-term pharmacological therapy (see the non-pharmacological approach section). Physicians must find the best approach to fit a patient’s needs, which include not only pain control, but also patient’s compliance; for example, some patients do not accept to have an implantable device even if it could be the right treatment for their pain as well as other patients who could not be able to manage a complex multidrug therapy.
Drug choice should be led primarily by the quality of pain: inflammatory pain would benefit from the use of NSAIDs, nociceptive pain from opioids, whereas neuropathic pain is generally treated with anticonvulsants and inhibitors of serotonin and noradrenaline reuptake [15]. It is difficult to identify a specific drug among a class which could adequately fit the patient’s needs and the choice is often based on the personal experience of the physician. Different molecules of the same class can have different effects, probably depending on receptor isoforms that could alter the pharmacological response: this is the concept underlying opioid rotation, i.e., the change of an opioid with another opioid due to adverse effects or ineffectiveness [16].
2.4. Iterative Evaluation
An iterative assessment of the patient involves a periodical re-evaluation of pain intensity along with the assessment of treatment effectiveness. The timing of follow-up is based on the patient’s symptoms and therapy. Patients prescribed with opioid have to be assessed for the risk of addiction, while for patients who have recently started a new therapy it is fundamental to evaluate the efficacy of the treatment and its adverse effects [17]. COVID-19 lead to severe restrictions reducing patient access to the clinics, so the development of telehealth technology has been encouraged. Telemedicine can be very helpful in the follow-up, especially in patients who are stable or only need a few interventions to modify their current therapy [18]. Emerik et al. presented a clear description of the benefits and the appropriateness of telemedicine: one of the most important advantages is that patients living far from their reference hospitals or with disabling symptoms can easily be evaluated [19]. It is fundamental to establish which phases of patient management can be performed through telemedicine and when the traditional in-person visits are necessary; in fact, telemedicine could be inappropriate in patients with progressive symptoms, unclear diagnoses, complex medical and psychosocial conditions, and suspected drug abuse [20].
2.5. Education
Patient education can be considered as the process through which healthcare providers give the patient both the information about his pain and the underlying condition and all the tools required to influence patient behavior and skills in order to effectively cope with the symptoms. The correct knowledge of the pain and of its management is fundamental, also because it can help the patient not feel trapped in his condition.
The patient has to be guided to recognize the factors that can influence pain such as sleep, nutrition, physical activity, mood, social life, flare-ups, and medications. Roberts MB et al. found a correlation between chronic pain and sleep disorders, while Brain K et al. showed that several nutrition interventions are effective in reducing pain [21][22]. Physical activity seems to decrease neural firing and increase endogenous opioid and serotonin levels in pain inhibitory pathways. Mental status and chronic pain can adversely affect each other, and they both can influence personal relationships. Moreover, patients have to be taught to recognize flare-ups and know the most suitable medications to treat them [23].
Many tools have been proposed to make patients more conscious of their condition: the simplest way is the use of brochures with all the basic information [24]. Video-assisted education can more efficiently engage patients; video–audio tools can be used in didactic presentation, with healthcare providers giving the information, practice presentation, showing real people engaged in an activity, and narrative presentation, including filming patients talking about their experience [25].
Cognitive–behavioral and educational interventions can improve both patients’ outcomes and family caregivers’ outcomes (e.g., adherence to therapy, fewer concerns about pain management, quality of life, the ability to recognize symptoms, flare-ups, worsening of background pain, and therapy efficacy) [26].
2.6. Non-Pharmacological Approach
Non-pharmacological approaches include invasive or minimally invasive treatments which are used to manage chronic pain refractory to traditional therapies; these treatments can dramatically reduce the dose of analgesic drugs and, consequently, their adverse effects. Even if these techniques could imply higher initial costs, in the long-term management they demonstrated to be economically advantageous both for the reduction in analgesic drugs and for a lower request of visits and hospital admissions [27].
2.6.1. Spinal Cord Stimulation
Spinal Cord Stimulation (SCS) is the most used and successful electric neuromodulation approach to chronic pain. The exact mechanism of action of SCS is still undetermined and several theories have been so far advocated, including the gate-control theory, proposed by Melzack and Wall in 1965, and the involvement of wide dynamic range (WDR) neurons’ desensitization in the dorsal horn [28]. Spinal cord stimulation was initially characterized by the replacement of pain with paresthesia, whereas high-frequency 10 kHz (HF10) and burst stimulation (BS) are paresthesia-free; moreover, burst stimulation seems to modulate medial spino-thalamo-cortical pathways, which are responsible for the emotional and affective parts of painful sensation [29]. SCS probably acts not only through the inhibition of the nociceptive transmission and nociceptive neurons’ hyperactivity in the dorsal horn, but even through a supraspinal mechanism of stimulation of the inhibitory descending pathways [30].
Even if generally used in the case of pain refractory to pharmacological therapies, ever-growing evidence suggest the use of SCS as a first-line approach to some painful syndromes [31]. The most frequent indications are failed back surgery syndrome (FBSS), complex regional pain syndrome (CRPS), peripheral neuropathic pain, postherpetic neuralgia, intercostal neuralgia, refractory angina pectoris, phantom limb pain syndrome, epidural fibrosis, and cauda equina injury syndrome [32][33][34][35][36]. In FBSS, two RCTs have shown that tonic SCS was significantly superior to the best medical treatment alone and to repeated spine surgery, to alleviate lower limb pain, in terms of pain score (≥50% reduction), patient’s satisfaction, quality of life, and emotional impact [34][35]. In addition, the SENZA-RCT trial established the superiority and safety of HF10-SCS over conventional SCS in reducing chronic back and leg pain [37]. Similarly, in the SUNBURST study, BS-SCS met non-inferiority and superiority criteria compared to tonic stimulation for pain relief, patient’s quality of life, and safety profile [38].
2.6.2. Radiofrequency
Radiofrequency (RF) stimulation is a safe and effective treatment to control different types of chronic neuropathic pain; it is performed through a needle percutaneously placed next to the target nerve under fluoroscopy, CT guidance, or ultrasound guidance. The continuous radiofrequency (CRF) technique uses a continuous electrical stimulation at a frequency of 400–500 kHz for 2 min, reaching a temperature of 60–90 °C with a consequent irreversible thermoablation of the target nerve. In contrast, pulsed radiofrequency (PRF) produces short heat bursts, with long resting phases between them in order to keep the temperature under the limit of protein denaturation of 42 °C, thus preventing irreversible nervous injuries [39]. PRF is supposed to have neuromodulatory and anti-inflammatory effects such as the decrease in microglia activity and the increase in c-fos expression in the dorsal horn; furthermore, it seems to potentiate the noradrenergic and serotonergic descending pain inhibitory pathways. C and A∂ fibers, which carry nociceptive stimuli from the periphery to the dorsal horn, seem to be microscopically damaged by PRF, in contrast with the Aβ fibers, which carry no pain-related signals and which are rarely involved [40][41].
Significant evidence supports RF stimulation as an effective and safe treatment for cervical and lumbar radicular and facet joint pain; in particular, the CRF of medial branch seems to be more effective and long-lasting than PRF with no motor damage risks.
Recently, PRF has emerged as a safe and potentially effective treatment also for postherpetic neuralgia and occipital neuralgia and it resulted as being superior to oral medication or epidural infusion of anesthetics; however, the CRF procedure should be avoided because it may induce several adverse effects, including sensory loss, dysesthesia, and anesthesia dolorosa [42][43][44]. The PRF of the nervus suprascapularis may relieve shoulder pain and can improve joint mobility, whereas the ablation of genicular nerves can be effectively used to treat knee pain [45]. The effectiveness of radiofrequency in other spinal disorders such as cervicogenic headaches, discogenic pain, thoracic facet joint pain, and coccydynia, is still uncertain; similarly, additional prospective clinical trials are necessary for clarifying the usefulness of PRF in meralgia paresthetica, carpal tunnel syndrome, tarsal tunnel syndrome, and Morton’s neuroma [39][46].
Cooled radiofrequency is a novel technique which uses a cooled needle to generate a larger ablation area: in fact, CRF, due to the high temperature reached around the needle, creates a charred layer which acts as an insulator, hindering the spreading of radiofrequency through the tissue [47].
Radiofrequency techniques are characterized by great potential applications, a high level of safety, low cost, reduction in analgesic drug use, and long-lasting effects, but, on the other hand, they are still not supported by consistent scientific evidence.
2.7. Team
The multi-dimensional aspect of pain suggests that its optimal assessment and management may be best achieved using a multidisciplinary approach [48]. Unfortunately, pain specialists are often not involved in patient management early enough, whereas they should have a central coordinating role in the pain team. The pain team should be composed of different figures according to patient pain characteristics and comorbidity, such as oncologists, clinical psychologists, physiotherapists, geriatricians, rheumatologists, orthopedic surgeons, neurosurgeons, and nutritionists. The multidisciplinary approach must involve pharmacological, invasive and mini-invasive interventions, physical rehabilitation, cognitive–behavioral and occupational therapy, and psychological interventions to manage depression and anxiety.
Older patients are at high risk for polypharmacy and medication mismanagement and require coordination between physicians and patients’ caregivers or long-term care facilities [49][50]. The aim is to offer a treatment that is tailored on patient-specific needs and to maximize the degree of satisfaction and quality of life while minimizing the risks of adverse psycho-physical consequences.
References
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic Pain: An Update on Burden, Best Practices, and New Advances. Lancet 2021, 397, 2082–2097.
- Kawai, K.; Kawai, A.T.; Wollan, P.; Yawn, B.P. Adverse Impacts of Chronic Pain on Health-Related Quality of Life, Work Productivity, Depression and Anxiety in a Community-Based Study. Fam. Pract. 2017, 34, 656–661.
- Mackey, S. Future Directions for Pain Management: Lessons from the Institute of Medicine Pain Report and the National Pain Strategy. Hand Clin. 2016, 32, 91–98.
- Fillingim, R.B.; Loeser, J.D.; Baron, R.; Edwards, R.R. Assessment of Chronic Pain: Domains, Methods, and Mechanisms. J. Pain 2016, 17, T10–T20.
- Campbell, J.N. APS 1995 Presidential Address. Pain Forum. 1996, 5, 85–88.
- Gibson, S.J.; Helme, R.D. Age-Related Differences in Pain Perception and Report. Clin. Geriatr. Med. 2001, 17, 433–456.
- Lautenbacher, S.; Peters, J.H.; Heesen, M.; Scheel, J.; Kunz, M. Age Changes in Pain Perception: A Systematic-Review and Meta-Analysis of Age Effects on Pain and Tolerance Thresholds. Neurosci. Biobehav. Rev. 2017, 75, 104–113.
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982.
- Glowacki, D. Effective Pain Management and Improvements in Patients’ Outcomes and Satisfaction. Crit. Care Nurse 2015, 35, 33–41.
- Balagué, F.; Mannion, A.F.; Pellisé, F.; Cedraschi, C. Non-Specific Low Back Pain. Lancet 2012, 379, 482–491.
- Breivik, H.; Borchgrevink, P.C.; Allen, S.M.; Rosseland, L.A.; Romundstad, L.; Hals, E.K.B.; Kvarstein, G.; Stubhaug, A. Assessment of Pain. Br. J. Anaesth. 2008, 101, 17–24.
- Gelot, S.; Nakhla, E. Opioid Dosing in Renal and Hepatic Impairment. U.S. Pharm. 2014, 39, 34–38.
- Knotkova, H.; Fine, P.G.; Portenoy, R.K. Opioid Rotation: The Science and the Limitations of the Equianalgesic Dose Table. J. Pain Symptom Manag. 2009, 38, 426–439.
- Moseley, G.L. Evidence for a Direct Relationship between Cognitive and Physical Change during an Education Intervention in People with Chronic Low Back Pain. Eur. J. Pain 2004, 8, 39–45.
- Attal, N. Pharmacological Treatments of Neuropathic Pain: The Latest Recommendations. Rev. Neurol. 2019, 175, 46–50.
- Fine, P.G.; Portenoy, R.K. Ad Hoc Expert Panel on Evidence Review and Guidelines for Opioid Rotation. Establishing “Best Practices” for Opioid Rotation: Conclusions of an Expert Panel. J. Pain Symptom Manag. 2009, 38, 418–425.
- Mücke, M.; Phillips, T.; Radbruch, L.; Petzke, F.; Häuser, W. Cannabis-Based Medicines for Chronic Neuropathic Pain in Adults. Cochrane Database Syst. Rev. 2018, 3, CD012182.
- Karran, E.L.; Grant, A.R.; Moseley, G.L. Low Back Pain and the Social Determinants of Health: A Systematic Review and Narrative Synthesis. Pain 2020, 161, 2476–2493.
- Emerick, T.; Alter, B.; Jarquin, S.; Brancolini, S.; Bernstein, C.; Luong, K.; Morrisseyand, S.; Wasan, A. Telemedicine for Chronic Pain in the COVID-19 Era and Beyond. Pain Med. 2020, 21, 1743–1748.
- Cascella, M.; Marinangeli, F.; Vittori, A.; Scala, C.; Piccinini, M.; Braga, A.; Miceli, L.; Vellucci, R. Open Issues and Practical Suggestions for Telemedicine in Chronic Pain. Int. J. Environ. Res. Public Health 2021, 18, 12416.
- Brain, K.; Burrows, T.L.; Rollo, M.E.; Chai, L.K.; Clarke, E.D.; Hayes, C.; Hodson, F.J.; Collins, C.E. A Systematic Review and Meta-Analysis of Nutrition Interventions for Chronic Noncancer Pain. J. Hum. Nutr. Diet. 2019, 32, 198–225.
- Roberts, M.B.; Drummond, P.D. Sleep Problems Are Associated with Chronic Pain Over and Above Mutual Associations with Depression and Catastrophizing. Clin. J. Pain 2016, 32, 792–799.
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical Activity and Exercise for Chronic Pain in Adults: An Overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 4, CD011279.
- Rantonen, J.; Vehtari, A.; Karppinen, J.; Luoto, S.; Viikari-Juntura, E.; Hupli, M.; Malmivaara, A.; Taimela, S. Face-to-Face Information Combined with a Booklet versus a Booklet Alone for Treatment of Mild Low-Back Pain: A Randomized Controlled Trial. Scand J. Work Environ. Health 2014, 40, 156–166.
- Abu Abed, M.; Himmel, W.; Vormfelde, S.; Koschack, J. Video-Assisted Patient Education to Modify Behavior: A Systematic Review. Patient Educ. Couns. 2014, 97, 16–22.
- Chi, N.-C.; Barani, E.; Fu, Y.-K.; Nakad, L.; Gilbertson-White, S.; Herr, K.; Saeidzadeh, S. Interventions to Support Family Caregivers in Pain Management: A Systematic Review. J. Pain Symptom Manag. 2020, 60, 630–656.e31.
- Kumar, K.; Rizvi, S. Cost-Effectiveness of Spinal Cord Stimulation Therapy in Management of Chronic Pain. Pain Med. 2013, 14, 1631–1649.
- Echeverria-Villalobos, M.; Mitchell, J.; Fiorda-Diaz, J.; Weaver, T. Effects of Dorsal Column Spinal Cord Stimulation on Neuroinflammation: Revisiting Molecular Mechanisms and Clinical Outcomes on Chronic Lumbar/Leg Pain and Failed Back Surgery Syndrome. J. Pain Res. 2021, 14, 2337–2345.
- Chakravarthy, K.; Fishman, M.A.; Zuidema, X.; Hunter, C.W.; Levy, R. Mechanism of Action in Burst Spinal Cord Stimulation: Review and Recent Advances. Pain Med. 2019, 20, S13–S22.
- Sdrulla, A.D.; Guan, Y.; Raja, S.N. Spinal Cord Stimulation: Clinical Efficacy and Potential Mechanisms. Pain Pract. 2018, 18, 1048–1067.
- Van Boxem, K.; Cheng, J.; Patijn, J.; Van Kleef, M.; Lataster, A.; Mekhail, N.; Van Zundert, J. Lumbosacral Radicular Pain. Pain Pract. 2010, 10, 339–358.
- Aryal, V.; Poudel, S.; Zulfiqar, F.; Shrestha, T.; Singh, A.; Shah, S.A.; Soomro, U.; Choudhari, J.; Quinonez, J.; Ruxmohan, S.; et al. Updates on the Role of Spinal Cord Stimulation in the Management of Non-Surgical Chronic Lower Back Pain. Cureus 2021, 13, e18928.
- Dones, I.; Levi, V. Spinal Cord Stimulation for Neuropathic Pain: Current Trends and Future Applications. Brain Sci. 2018, 8, 138.
- Kumar, K.; Taylor, R.S.; Jacques, L.; Eldabe, S.; Meglio, M.; Molet, J.; Thomson, S.; O’Callaghan, J.; Eisenberg, E.; Milbouw, G.; et al. Spinal Cord Stimulation versus Conventional Medical Management for Neuropathic Pain: A Multicentre Randomised Controlled Trial in Patients with Failed Back Surgery Syndrome. Pain 2007, 132, 179–188.
- North, R.B.; Kidd, D.H.; Farrokhi, F.; Piantadosi, S.A. Spinal Cord Stimulation versus Repeated Lumbosacral Spine Surgery for Chronic Pain: A Randomized, Controlled Trial. Neurosurgery 2005, 56, 98–107.
- Pollard, E.M.; Lamer, T.J.; Moeschler, S.M.; Gazelka, H.M.; Hooten, W.M.; Bendel, M.A.; Warner, N.S.; Murad, M.H. The Effect of Spinal Cord Stimulation on Pain Medication Reduction in Intractable Spine and Limb Pain: A Systematic Review of Randomized Controlled Trials and Meta-Analysis. J. Pain Res. 2019, 12, 1311–1324.
- Kapural, L.; Yu, C.; Doust, M.W.; Gliner, B.E.; Vallejo, R.; Sitzman, B.T.; Amirdelfan, K.; Morgan, D.M.; Yearwood, T.L.; Bundschu, R.; et al. Comparison of 10-KHz High-Frequency and Traditional Low-Frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: 24-Month Results From a Multicenter, Randomized, Controlled Pivotal Trial. Neurosurgery 2016, 79, 667–677.
- Deer, T.; Slavin, K.V.; Amirdelfan, K.; North, R.B.; Burton, A.W.; Yearwood, T.L.; Tavel, E.; Staats, P.; Falowski, S.; Pope, J.; et al. Success Using Neuromodulation With BURST (SUNBURST) Study: Results From a Prospective, Randomized Controlled Trial Using a Novel Burst Waveform. Neuromodulation Technol. Neural Interface 2018, 21, 56–66.
- Yang, S.; Chang, M.C. Efficacy of Pulsed Radiofrequency in Controlling Pain Caused by Spinal Disorders: A Narrative Review. Ann. Palliat. Med. 2020, 9, 3528–3536.
- Erdine, S.; Bilir, A.; Cosman, E.R.; Cosman, E.R. Ultrastructural Changes in Axons Following Exposure to Pulsed Radiofrequency Fields. Pain Pract. 2009, 9, 407–417.
- Hagiwara, S.; Iwasaka, H.; Takeshima, N.; Noguchi, T. Mechanisms of Analgesic Action of Pulsed Radiofrequency on Adjuvant-Induced Pain in the Rat: Roles of Descending Adrenergic and Serotonergic Systems. Eur. J. Pain 2009, 13, 249–252.
- Chang, M.C. Efficacy of Pulsed Radiofrequency Stimulation in Patients with Peripheral Neuropathic Pain: A Narrative Review. Pain Physician 2018, 21, E225–E234.
- Ma, K.; Fan, Y.; Yi, J.; Huang, X.; Liu, X.; Cheng, Z.; Huang, C.; Wang, Y. Efficacy of Pulsed Radiofrequency in the Treatment of Thoracic Postherpetic Neuralgia from the Angulus Costae: A Randomized, Double-Blinded, Controlled Trial. Pain Physician 2013, 16, 15–25.
- Pi, Z.B.; Lin, H.; He, G.D.; Cai, Z.; Xu, X.Z. Randomized and Controlled Prospective Trials of Ultrasound-Guided Spinal Nerve Posterior Ramus Pulsed Radiofrequency Treatment for Lower Back Post-Herpetic Neuralgia. Clin. Ter. 2015, 166, e301–e305.
- Kim, E.D.; Lee, Y.I.; Park, H.J. Comparison of Efficacy of Continuous Epidural Block and Pulsed Radiofrequency to the Dorsal Root Ganglion for Management of Pain Persisting beyond the Acute Phase of Herpes Zoster. PLoS ONE 2017, 12, e0183559.
- Merskey, H. Logic, Truth and Language in Concepts of Pain. Qual. Life Res. 1994, 3 (Suppl. 1), S69–S76.
- Kapural, L.; Deering, J.P. A Technological Overview of Cooled Radiofrequency Ablation and Its Effectiveness in the Management of Chronic Knee Pain. Pain Manag. 2020, 10, 133–140.
- Gauthier, K.; Dulong, C.; Argáez, C. Multidisciplinary Programs for Chronic Non-Malignant Pain Cite As: Multidisciplinary Treatment Programs for Patients with Chronic Non-Malignant Pain: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines. In Summary with Critical Appraisal; National Institutes of Health: Bethesda, MD, USA, 2017.
- Marra, A.; Hayhurst, C.J.; Hughes, C.G.; Merengoni, A.; Bellelli, G.; Pandharipande, P.; Morandi, A. Avoiding Inappropriate Medication Prescription in Older Intensive Care Survivors. JCOM 2018, 25, 67–83.
- Schwan, J.; Sclafani, J.; Tawfik, V.L. Chronic Pain Management in the Elderly. Anesthesiol. Clin. 2019, 37, 547–560.
More
Information
Subjects:
Anesthesiology; Medicine, General & Internal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
540
Revisions:
2 times
(View History)
Update Date:
02 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




