
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Igor Kovalchuk | -- | 4583 | 2023-11-01 15:40:32 | | | |
| 2 | Lindsay Dong | -1 word(s) | 4582 | 2023-11-02 01:49:05 | | |
Video Upload Options
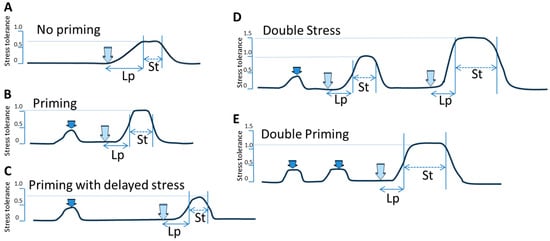
All species are well adapted to their environment. Stress causes a magnitude of biochemical and molecular responses in plants, leading to physiological or pathological changes. The response to various stresses is genetically predetermined, but is also controlled on the epigenetic level. Most plants are adapted to their environments through generations of exposure to all elements. Many plant species have the capacity to acclimate or adapt to certain stresses using the mechanism of priming. In most cases, priming is a somatic response allowing plants to deal with the same or similar stress more efficiently, with fewer resources diverted from growth and development. Priming likely relies on multiple mechanisms, but the differential expression of non-coding RNAs, changes in DNA methylation, histone modifications, and nucleosome repositioning play a crucial role.
1. Introduction
2. General Stress Response
3. Epigenetic Regulation of Response to Stress
3.1. Changes in Chromatin Structure in Response to Stress
3.1.1. Heterochromatin Decondensation and Activation of Transposable Elements in Response to Stress
3.1.2. The Role of CRFs in Response to Stress
3.1.3. The Role of Chromatin-modifying Proteins Lacking ATPase Domain
3.2. The Role of Histone Modifications in the Response to Stress
3.3. The Role of DNA Methylation in Response to Stress
3.4. Role of siRNAs and RdDM in Stress Response
3.5. Potential Role of Methylated RNA in Stress Response
References
- Pedroza-Garcia, J.A.; Xiang, Y.; De Veylder, L. Cell cycle checkpoint control in response to DNA damage by environmental stresses. Plant J. 2021, 109, 490–507.
- Tasset, C.; Yadav, A.S.; Sureshkumar, S.; Singh, R.; van der Woude, L.; Nekrasov, M.; Tremethick, D.; van Zanten, M.; Balasubramanian, S. POWERDRESS-mediated histone deacetylation is essential for thermomorphogenesis in Arabidopsis thaliana. PLoS Genet. 2018, 14, e1007280.
- Ito, T.; Nishio, H.; Tarutani, Y.; Emura, N.; Honjo, M.N.; Toyoda, A.; Fujiyama, A.; Kakutani, T.; Kudoh, H. Seasonal Stability and Dynamics of DNA Methylation in Plants in a Natural Environment. Genes 2019, 10, 544.
- Amaral, M.N.D.; Auler, P.A.; Rossatto, T.; Barros, P.M.; Oliveira, M.M.; Braga, E.J.B. Long-term somatic memory of salinity unveiled from physiological, biochemical and epigenetic responses in two contrasting rice genotypes. Physiol. Plant. 2020, 170, 248–268.
- Srivastava, A.K.; Suresh Kumar, J.; Suprasanna, P. Seed ‘primeomics’: Plants memorize their germination under stress. Biol. Rev. Camb. Philos. Soc. 2021, 96, 1723–1743.
- Ding, Y.; Shi, Y.; Yang, S. Molecular Regulation of Plant Responses to Environmental Temperatures. Mol. Plant 2020, 13, 544–564.
- Woodson, J.D. Chloroplast stress signals: Regulation of cellular degradation and chloroplast turnover. Curr. Opin. Plant Biol. 2019, 52, 30–37.
- Van Breusegem, F.; Remacle, C. Oxidative stress responses in plants. Free Radic. Biol. Med. 2023, 204, 394.
- Xu, Y.; Freund, D.M.; Hegeman, A.D.; Cohen, J.D. Metabolic signatures of Arabidopsis thaliana abiotic stress responses elucidate patterns in stress priming, acclimation, and recovery. Stress Biol. 2022, 2, 11.
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694.
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543.
- Leuendorf, J.E.; Frank, M.; Schmulling, T. Acclimation, priming and memory in the response of Arabidopsis thaliana seedlings to cold stress. Sci. Rep. 2020, 10, 689.
- Boulc’h, P.-N.; Caullireau, E.; Faucher, E.; Gouerou, M.; Guérin, A.; Miray, R.; Couée, I. Abiotic stress signalling in extremophile land plants. J. Exp. Bot. 2020, 71, 5771–5785.
- Ahn, I.-P.; Kim, S.; Lee, Y.-H.; Suh, S.-C. Vitamin B1-Induced Priming Is Dependent on Hydrogen Peroxide and the NPR1 Gene in Arabidopsis. Plant Physiol. 2006, 143, 838–848.
- Gamir, J.; Pastor, V.; Cerezo, M.; Flors, V. Identification of indole-3-carboxylic acid as mediator of priming against Plectosphaerella cucumerina. Plant Physiol. Biochem. 2012, 61, 169–179.
- Harris, C.J.; Amtmann, A.; Ton, J. Epigenetic processes in plant stress priming: Open questions and new approaches. Curr. Opin. Plant Biol. 2023, 75, 102432.
- Charng, Y.-Y.; Mitra, S.; Yu, S.-J. Maintenance of abiotic stress memory in plants: Lessons learned from heat acclimation. Plant Cell 2022, 35, 187–200.
- Liu, H.; Able, A.J.; Able, J.A. Priming crops for the future: Rewiring stress memory. Trends Plant Sci. 2021, 27, 699–716.
- Miryeganeh, M. Plants’ Epigenetic Mechanisms and Abiotic Stress. Genes 2021, 12, 1106.
- Chang, Y.; Zhu, C.; Jiang, J.; Zhang, H.; Zhu, J.; Duan, C. Epigenetic regulation in plant abiotic stress responses. J. Integr. Plant Biol. 2020, 62, 563–580.
- Xie, W.; Tang, Q.; Yan, F.; Tao, Z. Transcriptional memory and response to adverse temperatures in plants. J. Zhejiang Univ. B 2021, 22, 791–804.
- Yadav, V.K.; Santos-González, J.; Köhler, C. INT-Hi-C reveals distinct chromatin architecture in endosperm and leaf tissues of Arabidopsis. Nucleic Acids Res. 2021, 49, 4371–4385.
- Iberg, A.N.; Espejo, A.; Cheng, D.; Kim, D.; Michaud-Levesque, J.; Richard, S.; Bedford, M.T. Arginine Methylation of the Histone H3 Tail Impedes Effector Binding. J. Biol. Chem. 2008, 283, 3006–3010.
- Song, Z.; Liu, J.; Han, J. Chromatin remodeling factors regulate environmental stress responses in plants. J. Integr. Plant Biol. 2021, 63, 438–450.
- Brzezinka, K.; Altmann, S.; Czesnick, H.; Nicolas, P.; Gorka, M.; Benke, E.; Kabelitz, T.; Jähne, F.; Graf, A.; Kappel, C.; et al. Arabidopsis FORGETTER1 mediates stress-induced chromatin memory through nucleosome remodeling. Elife 2016, 5, e17061.
- Wang, Y.; Liu, Y.; Qu, S.; Liang, W.; Sun, L.; Ci, D.; Ren, Z.; Fan, L.; Qian, W. Nitrogen starvation induces genome-wide activation of transposable elements in Arabidopsis. J. Integr. Plant Biol. 2022, 64, 2374–2384.
- Pecinka, A.; Dinh, H.Q.; Baubec, T.; Rosa, M.; Lettner, N.; Scheid, O.M. Epigenetic Regulation of Repetitive Elements Is Attenuated by Prolonged Heat Stress in Arabidopsis. Plant Cell 2010, 22, 3118–3129.
- Thouly, C.; Le Masson, M.; Lai, X.; Carles, C.C.; Vachon, G. Unwinding BRAHMA Functions in Plants. Genes 2020, 11, 90.
- Charng, Y.-Y.; Liu, H.-C.; Liu, N.-Y.; Hsu, F.-C.; Ko, S.-S. Arabidopsis Hsa32, a Novel Heat Shock Protein, Is Essential for Acquired Thermotolerance during Long Recovery after Acclimation. Plant Physiol. 2006, 140, 1297–1305.
- Aslam, M.M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of Abscisic Acid-Mediated Drought Stress Responses in Plants. Int. J. Mol. Sci. 2022, 23, 1084.
- Peirats-Llobet, M.; Han, S.-K.; Gonzalez-Guzman, M.; Jeong, C.W.; Rodriguez, L.; Belda-Palazon, B.; Wagner, D.; Rodriguez, P.L. A Direct Link between Abscisic Acid Sensing and the Chromatin-Remodeling ATPase BRAHMA via Core ABA Signaling Pathway Components. Mol. Plant 2016, 9, 136–147.
- Han, S.K.; Sang, Y.; Rodrigues, A.; BIOL425 F2010; Wu, M.F.; Rodriguez, P.L.; Wagner, D. The SWI2/SNF2 chromatin remodeling ATPase BRAHMA represses abscisic acid responses in the absence of the stress stimulus in Arabidopsis. Plant Cell 2012, 24, 4892–4906.
- Dong, Y.; Uslu, V.V.; Berr, A.; Singh, G.; Papdi, C.; A Steffens, V.; Heitz, T.; A Ryabova, L. TOR represses stress responses through global regulation of H3K27 trimethylation in plants. J. Exp. Bot. 2022, 74, 1420–1431.
- Liu, J.; Feng, L.; Gu, X.; Deng, X.; Qiu, Q.; Li, Q.; Zhang, Y.; Wang, M.; Deng, Y.; Wang, E.; et al. An H3K27me3 demethylase-HSFA2 regulatory loop orchestrates transgenerational thermomemory in Arabidopsis. Cell Res. 2019, 29, 379–390.
- Iwasaki, M.; Paszkowski, J. Identification of genes preventing transgenerational transmission of stress-induced epigenetic states. Proc. Natl. Acad. Sci. USA 2014, 111, 8547–8552.
- Hyun, K.-G.; Lee, Y.; Yoon, J.; Yi, H.; Song, J.-J. Crystal structure of Arabidopsis thaliana SNC1 TIR domain. Biochem. Biophys. Res. Commun. 2016, 481, 146–152.
- Yokthongwattana, C.; Bucher, E.; Čaikovski, M.; Vaillant, I.; Nicolet, J.; Scheid, O.M.; Paszkowski, J. MOM1 and Pol-IV/V interactions regulate the intensity and specificity of transcriptional gene silencing. EMBO J. 2009, 29, 340–351.
- Jeddeloh, J.A.; Stokes, T.L.; Richards, E.J. Maintenance of genomic methylation requires a SWI2/SNF2-like protein. Nat. Genet. 1999, 22, 94–97.
- Kakutani, T.; Munakata, K.; Richards, E.J.; Hirochika, H. Meiotically and Mitotically Stable Inheritance of DNA Hypomethylation Induced by ddm1 Mutation of Arabidopsis thaliana. Genetics 1999, 151, 831–838.
- Long, J.C.; Xia, A.A.; Liu, J.H.; Jing, J.L.; Wang, Y.Z.; Qi, C.Y.; He, Y. Decrease in DNA methylation 1 (DDM1) is required for the formation of (m) CHH islands in maize. J. Integr. Plant Biol. 2019, 61, 749–764.
- Zemach, A.; Li, Y.; Wayburn, B.; Ben-Meir, H.; Kiss, V.; Avivi, Y.; Kalchenko, V.; Jacobsen, S.E.; Grafi, G. DDM1 binds Arabidopsis methyl-CpG binding domain proteins and affects their subnuclear localization. Plant Cell 2005, 17, 1549–1558.
- Lee, S.C.; Adams, D.W.; Ipsaro, J.J.; Cahn, J.; Lynn, J.; Kim, H.-S.; Berube, B.; Major, V.; Calarco, J.P.; LeBlanc, C.; et al. Chromatin remodeling of histone H3 variants by DDM1 underlies epigenetic inheritance of DNA methylation. Cell 2023, 186, 4100–4116.e15.
- Yao, Y.; Bilichak, A.; Golubov, A.; Kovalchuk, I. ddm1 plants are sensitive to methyl methane sulfonate and NaCl stresses and are deficient in DNA repair. Plant Cell Rep. 2012, 31, 1549–1561.
- Kanno, T.; Mette, M.F.; Kreil, D.P.; Aufsatz, W.; Matzke, M.; Matzke, A.J. Involvement of putative SNF2 chromatin remodeling protein DRD1 in RNA-directed DNA methylation. Curr. Biol. 2004, 14, 801–805.
- Penterman, J.; Uzawa, R.; Fischer, R.L. Genetic Interactions between DNA Demethylation and Methylation in Arabidopsis. Plant Physiol. 2007, 145, 1549–1557.
- Habu, Y. Epigenetic silencing of endogenous repetitive sequences by MORPHEUS’ MOLECULE1 in Arabidopsis thaliana. Epigenetics 2010, 5, 562–565.
- Vaillant, I.; Schubert, I.; Tourmente, S.; Mathieu, O. MOM1 mediates DNA-methylation-independent silencing of repetitive sequences in Arabidopsis. EMBO Rep. 2006, 7, 1273–1278.
- Charng, Y.Y.; Liu, H.C.; Liu, N.Y.; Chi, W.T.; Wang, C.N.; Chang, S.H.; Wang, T.T. A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol. 2007, 143, 251–262.
- Lämke, J.; Brzezinka, K.; Altmann, S.; Bäurle, I. A hit-and-run heat shock factor governs sustained histone methylation and transcriptional stress memory. EMBO J. 2015, 35, 162–175.
- Takeda, S.; Tadele, Z.; Hofmann, I.; Probst, A.V.; Angelis, K.J.; Kaya, H.; Araki, T.; Mengiste, T.; Scheid, O.M.; Shibahara, K.I.; et al. BRU1, a novel link between responses to DNA damage and epigenetic gene silencing in Arabidopsis. Genes Dev. 2004, 18, 782–793.
- Brzezinka, K.; Altmann, S.; Bäurle, I. BRUSHY1/TONSOKU/MGOUN3 is required for heat stress memory. Plant Cell Environ. 2018, 42, 771–781.
- Sani, E.; Herzyk, P.; Perrella, G.; Colot, V.; Amtmann, A. Hyperosmotic priming of Arabidopsis seedlings establishes a long-term somatic memory accompanied by specific changes of the epigenome. Genome Biol. 2013, 14, R59.
- Vriet, C.; Hennig, L.; Laloi, C. Stress-induced chromatin changes in plants: Of memories, metabolites and crop improvement. Cell. Mol. Life Sci. 2015, 72, 1261–1273.
- Lämke, J.; Bäurle, I. Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol. 2017, 18, 124.
- Friedrich, T.; Faivre, L.; Bäurle, I.; Schubert, D. Chromatin-based mechanisms of temperature memory in plants. Plant Cell Environ. 2018, 42, 762–770.
- Singh, A.K.; Dhanapal, S.; Finkelshtein, A.; Chamovitz, D.A. CSN5A Subunit of COP9 Signalosome Is Required for Resetting Transcriptional Stress Memory after Recurrent Heat Stress in Arabidopsis. Biomolecules 2021, 11, 668.
- Kim, J.-M.; Sasaki, T.; Ueda, M.; Sako, K.; Seki, M. Chromatin changes in response to drought, salinity, heat, and cold stresses in plants. Front. Plant Sci. 2015, 6, 114.
- Song, Z.; Zhang, L.; Han, J.; Zhou, M.; Liu, J. Histone H3K4 methyltransferases SDG25 and ATX1 maintain heat-stress gene expression during recovery in Arabidopsis. Plant J. 2020, 105, 1326–1338.
- Lim, C.J.; Park, J.; Shen, M.; Park, H.J.; Cheong, M.S.; Park, K.S.; Baek, D.; Bae, M.J.; Ali, A.; Jan, M.; et al. The Histone-Modifying Complex PWR/HOS15/HD2C Epigenetically Regulates Cold Tolerance. Plant Physiol. 2020, 184, 1097–1111.
- Park, J.; Lim, C.J.; Shen, M.; Park, H.J.; Cha, J.-Y.; Iniesto, E.; Rubio, V.; Mengiste, T.; Zhu, J.-K.; Bressan, R.A.; et al. Epigenetic switch from repressive to permissive chromatin in response to cold stress. Proc. Natl. Acad. Sci. USA 2018, 115, E5400–E5409.
- Pavangadkar, K.; Thomashow, M.F.; Triezenberg, S.J. Histone dynamics and roles of histone acetyltransferases during cold-induced gene regulation in Arabidopsis. Plant Mol. Biol. 2010, 74, 183–200.
- Zhu, J.; Jeong, J.C.; Zhu, Y.; Sokolchik, I.; Miyazaki, S.; Zhu, J.K.; Bressan, R.A. Involvement of Arabidopsis HOS15 in histone deacetylation and cold tolerance. Proc. Natl. Acad. Sci. USA 2008, 105, 4945–4950.
- Dong, W.; Gao, T.; Wang, Q.; Chen, J.; Lv, J.; Song, Y. Salinity stress induces epigenetic alterations to the promoter of MsMYB4 encoding a salt-induced MYB transcription factor. Plant Physiol. Biochem. 2020, 155, 709–715.
- Sokol, A.; Kwiatkowska, A.; Jerzmanowski, A.; Prymakowska-Bosak, M. Up-regulation of stress-inducible genes in tobacco and Arabidopsis cells in response to abiotic stresses and ABA treatment correlates with dynamic changes in histone H3 and H4 modifications. Planta 2007, 227, 245–254.
- Zheng, M.; Liu, X.; Lin, J.; Liu, X.; Wang, Z.; Xin, M.; Yao, Y.; Peng, H.; Zhou, D.; Ni, Z.; et al. Histone acetyltransferase GCN5 contributes to cell wall integrity and salt stress tolerance by altering the expression of cellulose synthesis genes. Plant J. 2018, 97, 587–602.
- Wang, Q.; Liu, P.; Jing, H.; Zhou, X.F.; Zhao, B.; Li, Y.; Jin, J.B. JMJ27-mediated histone H3K9 demethylation positively regulates drought-stress responses in Arabidopsis. New Phytol. 2021, 232, 221–236.
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704.
- Kim, J.-M.; To, T.K.; Ishida, J.; Morosawa, T.; Kawashima, M.; Matsui, A.; Toyoda, T.; Kimura, H.; Shinozaki, K.; Seki, M. Alterations of Lysine Modifications on the Histone H3 N-Tail under Drought Stress Conditions in Arabidopsis thaliana. Plant Cell Physiol. 2008, 49, 1580–1588.
- Matsui, A.; Ishida, J.; Morosawa, T.; Mochizuki, Y.; Kaminuma, E.; Endo, T.A.; Okamoto, M.; Nambara, E.; Nakajima, M.; Kawashima, M.; et al. Arabidopsis Transcriptome Analysis under Drought, Cold, High-Salinity and ABA Treatment Conditions using a Tiling Array. Plant Cell Physiol. 2008, 49, 1135–1149.
- Wight, W.D.; Kim, K.-H.; Lawrence, C.B.; Walton, J.D. Biosynthesis and Role in Virulence of the Histone Deacetylase Inhibitor Depudecin from Alternaria brassicicola. Mol. Plant-Microbe Interactions® 2009, 22, 1258–1267.
- Zhi, P.; Kong, L.; Liu, J.; Zhang, X.; Wang, X.; Li, H.; Sun, M.; Li, Y.; Chang, C. Histone Deacetylase TaHDT701 Functions in TaHDA6-TaHOS15 Complex to Regulate Wheat Defense Responses to Blumeria graminis f.sp. tritici. Int. J. Mol. Sci. 2020, 21, 2640.
- Yang, L.; Chen, X.; Wang, Z.; Sun, Q.; Hong, A.; Zhang, A.; Zhong, X.; Hua, J. HOS15 and HDA9 negatively regulate immunity through histone deacetylation of intracellular immune receptor NLR genes in Arabidopsis. New Phytol. 2019, 226, 507–522.
- Wang, Z.; Casas-Mollano, J.A.; Xu, J.; Riethoven, J.-J.M.; Zhang, C.; Cerutti, H. Osmotic stress induces phosphorylation of histone H3 at threonine 3 in pericentromeric regions of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2015, 112, 8487–8492.
- Chen, H.; Feng, H.; Zhang, X.; Zhang, C.; Wang, T.; Dong, J. An Arabidopsis E3 ligase HUB2 increases histone H2B monoubiquitination and enhances drought tolerance in transgenic cotton. Plant Biotechnol. J. 2018, 17, 556–568.
- Sun, Y.; Zhao, J.; Li, X.; Li, Y. E2 conjugases UBC1 and UBC2 regulate MYB42-mediated SOS pathway in response to salt stress in Arabidopsis. New Phytol. 2020, 227, 455–472.
- Castellanos, R.U.; Friedrich, T.; Petrovic, N.; Altmann, S.; Brzezinka, K.; Gorka, M.; Graf, A.; Bäurle, I. FORGETTER2 protein phosphatase and phospholipase D modulate heat stress memory in Arabidopsis. Plant J. 2020, 104, 7–17.
- Zhang, H.; Lang, Z.; Zhu, J.-K. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol. 2018, 19, 489–506.
- Du, J.; Johnson, L.M.; Jacobsen, S.E.; Patel, D.J. DNA methylation pathways and their crosstalk with histone methylation. Nat. Rev. Mol. Cell Biol. 2015, 16, 519–532.
- Sun, R.-Z.; Liu, J.; Wang, Y.-Y.; Deng, X. DNA methylation-mediated modulation of rapid desiccation tolerance acquisition and dehydration stress memory in the resurrection plant Boea hygrometrica. PLOS Genet. 2021, 17, e1009549.
- Sharma, R.; Vishal, P.; Kaul, S.; Dhar, M.K. Epiallelic changes in known stress-responsive genes under extreme drought conditions in Brassica juncea (L.) Czern. Plant Cell Rep. 2016, 36, 203–217.
- Skorupa, M.; Szczepanek, J.; Mazur, J.; Domagalski, K.; Tretyn, A.; Tyburski, J. Salt stress and salt shock differently affect DNA methylation in salt-responsive genes in sugar beet and its wild, halophytic ancestor. PLoS ONE 2021, 16, e0251675.
- Li, Y.; Kumar, S.; Qian, W. Active DNA demethylation: Mechanism and role in plant development. Plant Cell Rep. 2017, 37, 77–85.
- Steward, N.; Kusano, T.; Sano, H. Expression of ZmMET1, a gene encoding a DNA methyltransferase from maize, is associated not only with DNA replication in actively proliferating cells, but also with altered DNA methylation status in cold-stressed quiescent cells. Nucleic. Acids. Res. 2000, 28, 3250–3259.
- Wang, L.; Cao, S.; Wang, P.; Lu, K.; Song, Q.; Zhao, F.-J.; Chen, Z.J. DNA hypomethylation in tetraploid rice potentiates stress-responsive gene expression for salt tolerance. Proc. Natl. Acad. Sci. USA 2021, 118, e2023981118.
- Song, Y.; Jia, Z.; Hou, Y.; Ma, X.; Li, L.; Jin, X.; An, L. Roles of DNA Methylation in Cold Priming in Tartary Buckwheat. Front. Plant Sci. 2020, 11, 608540.
- Erdmann, R.M.; Picard, C.L. RNA-directed DNA Methylation. PLoS Genet. 2020, 16, e1009034.
- He, L.; Zhao, C.; Zhang, Q.; Zinta, G.; Wang, D.; Lozano-Durán, R.; Zhu, J.K. Pathway conversion enables a double-lock mechanism to maintain DNA methylation and genome stability. Proc. Natl. Acad. Sci. USA 2021, 118, e2107320118.
- Ito, H.; Gaubert, H.; Bucher, E.; Mirouze, M.; Vaillant, I.; Paszkowski, J. An siRNA pathway prevents transgenerational retrotransposition in plants subjected to stress. Nature 2011, 472, 115–119.
- Popova, O.V.; Dinh, H.Q.; Aufsatz, W.; Jonak, C. The RdDM Pathway Is Required for Basal Heat Tolerance in Arabidopsis. Mol. Plant 2013, 6, 396–410.
- Li, J.; Yang, D.-L.; Huang, H.; Zhang, G.; He, L.; Pang, J.; Lozano-Durán, R.; Lang, Z.; Zhu, J.-K. Epigenetic memory marks determine epiallele stability at loci targeted by de novo DNA methylation. Nat. Plants 2020, 6, 661–674.
- Zhong, S.-H.; Liu, J.-Z.; Jin, H.; Lin, L.; Li, Q.; Chen, Y.; Yuan, Y.-X.; Wang, Z.-Y.; Huang, H.; Qi, Y.-J.; et al. Warm temperatures induce transgenerational epigenetic release of RNA silencing by inhibiting siRNA biogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 9171–9176.
- Shinde, H.; Dudhate, A.; Kadam, U.S.; Hong, J.C. RNA methylation in plants: An overview. Front. Plant Sci. 2023, 14, 1132959.
- Cai, J.; Hu, J.; Amara, U.; Park, S.J.; Li, Y.; Jeong, D.; Lee, I.; Xu, T.; Kang, H. Arabidopsis N6-methyladenosine methyltransferase FIONA1 regulates floral transition by affecting the splicing of FLC and the stability of floral activators SPL3 and SEP3. J. Exp. Bot. 2022, 74, 864–877.
- Shen, L.; Liang, Z.; Gu, X.; Chen, Y.; Teo, Z.W.N.; Hou, X.; Cai, W.M.; Dedon, P.C.; Liu, L.; Yu, H. N6-Methyladenosine RNA Modification Regulates Shoot Stem Cell Fate in Arabidopsis. Dev. Cell 2016, 38, 186–200.
- Martínez-Pérez, M.; Aparicio, F.; López-Gresa, M.P.; Bellés, J.M.; Sánchez-Navarro, J.A.; Pallás, V. Arabidopsis m(6)A demethylase activity modulates viral infection of a plant virus and the m(6)A abundance in its genomic RNAs. Proc. Natl. Acad. Sci. USA 2017, 114, 10755–10760.
- Li, Y.; Wang, X.; Li, C.; Hu, S.; Yu, J.; Song, S. Transcriptome-wide N6-methyladenosine profiling of rice callus and leaf reveals the presence of tissue-specific competitors involved in selective mRNA modification. RNA Biol. 2014, 11, 1180–1188.
- Wang, Z.; Tang, K.; Zhang, D.; Wan, Y.; Wen, Y.; Lu, Q.; Wang, L. High-throughput m6A-seq reveals RNA m6A methylation patterns in the chloroplast and mitochondria transcriptomes of Arabidopsis thaliana. PLoS ONE 2017, 12, e0185612.
- Tieu Ngoc, L.N.; Jung Park, S.; Thi Huong, T.; Lee, K.H.; Kang, H. N4-methylcytidine ribosomal RNA methylation in chloroplasts is crucial for chloroplast function, development, and abscisic acid response in Arabidopsis. J. Integr. Plant Biol. 2021, 63, 570–582.
- Yang, L.; Perrera, V.; Saplaoura, E.; Apelt, F.; Bahin, M.; Kramdi, A.; Olas, J.; Mueller-Roeber, B.; Sokolowska, E.; Zhang, W.; et al. m5C Methylation Guides Systemic Transport of Messenger RNA over Graft Junctions in Plants. Curr. Biol. 2019, 29, 2465–2476.e5.
- Hu, J.; Cai, J.; Park, S.J.; Lee, K.; Li, Y.; Chen, Y.; Kang, H. N(6)-Methyladenosine mRNA methylation is important for salt stress tolerance in Arabidopsis. Plant J. 2021, 106, 1759–1775.




