
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Finosh G Thankam | -- | 3128 | 2023-11-01 19:53:13 | | | |
| 2 | Peter Tang | Meta information modification | 3128 | 2023-11-02 02:22:19 | | |
Video Upload Options
Increased prevalence of cardiovascular disease and potentially life-threatening complications of myocardial infarction (MI) has led to emerging therapeutic approaches focusing on myocardial regeneration and restoration of physiologic function following infarction. Extracellular vesicle (EV) technology has gained attention owing to the biological potential to modulate cellular immune responses and promote the repair of damaged tissue. Also, EVs are involved in local and distant cellular communication following damage and play an important role in initiating the repair process. Vesicles derived from stem cells and cardiomyocytes (CM) are of particular interest due to their ability to promote cell growth, proliferation, and angiogenesis following MI.
1. Introduction
2. Vesicle-Mediated Cardiac Regeneration
3. Immune Cell-Derived EVs
4. Mesenchymal Stem Cells (MSCs)-Derived EVs
5. EVs from Cardiomyocytes (CMs) and Cardiac Fibroblasts (CF)
6. EVs from Cardiosphere-Derived Cells (CDCs)
7. Cardiac Endothelial Cell (CEC)-Derived EVs
8. Adipose-Derived Stem Cells (ADSC)-Derived EVs
9. Bone Marrow-Derived Stem Cells (BMSC)-Derived EVs
10. Epicardial Adipose-Derived Stem Cells-Derived EVs
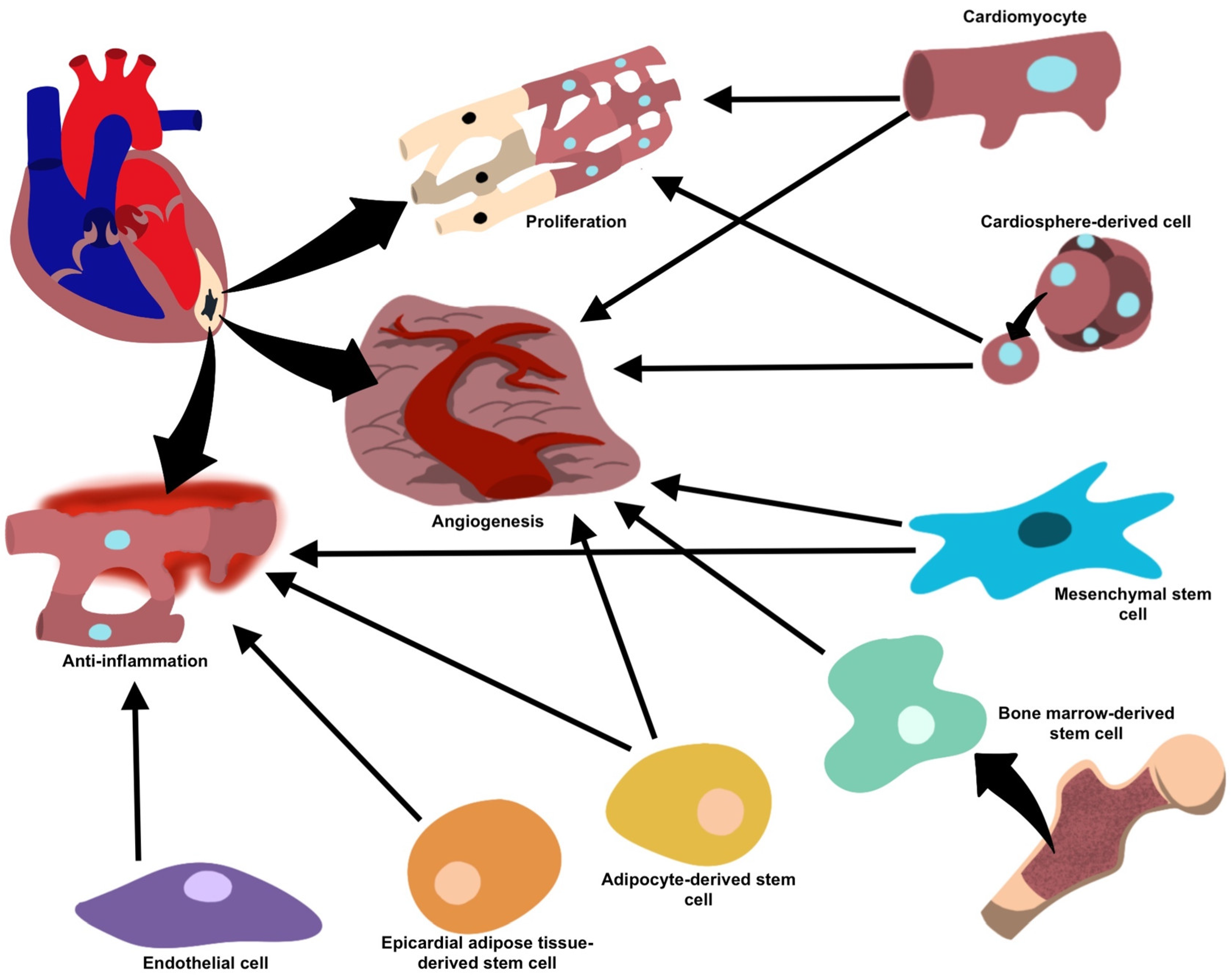
|
Target Cell |
Mechanism Of Repair/Regeneration and Mediators |
References |
|---|---|---|
|
Mesenchymal stem cells |
Anti-inflammatory (miR-182, miR-233, miR-181c, miR-19a, miR-22, miR-199a, miR-214), anti-fibrotic (miR-19a, miR-29, miR-133) and pro-angiogenic (miR-126, miR-210, miR-20a, VEGF) processes facilitating repair and regeneration and inhibits the formation of fibrotic scar tissue. |
[24] |
|
Cardiomyocytes |
Cell growth (miR-17, miR-20a, miR-23b) under normal conditions, and enhanced angiogenesis and decreased collagen deposition (miR-16, miR-19a, miR-19b, miR-23a, miR-23b) under stress. |
[30] |
|
Cardiosphere-derived cells |
Pro-angiogenic and pro-apoptotic properties (miR-210, miR-132, miR-146a-3p). |
[23] |
|
Endothelial cells |
Reduce inflammation and facilitate healing (integrin avβ6). |
[37] |
|
Adipose-derived stem cells |
Anti-inflammatory properties (miR-126) prevent fibrosis and favor angiogenesis, facilitating repair. |
[39] |
|
Bone marrow-derived stem cells |
Neovascularization and vasculogenesis, once implanted into ischemic cardiac tissue. |
|
|
Epicardial adipose tissue-derived stem cells |
Upregulation in regenerative properties and proliferative/anti-inflammatory proteins during periods of cellular stress or ischemia, as well as differentiation of cell types. |
[48] |
References
- Tzahor, E.; Poss, K.D. Cardiac Regeneration Strategies: Staying Young at Heart. Science 2017, 356, 1035–1039.
- Sheng, K.; Nie, Y.; Gao, B. Recent Advances in Myocardial Regeneration Strategy. J. Int. Med. Res. 2019, 47, 5453–5464.
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977.
- Joladarashi, D.; Kishore, R. Mesenchymal Stromal Cell Exosomes in Cardiac Repair. Curr. Cardiol. Rep. 2022, 24, 405–417.
- Shen, M.; Chen, T. Mesenchymal Stem Cell-Derived Exosomes and Their Potential Agents in Hematological Diseases. Oxid. Med. Cell Longev. 2021, 2021, 4539453.
- Lee, T.L.; Lai, T.C.; Lin, S.R.; Lin, S.W.; Chen, Y.C.; Pu, C.M.; Lee, I.T.; Tsai, J.S.; Lee, C.W.; Chen, Y.L. Conditioned Medium from Adipose-Derived Stem Cells Attenuates Ischemia/Reperfusion-Induced Cardiac Injury through the MicroRNA-221/222/PUMA/ETS-1 Pathway. Theranostics 2021, 11, 3131–3149.
- Sahoo, S.; Losordo, D. Cardiac Repair and Regeneration after MI: What Is Known? Circ. Res. 2014, 114, 333–344.
- Ong, S.G.; Wu, J.C. Exosomes as Potential Alternatives to Stem Cell Therapy in Mediating Cardiac Regeneration. Circ. Res. 2015, 117, 7–9.
- Kolios, G.; Moodley, Y. Introduction to Stem Cells and Regenerative Medicine. Respiration 2012, 85, 3–10.
- Matsuzaka, Y.; Yashiro, R. Therapeutic Strategy of Mesenchymal-Stem-Cell-Derived Extracellular Vesicles as Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 6480.
- Hegyesi, H.; Pallinger, É.; Mecsei, S.; Hornyák, B.; Kovácsházi, C.; Brenner, G.B.; Giricz, Z.; Pálóczi, K.; Kittel, Á.; Tóvári, J.; et al. Circulating Cardiomyocyte-Derived Extracellular Vesicles Reflect Cardiac Injury during Systemic Inflammatory Response Syndrome in Mice. Cell. Mol. Life Sci. 2022, 79, 84.
- Ju, Y.; Hu, Y.; Yang, P.; Xie, X.; Fang, B. Extracellular Vesicle-Loaded Hydrogels for Tissue Repair and Regeneration. Mater. Today Bio 2023, 18, 100522.
- Wang, X.; Gu, H.; Qin, D.; Yang, L.; Huang, W.; Essandoh, K.; Wang, Y.; Caldwell, C.C.; Peng, T.; Zingarelli, B.; et al. Exosomal MIR-223 Contributes to Mesenchymal Stem Cell-Elicited Cardioprotection in Polymicrobial Sepsis. Sci. Rep. 2015, 5, 13721.
- Wang, X.; Chen, Y.; Zhao, Z.; Meng, Q.; Yu, Y.; Sun, J.; Yang, Z.; Chen, Y.; Li, J.; Ma, T.; et al. Engineered Exosomes with Ischemic Myocardium-Targeting Peptide for Targeted Therapy in Myocardial Infarction. J. Am. Heart Assoc. 2018, 7, e008737.
- Wang, H.; Xie, Y.; Salvador, A.M.; Zhang, Z.; Chen, K.; Li, G.; Xiao, J. Exosomes: Multifaceted Messengers in Atherosclerosis. Curr. Atheroscler. Rep. 2020, 22, 57.
- Wang, X.; Bai, L.; Liu, X.; Shen, W.; Tian, H.; Liu, W.; Yu, B. Cardiac Microvascular Functions Improved by MSC-Derived Exosomes Attenuate Cardiac Fibrosis after Ischemia–Reperfusion via PDGFR-β Modulation. Int. J. Cardiol. 2021, 344, 13–24.
- Dong, J.; Wu, B.; Tian, W. Exosomes Derived from Hypoxia-Preconditioned Mesenchymal Stem Cells (HypoMSCs-Exo): Advantages in Disease Treatment. Cell Tissue Res. 2023, 392, 621–629.
- Wen, H.; Peng, L.; Chen, Y. The Effect of Immune Cell-Derived Exosomes in the Cardiac Tissue Repair after Myocardial Infarction: Molecular Mechanisms and Pre-Clinical Evidence. J. Cell. Mol. Med. 2021, 25, 6500–6510.
- Wu, Y.; Peng, W.; Fang, M.; Wu, M.; Wu, M. MSCs-Derived Extracellular Vesicles Carrying MiR-212-5p Alleviate Myocardial Infarction-Induced Cardiac Fibrosis via NLRC5/VEGF/TGF-Β1/SMAD Axis. J. Cardiovasc. Transl. Res. 2022, 15, 302–316.
- Fang, W.H.; Agrawal, D.K.; Thankam, F.G. Smart Exosomes: A Smart Approach for Tendon Regeneration. Tissue Eng. Part. B Rev. 2022, 28, 613–625.
- Wu, R.; Gao, W.; Yao, K.; Ge, J. Roles of Exosomes Derived from Immune Cells in Cardiovascular Diseases. Front. Immunol. 2019, 10, 648.
- Mihailovic, P.M.; Lio, W.M.; Herscovici, R.; Chyu, K.Y.; Yano, J.; Zhao, X.; Zhou, J.; Zhou, B.; Freeman, M.R.; Yang, W.; et al. Keratin 8 Is a Potential Self-Antigen in the Coronary Artery Disease Immunopeptidome: A Translational Approach. PLoS ONE 2019, 14, e0213025.
- Lazar, E.; Benedek, T.; Korodi, S.; Rat, N.; Lo, J.; Benedek, I. Stem Cell-Derived Exosomes—An Emerging Tool for Myocardial Regeneration. World J. Stem Cells 2018, 10, 106–115.
- Shafei, S.; Khanmohammadi, M.; Ghanbari, H.; Nooshabadi, V.T.; Tafti, S.H.A.; Rabbani, S.; Kasaiyan, M.; Basiri, M.; Tavoosidana, G. Effectiveness of Exosome Mediated MiR-126 and MiR-146a Delivery on Cardiac Tissue Regeneration. Cell Tissue Res. 2022, 390, 71–92.
- Nasser, M.I.; Masood, M.; Adlat, S.; Gang, D.; Zhu, S.; Li, G.; Li, N.; Chen, J.; Zhu, P. Mesenchymal Stem Cell-Derived Exosome MicroRNA as Therapy for Cardiac Ischemic Injury. Biomed. Pharmacother. 2021, 143, 112118.
- Sun, S.J.; Wei, R.; Li, F.; Liao, S.Y.; Tse, H.F. Mesenchymal Stromal Cell-Derived Exosomes in Cardiac Regeneration and Repair. Stem Cell Rep. 2021, 16, 1662–1673.
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8, 237–255.
- Zhang, P.; Su, J.; Mende, U. Cross Talk between Cardiac Myocytes and Fibroblasts: From Multiscale Investigative Approaches to Mechanisms and Functional Consequences. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1385–H1396.
- Yang, W.; Tu, H.; Tang, K.; Huang, H.; Ou, S.; Wu, J. MiR-3064 in Epicardial Adipose-Derived Exosomes Targets Neuronatin to Regulate Adipogenic Differentiation of Epicardial Adipose Stem Cells. Front. Cardiovasc. Med. 2021, 8, 709079.
- Wojciechowska, A.; Braniewska, A.; Kozar-Kamińska, K. MicroRNA in Cardiovascular Biology and Disease. Adv. Clin. Exp. Med. 2017, 26, 865–874.
- Mancuso, T.; Barone, A.; Salatino, A.; Molinaro, C.; Marino, F.; Scalise, M.; Torella, M.; De Angelis, A.; Urbanek, K.; Torella, D.; et al. Unravelling the Biology of Adult Cardiac Stem Cell-Derived Exosomes to Foster Endogenous Cardiac Regeneration and Repair. Int. J. Mol. Sci. 2020, 21, 3725.
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W.; Anchordoquy, T.J. Biodistribution and Delivery Efficiency of Unmodified Tumor-Derived Exosomes. J. Control. Release 2015, 199, 145–155.
- Maring, J.A.; Beez, C.M.; Falk, V.; Seifert, M.; Stamm, C. Myocardial Regeneration via Progenitor Cell-Derived Exosomes. Stem Cells Int. 2017, 2017, 7849851.
- Wang, Y.; Wang, C.; Ma, J. Role of Cardiac Endothelial Cells-Derived MicroRNAs in Cardiac Remodeling. Discov. Med. 2019, 28, 95–105.
- Roncarati, R.; Viviani Anselmi, C.; Losi, M.A.; Papa, L.; Cavarretta, E.; Da Costa Martins, P.; Contaldi, C.; Saccani Jotti, G.; Franzone, A.; Galastri, L.; et al. Circulating MiR-29a, among Other up-Regulated MicroRNAs, Is the Only Biomarker for Both Hypertrophy and Fibrosis in Patients with Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2014, 63, 920–927.
- Ono, K.; Kuwabara, Y.; Han, J. MicroRNAs and Cardiovascular Diseases. FEBS J. 2011, 278, 1619–1633.
- Song, J.; Chen, X.; Wang, M.; Xing, Y.; Zheng, Z.; Hu, S. Cardiac Endothelial Cell-Derived Exosomes Induce Specific Regulatory B Cells. Sci. Rep. 2014, 4, 7583.
- Lee, C.; Mitsialis, S.A.; Aslam, M.; Vitali, S.H.; Vergadi, E.; Konstantinou, G.; Sdrimas, K.; Fernandez-Gonzalez, A.; Kourembanas, S. Exosomes Mediate the Cytoprotective Action of Mesenchymal Stromal Cells on Hypoxia-Induced Pulmonary Hypertension. Circulation 2012, 126, 2601–2611.
- Luo, Q.; Guo, D.; Liu, G.; Chen, G.; Hang, M.; Jin, M. Exosomes from MiR-126-Overexpressing Adscs Are Therapeutic in Relieving Acute Myocardial Ischaemic Injury. Cell. Physiol. Biochem. 2017, 44, 2105–2116.
- Lee, N.; Thorne, T.; Losordo, D.W.; Yoon, Y.S. Repair of Ischemic Heart Disease with Novel Bone Marrow-Derived Multipotent Stem Cells. Cell Cycle 2005, 4, 861–864.
- Müller, P.; Lemcke, H.; David, R. Stem Cell Therapy in Heart Diseases-Cell Types, Mechanisms and Improvement Strategies. Cell. Physiol. Biochem. 2018, 48, 2607–2655.
- Azari, Z.; Nazarnezhad, S.; Webster, T.J.; Hoseini, S.J.; Brouki Milan, P.; Baino, F.; Kargozar, S. Stem cell-mediated Angiogenesis in Skin Tissue Engineering and Wound Healing. Wound Repair. Regen. 2022, 30, 421–435.
- Mangialardi, G.; Madeddu, P. Bone Marrow-Derived Stem Cells: A Mixed Blessing in the Multifaceted World of Diabetic Complications. Curr. Diabetes Rep. 2016, 16, 43.
- Xu, M.; Shaw, G.; Murphy, M.; Barry, F. Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells Are Functionally and Genetically Different From Bone Marrow-Derived Mesenchymal Stromal Cells. Stem Cells 2019, 37, 754–765.
- Korf-Klingebiel, M.; Reboll, M.R.; Klede, S.; Brod, T.; Pich, A.; Polten, F.; Napp, L.C.; Bauersachs, J.; Ganser, A.; Brinkmann, E.; et al. Myeloid-Derived Growth Factor (C19orf10) Mediates Cardiac Repair Following Myocardial Infarction. Nat. Med. 2015, 21, 140–149.
- Wang, Y.; Li, Y.; Feng, J.; Liu, W.; Li, Y.; Liu, J.; Yin, Q.; Lian, H.; Liu, L.; Nie, Y. Mydgf Promotes Cardiomyocyte Proliferation and Neonatal Heart Regeneration. Theranostics 2020, 10, 9100–9112.
- Özkaynak, B.; Şahin, I.; Özenc, E.; Subaşı, C.; Oran, D.S.; Totoz, T.; Tetikkurt, Ü.S.; Mert, B.; Polat, A.; Okuyan, E.; et al. Mesenchymal Stem Cells Derived from Epicardial Adipose Tissue Reverse Cardiac Remodeling in a Rabbit Model of Myocardial Infarction. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4372–4384.
- Thankam, F.G.; Agrawal, D.K. Single Cell Genomics Identifies Unique Cardioprotective Phenotype of Stem Cells Derived from Epicardial Adipose Tissue under Ischemia. Stem Cell Rev. Rep. 2022, 18, 294–335.
- Lambert, C.; Arderiu, G.; Bejar, M.T.; Crespo, J.; Baldellou, M.; Juan-Babot, O.; Badimon, L. Stem Cells from Human Cardiac Adipose Tissue Depots Show Different Gene Expression and Functional Capacities. Stem Cell Res. Ther. 2019, 10, 361.




