
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Juan Eduardo Sosa-Hernández | -- | 3324 | 2023-10-31 16:17:46 | | | |
| 2 | Catherine Yang | Meta information modification | 3324 | 2023-11-01 02:14:34 | | |
Video Upload Options
The use of macroalgae or macroalgae extracts in animal feed has garnered significant attention due to the increasing demand for renewable and sustainable sources of animal protein, reducing the strain on land resources. Numerous studies have investigated the incorporation of fresh or dried macroalgae and its extracts in feeding animals, focusing on aquatic organisms. Macroalgae metabolites have been found to enhance growth, boost immunity, reduce microbial load, and improve meat quality. However, it is essential to note that macroalgae are primarily used as fortifiers in basal animal feed rather than as a whole feed source due to their essential amino acid content being considerably lower than that of traditional ingredients such as animal and soybean protein, fishmeal, and fish oil. The composition of macroalgae metabolites can vary depending on factors such as species, geographic location, season, external conditions (pH, water temperature, sunlight intensity), and nutrient concentration in the water. This variability provides ample opportunities to enhance feeding techniques by identifying ingredients with beneficial characteristics such as high nutritional profiles (amino acids, fatty acids, polysaccharides, vitamins, and minerals), digestibility, environmental and consumer safety, low production costs, year-round availability, and suitability as alternatives to fishmeal, animal protein, antibiotics, and immunostimulants.
1. Aquatic Organisms
| Seaweed Specie |
Used Compound |
Test Organism | Trial Dose Days | Pathogen/ Stress |
Results | Reference |
|---|---|---|---|---|---|---|
| Brown seaweed (Phaeophyta) | ||||||
| Cystoseira trinodis |
Polysaccharide—fucoidan | Litopenaeus vannamei | 0, 0.1, 0.2 and 0.4%—60 days | WSSV | FW, WG, SGR, expression rate of genes: proPO I, SOD, LYZ, and resistance against WSSV +; GPx and FCR − | [28] |
| Sargassum horneri |
Hot water extract | L. vannamei | 0, 2.5, 5.0, 10 g kg−1—28 days | NA | PO, THC, phagocytic rate, WG, and expression rate of genes: ProPO I, ProPO II, peroxinectin, α2macroglobulin, clotting protein, LYZ, SOD, GPx, penaiedin2-4, crusting + | [29] |
| U. pinnatifida | This is a hot water extract that contains a significant amount of mannitol and fucoidan | Pleoticus muelleri | 0, 3.0 g 100 g−1—30 days | UVR stress | Resistance against UVR stress, concentrations of UV-absorbing compounds, and TAS +; carotenoid concentration − | [30] |
| Iyengaria stellata |
Water extract | L. vannamei | 0, 0.5, 1, 1.5 g kg−1—56 days | Photobacterium damselae | WG, SGR, FE, PUFA, TAS, PO, CAT, SOD and GPX activities, and resistance against P. damselae + | [19] |
| Padina tetrastromatica and Sargassum ilicifolium |
Ethyl acetate, ethanol, and methanol extracts | Penaeus monodon |
0, 2.5, and 5 g kg−1—45 days | Vibrio parahaemolyticus | WG, SGR, SR, antibacterial activity, PO, SOD, resistance against V. parahaemolyticus +; minimal time required for hemolymph clotting − | [31] |
| S. cristaefolium | Wholemeal | L. vannamei | 0, 5, 10, 15, 20 and 40 g kg−1—60 days | NA | FW, muscle total protein =; muscle cholesterol and triglyceride level, and Vibrio counts in the intestine −; THC + | [32] |
| Sargassum polycystum |
Powdered seaweed flour | L. vannamei | 0.5 g kg−1—56 days | Cold stress | Body proximate composition: protein, ether extract, ash, carcass energy, FCR −; WG, ADG, SGR, SR, nonspecific immune responses, activation of the hepatic glandular duct system, hemocyte infiltration, and expression rate of genes: SOD, penaeidin4, HSP-70 + | [33] |
| Red seaweed (Rhodophyta) | ||||||
| Amphiroa fragilissima |
Crude polysaccharides, encapsulated Artemia nauplii |
L. vannamei | 0, 0.1, 0.15, and 0.20 g L−1—45 days | NA | WG, SGR, protease/amylase activities, and: protein, amino acid, free sugar, lipid, SOD, and CAT activities + | [34] |
| Asparagopsis armata |
Ethanolic extract | L. vannamei | 0, 1.5, 3.5, 7.5 g kg−1—40 days | V. parahaemolyticus | WG, SR, TAS, antimicrobial activity, and resistance against V. parahaemolyticus +; FCR − | [22] |
| Gracilaria birdiae |
Sulfated polysaccharide | L. vannamei | 0, 0.3%—32 days | WSSV | Agglutinating capacity, PO, resistance against WSSV, and THC + | [20] |
| Gracilaria verrucosa |
Ethyl acetate extracts (alkaloids, saponins, phenolics, flavonoids, triterpenoids, steroids, and glycosides) |
L. vannamei | 0—2.0 g per kg−1—14 days | Vibrio harveyi | Inhibition the growth of V. harveyi, THC, PO activity, phagocytosis activity, respiratory burst activity, and resistance against V. harveyi + | [27] |
| Porphyra haitanensis |
Wholemeal | L. vannamei | 0, 2.0%—56 days | WSSV | FCR -; WG, SGR, digestibility, TAS, expression of immune genes, ProPO and SOD activities + | [25] |
| Jania adherens |
Ethanolic extract | L. vannamei | 0, 0.5, 1.0, and 1.5 g kg−1—56 days | Photobacterium damselae | FCR −; SR =; WG, SGR, FE, PO, GPx, lipase, amylase, LYZ, respiratory burst activities, and resistance against P. damselae + | [19] |
| K. alvarezii | Fermented K. alvarezii powder | L. vannamei | 0, 0.5, 1.5%—15 days | NA | FW, SGR, and SR + | [35] |
| Kappaphycus alvarezii |
κ-carrageenan | P. monodon | 0.0, 0.15, 0.30, 0.45, and 0.60 g kg−1—30 days | Salinity stress | Attractability, FW, WG, SGR, FE, and immune-stimulating effects on salinity stress +; FCR − | [23] |
| Sarcodia suae |
S. suae powder | L. vannamei | 0, 2.5, 5, 7.5%—20 days | Vibrio alginolyticus | Anti-V. alginolyticus activity, phagocytic activity, THC, and expression of GPx + | [36] |
| Green seaweed (Chlorophyta) | ||||||
| Caulerpa lentillifera |
Seaweed flour |
P. monodon | 0, 10, 20, 30, 40 and 50 g kg−1—60 days | NA | WG, SGR, and FR+; FCR −; SR = | [37] |
| Caulerpa sp. | Caulerpa sp. flour | White leg shrimp | 0, 2, 4 and 6 g kg−1—30 days | NA | FW and FE +; FCR − | [38] |
| Enteromorpha | Polysaccharides | Fenneropenaeus merguiensis |
0, 1, 2 and 3 g kg−1—42 days | NA | FCR, relative abundance of Vibrio spp., and malondialdehyde content −; expression of immune genes, relative abundance of Firmicutes, FW, WG, SGR, TAS, GPx, S-transferase, SOD, LYZ, and PO activities + | [39] |
| U. lactuca | Water extract | L. vannamei | 0, 5, 10 15%—28 days | NA | FW, WG, SGR, FE, chymotrypsin, lipase, and amylase enzyme activity + | [40] |
| U. lactuca | U. lactuca powder |
L. vannamei | 0, 4 g 100 g−1—36 days | NA | SGR, ADG, and relative abundance of: Agarivorans, Sphingomonas, Lactobacillus, Leuconostoc, Peredibacter, Bdellovibrios +; relative abundance of: V. alginolyticus and Photobacterium sp. − | [41] |
| Ulva clathrata and U. lactuca | Wholemeal | L. vannamei | 100%—28 days | NA | Relative abundance of Rubritalea, Lysinibacillus, Acinetobacter, Blastopirellula and Litoreibacter spp. +; FW, SR =; relative abundance of Vibrio − | [42] |
| Ulva intestinalis |
Hot water crude extract | L. vannamei | 0, 1, 5, and 10 g kg−1—28 days | WSSV and YHV | WG, ADG, SGR, villus height, phagocytic activity, resistance against YHV, and expression of immune genes +; resistance against WSSV = | [43] |
| Ulva prolifera |
Polysaccharides and filtered residue | L. vannamei | 0, 0.78, 1.33, 31.7 g kg−1—21 days | V. parahaemolyticus | FCR −; FW, LYZ, PO, resistance against V. parahaemolyticus, and immune protective rate + | [44] |
| Combined seaweed | ||||||
| Sargassum filipendula and U. pinnatifida |
Combined seaweed dry biomass | L. vannamei | 0, 15, 25, 45 g kg−1—15 and 21 days | Thermal shock/WSSV | Resistance against WSSV, hemocyte infiltration, and positive changes in shrimp gut microbiome + | [45] |
| S. filipendula and U. pinnatifida |
Combined seaweed dry biomass | L. vannamei | 0, 25, 30, 45, 50 g kg−1—15 and 49 days | Thermal shock/Vibrio spp. | Resistance against Vibrio spp. and against thermal shock FW, and heterotrophic bacteria count in organisms + | [46] |
| U. lactuca and Jania rubens |
Combined seaweed powder |
Procambarus clarkii | 0, 10, 15%—56 days | NA | Frequency of molting and FW+; FCR −; SR = | [47] |
| U. pinnatifida and S. filipendula | Combined seaweed dry biomass | L. vannamei | 0, 3 and 5%—35 days | Thermal stress/WSSV | Gut bacterial diversity, THC, and resistance against thermal stress +; abundance of Vibrionaceae spp. −; SR, FCR, and FW = | [21] |
| U. lactuca, Eisenia sp., and Porphyra sp. |
Mix in equal parts of extracts | L. vannamei | 0.30 g kg−1—28 days | WSSV | Resistance against WSSV (genes related to WSSV resistance) + | [48] |
2. Poultry
3. Ruminants
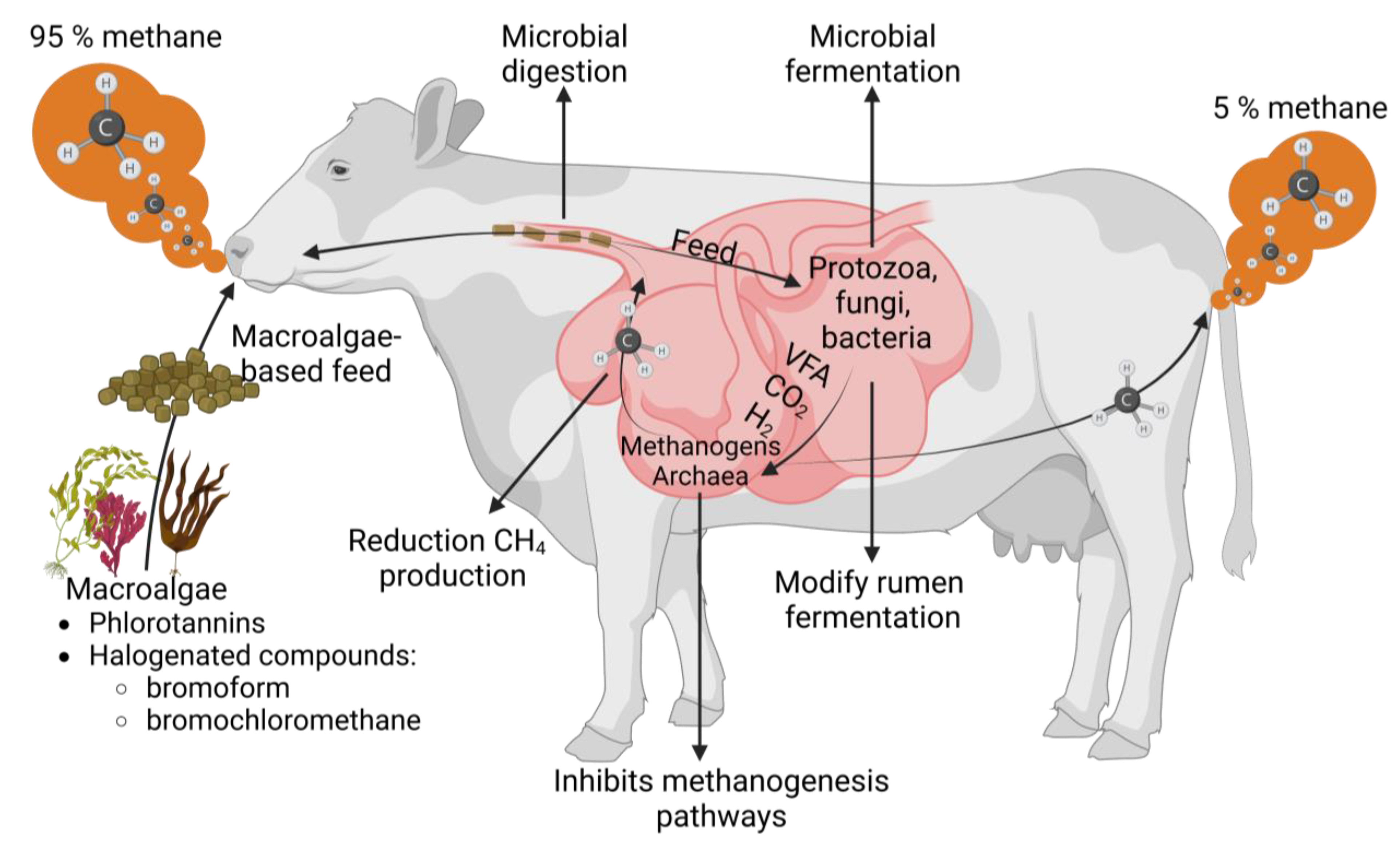
4. Pigs
5. Other Animals
References
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K.; et al. The Future of Aquatic Protein: Implications for Protein Sources in Aquaculture Diets. One Earth 2019, 1, 316–329.
- Jannathulla, R.; Rajaram, V.; Kalanjiam, R.; Ambasankar, K.; Muralidhar, M.; Dayal, J.S. Fishmeal Availability in the Scenarios of Climate Change: Inevitability of Fishmeal Replacement in Aquafeeds and Approaches for the Utilization of Plant Protein Sources. Aquac. Res. 2019, 50, 3493–3506.
- Duarte, C.M.; Bruhn, A.; Krause-Jensen, D. A Seaweed Aquaculture Imperative to Meet Global Sustainability Targets. Nat. Sustain. 2021, 5, 185–193.
- Hodar, A.R.; Vasava, R.; Joshi, N.H.; Mahavadiya, D.R. Fish Meal and Fish Oil Replacement for Alternative Sources: A Review. J. Exp. Zool. India 2020, 23, 13–21.
- Melchor-Martínez, E.M.; Reyes, A.G.; Morreeuw, Z.P.; Flores-Contreras, E.A.; Araújo, R.G.; Ramírez-Gamboa, D.; Sosa-Hernández, J.E.; Iqbal, H.M.N.; González-Meza, G.M.; Bonaccorso, A.D.; et al. Comparative Study on the Valorization of Sargassum from the Mexican Caribbean Coast and Gulf of California as an Ingredient on Healthy Diets for Shrimp Farming. Aquac. Rep. 2023, 32, 101709.
- Wan, A.H.L.; Davies, S.J.; Soler-Vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a Sustainable Aquafeed Ingredient. Rev. Aquac. 2019, 11, 458–492.
- Vijayaram, S.; Ringø, E.; Zuorro, A.; van Doan, H.; Sun, Y. Beneficial Roles of Nutrients as Immunostimulants in Aquaculture: A Review. Aquac. Fish 2023.
- Carreon, J.M.; Purnamasari, L.; Dela Cruz, J.F. Benefits of Green Seaweed As Protein Source for Broiler: A Review. J. Livest. Sci. Prod. 2022, 6, 381–400.
- Tadele, Y. Important Anti-Nutritional Substances and Inherent Toxicants of Feeds. Food Sci. Qual. Manag. 2015, 36, 40–47.
- Krogdahl, Å.; Kortner, T.M.; Hardy, R.W. Antinutrients and Adventitious Toxins. In Fish Nutrition; Elsevier: Amsterdam, The Netherlands, 2022; pp. 775–821. ISBN 9780128195871.
- Ang, C.-Y.; Yong, A.S.K.; Al Azad, S.; Lim, L.-S.; Zuldin, W.H.; Lal, M.T.M. Valorization of Macroalgae through Fermentation for Aquafeed Production: A Review. Fermentation 2021, 7, 304.
- Kokou, F.; Fountoulaki, E. Aquaculture Waste Production Associated with Antinutrient Presence in Common Fish Feed Plant Ingredients. Aquaculture 2018, 495, 295–310.
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current Trends on Seaweeds: Looking at Chemical Composition, Phytopharmacology, and Cosmetic Applications. Molecules 2019, 24, 4182.
- Bakky, M.A.H.; Tran, N.T.; Zhang, Y.; Li, S. Utilization of Marine Macroalgae-derived Sulphated Polysaccharides as Dynamic Nutraceutical Components in the Feed of Aquatic Animals: A Review. Aquac. Res. 2022, 53, 5787–5808.
- Anisha, G.S.; Padmakumari, S.; Patel, A.K.; Pandey, A.; Singhania, R.R. Fucoidan from Marine Macroalgae: Biological Actions and Applications in Regenerative Medicine, Drug Delivery Systems and Food Industry. Bioengineering 2022, 9, 472.
- Abdel-Latif, H.M.R.; Dawood, M.A.O.; Alagawany, M.; Faggio, C.; Nowosad, J.; Kucharczyk, D. Health Benefits and Potential Applications of Fucoidan (FCD) Extracted from Brown Seaweeds in Aquaculture: An Updated Review. Fish Shellfish Immunol. 2022, 122, 115–130.
- Legarda, E.C.; Viana, M.T.; Zaragoza, O.B.D.R.; Skrzynska, A.K.; Braga, A.; de Lorenzo, M.A.; do Nascimento Vieira, F. Effects on Fatty Acids Profile of Seriola dorsalis Muscle Tissue Fed Diets Supplemented with Different Levels of Ulva fasciata from an Integration Multi-Trophic Aquaculture System. Aquaculture 2021, 535, 736414.
- Sultana, N.; Mohammad, M.; Lisa, S.A.; Saha, B. Effects of Dietary Inclusion of Seaweed in Regular Commercial Fish Feed on Proximate Composition, Minerals and Fatty Acid Profiling of Nile Tilapia Oreochromis niloticus (Linnaeus, 1758). Aquat. Food Stud. 2023, 2, AFS112.
- Akbary, P.; Adeshina, I.; Jahanbakhshi, A. Growth Performance, Digestive Enzymes, Antioxidant Activity and Immune Responses of Litopenaeus vannamei Fed with Jania adhaerens J.V. Supplemented Diet against Photobacterium damselae Infection. Anim. Feed Sci. Technol. 2020, 270, 114696.
- Cantelli, L.; Goncalves, P.; Guertler, C.; Kayser, M.; Pilotto, M.R.; Barracco, M.A.; Perazzolo, L.M. Dietary Supplementation with Sulfated Polysaccharides from Gracilaria Birdiae Promotes a Delayed Immunostimulation in Marine Shrimp Challenged by the White Spot Syndrome Virus. Aquac. Int. 2019, 27, 349–367.
- Costa Rezende, P.; Soares, M.; Guimarães, A.M.; da Rosa Coelho, J.; Seiffert, W.Q.; Dias Schleder, D.; Vieira, F. do N. Brown Seaweeds Added in the Diet Improved the Response to Thermal Shock and Reduced Vibrio Spp. in Pacific White Shrimp Post-larvae Reared in a Biofloc System. Aquac. Res. 2021, 52, 2852–2861.
- Félix, R.; Félix, C.; Januário, A.P.; Carmona, A.M.; Baptista, T.; Gonçalves, R.A.; Sendão, J.; Novais, S.C.; Lemos, M.F.L. Tailoring Shrimp Aquafeed to Tackle Acute Hepatopancreatic Necrosis Disease by Inclusion of Industry-Friendly Seaweed Extracts. Aquaculture 2020, 529, 735661.
- Jumah, Y.U.; Tumbokon, B.L.M.; Serrano, A.E. Effects of Dietary κ-Carrageenan on Growth and Resistance to Acute Salinity Stress in the Black Tiger Shrimp Penaeus monodon Post Larvae. Isr. J. Aquac. Bamidgeh 2020, 72, 1–11.
- Lee, J.-S.; Kang, S.; Kim, M.-J.; Han, S.-G.; Lee, H.-G. Dietary Supplementation with Combined Extracts from Garlic (Allium sativum), Brown Seaweed (Undaria pinnatifida), and Pinecone (Pinus koraiensis) Improves Milk Production in Holstein Cows under Heat Stress Conditions. Asian-Australas. J. Anim. Sci. 2020, 33, 111–119.
- Niu, J.; Xie, J.-J.; Guo, T.-Y.; Fang, H.-H.; Zhang, Y.-M.; Liao, S.-Y.; Xie, S.-W.; Liu, Y.-J.; Tian, L.-X. Comparison and Evaluation of Four Species of Macro-Algaes as Dietary Ingredients in Litopenaeus vannamei Under Normal Rearing and WSSV Challenge Conditions: Effect on Growth, Immune Response, and Intestinal Microbiota. Front. Physiol. 2019, 9, 1880.
- Omont, A.; Elizondo-González, R.; Escobedo-Fregoso, C.; Tovar-Ramírez, D.; Hinojosa-Baltazar, P.; Peña-Rodríguez, A. Bacterial Communities and Digestive Enzymatic Activities of Litopenaeus vannamei Shrimp Fed Pre-Digested Seaweeds as a Functional Ingredient. J. Appl. Phycol. 2021, 33, 1239–1251.
- Rudi, M.; Sukenda; Wahjuningrum, D.; Pasaribu, W.; Hidayatullah, D. Seaweed Extract of Gracilaria Verrucosa as an Antibacterial and Treatment against Vibrio Harveyi Infection of Litopenaeus vannamei. J. Akuakultur. Indones. 2019, 18, 120–129.
- Salehpour, R.; Amrollahi Biuki, N.; Mohammadi, M.; Dashtiannasab, A.; Ebrahimnejad, P. The Dietary Effect of Fucoidan Extracted from Brown Seaweed, Cystoseira trinodis (C. Agardh) on Growth and Disease Resistance to WSSV in Shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2021, 119, 84–95.
- Lee, P.-T.; Quan Tran, H.T.; Huang, H.-T.; Nan, F.-H.; Lee, M.-C. Sargassum Horneri Extracts Stimulate Innate Immunity, Enhance Growth Performance, and Upregulate Immune Genes in the White Shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2020, 102, 276–285.
- Marcoval, M.A.; Díaz, A.C.; Espino, M.L.; Arzoz, N.S.; Velurtas, S.M.; Fenucci, J.L. Role of Dietary Photoprotective Compounds on the Performance of Shrimp Pleoticus muelleri under UVR Stress. Aquaculture 2020, 515, 734564.
- AftabUddin, S.; Siddique, M.A.M.; Habib, A.; Akter, S.; Hossen, S.; Tanchangya, P.; Abdullah Al, M. Effects of Seaweeds Extract on Growth, Survival, Antibacterial Activities, and Immune Responses of Penaeus monodon against Vibrio parahaemolyticus. Ital. J. Anim. Sci. 2021, 20, 243–255.
- Jahromi, S.T.; Pourmozaffar, S.; Jahanbakhshi, A.; Rameshi, H.; Gozari, M.; Khodadadi, M.; Sohrabipour, J.; Behzadi, S.; Barzkar, N.; Nahavandi, R.; et al. Effect of Different Levels of Dietary Sargassum cristaefolium on Growth Performance, Hematological Parameters, Histological Structure of Hepatopancreas and Intestinal Microbiota of Litopenaeus vannamei. Aquaculture 2021, 533, 736130.
- Abdel-Rahim, M.; Bahattab, O.; Nossir, F.; Al-Awthan, Y.; Khalil, R.H.; Mohamed, R. Dietary Supplementation of Brown Seaweed and/or Nucleotides Improved Shrimp Performance, Health Status and Cold-Tolerant Gene Expression of Juvenile Whiteleg Shrimp during the Winter Season. Mar. Drugs 2021, 19, 175.
- Muttharasi, C.; Gayathri, V.; Muralisankar, T.; Mohan, K.; Uthayakumar, V.; Radhakrishnan, S.; Kumar, P.; Palanisamy, M. Growth Performance, Digestive Enzymes and Antioxidants Activities in the Shrimp Litopenaeus vannamei Fed with Amphiroa fragilissima Crude Polysaccharides Encapsulated Artemia nauplii. Aquaculture 2021, 545, 737263.
- Suantika, G.; Situmorang, M.L.; Saputra, F.I.; Alviredieta, U.; Aditiawati, P.; Putri, S.P. The Effect of Feed Supplementation with Fermented Red Seaweed (Kappaphycus alvarezii) on Growth and Survival of White Shrimp (Litopenaeus vannamei) Post-Larvae Culture. Hayati 2021, 28, 286–392.
- Kuo, C.-H.; Ballantyne, R.; Huang, P.-L.; Ding, S.; Hong, M.-C.; Lin, T.-Y.; Wu, F.-C.; Xu, Z.-Y.; Chiu, K.; Chen, B.; et al. Sarcodia Suae Modulates the Immunity and Disease Resistance of White Shrimp Litopenaeus vannamei against Vibrio Alginolyticus via the Purine Metabolism and Phenylalanine Metabolism. Fish Shellfish Immunol. 2022, 127, 766–777.
- Putra, D.F.; Rahmawati, M.; Abidin, M.Z.; Ramlan, R. Dietary Administration of Sea Grape Powder (Caulerpa lentillifera) Effects on Growth and Survival Rate of Black Tiger Shrimp (Penaeus monodon). IOP Conf. Ser. Earth Environ. Sci. 2019, 348, 012100.
- Nasmia; Natsir, S.; Rusaini; Tahya, A.M.; Nilawati, J.; Ismail, S.N. Utilization of Caulerpa Sp. as a Feed Ingredient for Growth and Survival of Whiteleg Shrimp and Chanos Chanos in Polyculture. Egypt. J. Aquat. Res. 2022, 48, 175–180.
- Liu, W.-C.; Zhou, S.-H.; Balasubramanian, B.; Zeng, F.-Y.; Sun, C.-B.; Pang, H.-Y. Dietary Seaweed (Enteromorpha) Polysaccharides Improves Growth Performance Involved in Regulation of Immune Responses, Intestinal Morphology and Microbial Community in Banana Shrimp Fenneropenaeus merguiensis. Fish Shellfish Immunol. 2020, 104, 202–212.
- Omont, A.; Quiroz-Guzman, E.; Tovar-Ramirez, D.; Peña-Rodríguez, A. Correction to: Effect of Diets Supplemented with Different Seaweed Extracts on Growth Performance and Digestive Enzyme Activities of Juvenile White Shrimp Litopenaeus vannamei. J. Appl. Phycol. 2019, 31, 1443.
- Mangott, A.; Nappi, J.; Delli Paoli Carini, A.; Goncalves, P.; Hua, K.; Domingos, J.A.; de Nys, R.; Thomas, T. Ulva Lactuca as a Functional Ingredient and Water Bioremediator Positively Influences the Hepatopancreas and Water Microbiota in the Rearing of Litopenaeus vannamei. Algal. Res. 2020, 51, 102040.
- Elizondo-González, R.; Quiroz-Guzmán, E.; Howe, A.; Yang, F.; Flater, J.; Gemin, M.; Palacios, E.; Peña-Rodríguez, A. Changes on the Intestinal Bacterial Community of White Shrimp Penaeus vannamei Fed with Green Seaweeds. J. Appl. Phycol. 2020, 32, 2061–2070.
- Klongklaew, N.; Praiboon, J.; Tamtin, M.; Srisapoome, P. Chemical Composition of a Hot Water Crude Extract (HWCE) from Ulva intestinalis and Its Potential Effects on Growth Performance, Immune Responses, and Resistance to White Spot Syndrome Virus and Yellowhead Virus in Pacific White Shrimp (Litopenaeus vannam). Fish Shellfish Immunol. 2021, 112, 8–22.
- Ge, H.; Ni, Q.; Chen, Z.; Li, J.; Zhao, F. Effects of Short Period Feeding Polysaccharides from Marine Macroalga, Ulva prolifera on Growth and Resistance of Litopenaeus vannamei against Vibrio parahaemolyticus Infection. J. Appl. Phycol. 2019, 31, 2085–2092.
- Schleder, D.D.; Blank, M.; Peruch, L.G.B.; Poli, M.A.; Gonçalves, P.; Rosa, K.V.; Fracalossi, D.M.; do Vieira, F.N.; Andreatta, E.R.; Hayashi, L. Impact of Combinations of Brown Seaweeds on Shrimp Gut Microbiota and Response to Thermal Shock and White Spot Disease. Aquaculture 2020, 519, 734779.
- Costa, M.; Cardoso, C.; Afonso, C.; Bandarra, N.M.; Prates, J.A.M. Current Knowledge and Future Perspectives of the Use of Seaweeds for Livestock Production and Meat Quality: A Systematic Review. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1075–1102.
- Mazlum, Y.; Yazıcı, M.; Sayin, S.; Habiboğlu, O.; Uğur, S. Effects of Two Different Macroalgae (Ulva lactuca and Jania rubens) Species on Growth and Survival of Red Swamp Crayfish (Procambarus clarkii) as Feed Additive. Mar. Sci. Technol. Bull. 2021, 10, 154–162.
- Cabrera-Stevens, M.J.; Sánchez-Paz, A.; Mendoza-Cano, F.; Escobedo-Fregoso, C.; Encinas-García, T.; Elizondo-González, R.; Peña-Rodríguez, A. Transcriptome Analysis Reveals Differential Gene Expression Associated with White Spot Syndrome Virus Resistance in the Shrimp Litopenaeus vannamei Fed on Functional Diets. Aquaculture 2022, 547, 737434.
- Hafez, H.M.; Attia, Y.A. Challenges to the Poultry Industry: Current Perspectives and Strategic Future After the COVID-19 Outbreak. Front. Vet. Sci. 2020, 7.
- Matshogo, T.B.; Mnisi, C.M.; Mlambo, V. Dietary Green Seaweed Compromises Overall Feed Conversion Efficiency but Not Blood Parameters and Meat Quality and Stability in Broiler Chickens. Agriculture 2020, 10, 547.
- Ribeiro, D.M.; Martins, C.F.; Costa, M.; Coelho, D.; Pestana, J.; Alfaia, C.; Lordelo, M.; de Almeida, A.M.; Freire, J.P.B.; Prates, J.A.M. Quality Traits and Nutritional Value of Pork and Poultry Meat from Animals Fed with Seaweeds. Foods 2021, 10, 2961.
- Bai, J.; Wang, R.; Yan, L.; Feng, J. Co-Supplementation of Dietary Seaweed Powder and Antibacterial Peptides Improves Broiler Growth Performance and Immune Function. Braz. J. Poult. Sci. 2019, 21.
- Nhlane, L.T.; Mnisi, C.M.; Mlambo, V.; Madibana, M.J. Nutrient Digestibility, Growth Performance, and Blood Indices of Boschveld Chickens Fed Seaweed-Containing Diets. Animals 2020, 10, 1296.
- Balasubramanian, B.; Shanmugam, S.; Park, S.; Recharla, N.; Koo, J.S.; Andretta, I.; Kim, I.H. Supplemental Impact of Marine Red Seaweed (Halymenia palmata) on the Growth Performance, Total Tract Nutrient Digestibility, Blood Profiles, Intestine Histomorphology, Meat Quality, Fecal Gas Emission, and Microbial Counts in Broilers. Animals 2021, 11, 1244.
- Choi, J.; Kim, W.K. Dietary Application of Tannins as a Potential Mitigation Strategy for Current Challenges in Poultry Production: A Review. Animals 2020, 10, 2389.
- Kulshreshtha, G.; Critchley, A.; Rathgeber, B.; Stratton, G.; Banskota, A.H.; Hafting, J.; Prithiviraj, B. Antimicrobial Effects of Selected, Cultivated Red Seaweeds and Their Components in Combination with Tetracycline, against Poultry Pathogen Salmonella Enteritidis. J. Mar. Sci. Eng. 2020, 8, 511.
- Coudert, E.; Baéza, E.; Berri, C. Use of Algae in Poultry Production: A Review. Worlds Poult. Sci. J. 2020, 76, 767–786.
- Michalak, I.; Mahrose, K. Seaweeds, Intact and Processed, as a Valuable Component of Poultry Feeds. J. Mar. Sci. Eng. 2020, 8, 620.
- Borzouie, S.; M Rathgeber, B.; M Stupart, C.; MacIsaac, J.; MacLaren, L.A. Effects of Dietary Inclusion of Seaweed, Heat Stress and Genetic Strain on Performance, Plasma Biochemical and Hematological Parameters in Laying Hens. Animals 2020, 10, 1570.
- Muizelaar, W.; Groot, M.; van Duinkerken, G.; Peters, R.; Dijkstra, J. Safety and Transfer Study: Transfer of Bromoform Present in Asparagopsis taxiformis to Milk and Urine of Lactating Dairy Cows. Foods 2021, 10, 584.
- McCauley, J.I.; Labeeuw, L.; Jaramillo-Madrid, A.C.; Nguyen, L.N.; Nghiem, L.D.; Chaves, A.V.; Ralph, P.J. Management of Enteric Methanogenesis in Ruminants by Algal-Derived Feed Additives. Curr. Pollut. Rep. 2020, 6, 188–205.
- Vijn, S.; Compart, D.P.; Dutta, N.; Foukis, A.; Hess, M.; Hristov, A.N.; Kalscheur, K.F.; Kebreab, E.; Nuzhdin, S.V.; Price, N.N.; et al. Key Considerations for the Use of Seaweed to Reduce Enteric Methane Emissions From Cattle. Front. Vet. Sci. 2020, 7, 1–9.
- Giamouri, E.; Zisis, F.; Mitsiopoulou, C.; Christodoulou, C.; Pappas, A.C.; Simitzis, P.E.; Kamilaris, C.; Galliou, F.; Manios, T.; Mavrommatis, A.; et al. Sustainable Strategies for Greenhouse Gas Emission Reduction in Small Ruminants Farming. Sustainability 2023, 15, 4118.
- Broucek, J. Production of Methane Emissions from Ruminant Husbandry: A Review. J. Environ. Prot. 2014, 5, 1482–1493.
- Moorby, J.M.; Fraser, M.D. Review: New Feeds and New Feeding Systems in Intensive and Semi-Intensive Forage-Fed Ruminant Livestock Systems. Animal 2021, 15, 100297.
- Sofyan, A.; Irawan, A.; Herdian, H.; Jasmadi; Harahap, M.A.; Sakti, A.A.; Suryani, A.E.; Novianty, H.; Kurniawan, T.; Darma, I.N.G.; et al. Effects of Various Macroalgae Species on Methane Production, Rumen Fermentation, and Ruminant Production: A Meta-Analysis from in Vitro and in Vivo Experiments. Anim. Feed Sci. Technol. 2022, 294, 115503.
- Glasson, C.R.K.; Kinley, R.D.; de Nys, R.; King, N.; Adams, S.L.; Packer, M.A.; Svenson, J.; Eason, C.T.; Magnusson, M. Benefits and Risks of Including the Bromoform Containing Seaweed Asparagopsis in Feed for the Reduction of Methane Production from Ruminants. Algal. Res. 2022, 64, 102673.
- Min, B.R.; Parker, D.; Brauer, D.; Waldrip, H.; Lockard, C.; Hales, K.; Akbay, A.; Augyte, S. The Role of Seaweed as a Potential Dietary Supplementation for Enteric Methane Mitigation in Ruminants: Challenges and Opportunities. Anim. Nutr. 2021, 7, 1371–1387.
- Pandey, D.; Mansouryar, M.; Novoa-Garrido, M.; Næss, G.; Kiron, V.; Hansen, H.H.; Nielsen, M.O.; Khanal, P. Nutritional and Anti-Methanogenic Potentials of Macroalgae for Ruminants; Burleigh Dodds Science Publishing: Cambridge, UK, 2021; pp. 195–228.
- Roque, B.M.; Salwen, J.K.; Kinley, R.; Kebreab, E. Inclusion of Asparagopsis Armata in Lactating Dairy Cows’ Diet Reduces Enteric Methane Emission by over 50 Percent. J. Clean Prod. 2019, 234, 132–138.
- Kinley, R.D.; Martinez-Fernandez, G.; Matthews, M.K.; de Nys, R.; Magnusson, M.; Tomkins, N.W. Mitigating the Carbon Footprint and Improving Productivity of Ruminant Livestock Agriculture Using a Red Seaweed. J. Clean Prod. 2020, 259, 120836.
- Neville, E.W.; Fahey, A.G.; Gath, V.P.; Molloy, B.P.; Taylor, S.J.; Mulligan, F.J. The Effect of Calcareous Marine Algae, with or without Marine Magnesium Oxide, and Sodium Bicarbonate on Rumen PH and Milk Production in Mid-Lactation Dairy Cows. J. Dairy Sci. 2019, 102, 8027–8039.
- Caroprese, M.; Ciliberti, M.G.; Marino, R.; Santillo, A.; Sevi, A.; Albenzio, M. Polyunsaturated Fatty Acid Supplementation: Effects of Seaweed Ascophyllum nodosum and Flaxseed on Milk Production and Fatty Acid Profile of Lactating Ewes during Summer. J. Dairy Res. 2016, 83, 289–297.
- Quigley, A.; Walsh, S.W.; Hayes, E.; Connolly, D.; Cummins, W. Effect of Seaweed Supplementation on Tocopherol Concentrations in Bovine Milk Using Dispersive Liquid-Liquid Microextraction. J. Chromatogr. B 2018, 1092, 152–157.
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed Potential in the Animal Feed: A Review. J. Mar. Sci. Eng. 2020, 8, 559.
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for Livestock Diets: A Review. Anim. Feed Sci. Technol. 2016, 212, 1–17.
- Mahfuz, S.; Mun, H.-S.; Dilawar, M.A.; Yang, C.-J. Applications of Smart Technology as a Sustainable Strategy in Modern Swine Farming. Sustainability 2022, 14, 2607.
- Delsart, M.; Pol, F.; Dufour, B.; Rose, N.; Fablet, C. Pig Farming in Alternative Systems: Strengths and Challenges in Terms of Animal Welfare, Biosecurity, Animal Health and Pork Safety. Agriculture 2020, 10, 261.
- Corino, C.; Di Giancamillo, A.; Modina, S.C.; Rossi, R. Prebiotic Effects of Seaweed Polysaccharides in Pigs. Animals 2021, 11, 1573.
- Ribeiro, D.; Alfaia, C.; Pestana, J.; Carvalho, D.; Costa, M.; Martins, C.; Lemos, J.; Mourato, M.; Gueifão, S.; Delgado, I.; et al. Influence of Feeding Weaned Piglets with Laminaria digitata on the Quality and Nutritional Value of Meat. Foods 2022, 11, 1024.
- Nguyen, T.X.; Agazzi, A.; Comi, M.; Bontempo, V.; Guido, I.; Panseri, S.; Sauerwein, H.; Eckersall, P.D.; Burchmore, R.; Savoini, G. Effects of Low Ω6:Ω3 Ratio in Sow Diet and Seaweed Supplement in Piglet Diet on Performance, Colostrum and Milk Fatty Acid Profiles, and Oxidative Status. Animals 2020, 10, 2049.
- Jerez-Timaure, N.; Sánchez-Hidalgo, M.; Pulido, R.; Mendoza, J. Effect of Dietary Brown Seaweed (Macrocystis pyrifera) Additive on Meat Quality and Nutrient Composition of Fattening Pigs. Foods 2021, 10, 1720.
- Shimazu, T.; Borjigin, L.; Katoh, K.; Roh, S.; Kitazawa, H.; Abe, K.; Suda, Y.; Saito, H.; Kunii, H.; Nihei, K.; et al. Addition of Wakame Seaweed (Undaria pinnatifida) Stalk to Animal Feed Enhances Immune Response and Improves Intestinal Microflora in Pigs. Anim. Sci. J. 2019, 90, 1248–1260.
- Bussy, F.; Matthieu, L.G.; Salmon, H.; Delaval, J.; Berri, M.; Pi, N.C. Immunomodulating Effect of a Seaweed Extract from Lva armoricana in Pig: Specific IgG and Total IgA in Colostrum, Milk, and Blood. Vet. Anim. Sci. 2019, 7, 100051.
- Walsh, A.M.; Sweeney, T.; O’Shea, C.J.; Doyle, D.N.; O’Doherty, J.V. Effect of Dietary Laminarin and Fucoidan on Selected Microbiota, Intestinal Morphology and Immune Status of the Newly Weaned Pig. Br. J. Nutr. 2013, 110, 1630–1638.
- Bouwhuis, M.A.; McDonnell, M.J.; Sweeney, T.; Mukhopadhya, A.; O’Shea, C.J.; O’Doherty, J.V. Seaweed Extracts and Galacto-Oligosaccharides Improve Intestinal Health in Pigs Following Salmonella typhimurium Challenge. Animal 2017, 11, 1488–1496.
- Corino, C.; Modina, S.C.; Di Giancamillo, A.; Chiapparini, S.; Rossi, R. Seaweeds in Pig Nutrition. Animals 2019, 9, 1126.
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse Applications of Marine Macroalgae. Mar. Drugs 2019, 18, 17.
- Mala, A.; Bhassu, S.; Taufek, N.M.; Sadali, N.M.; Wang, S.; Mohamed, E.; Nor, A.M. Review: Potential of Using Lactic Acid Bacteria as Inoculant for Seaweed Silage towards Sustainable Aquaculture. Aquac. Rep. 2023, 28, 101440.
- Isidori, M.; Rueca, F.; Trabalza-Marinucci, M. Palatability of Extruded Dog Diets Supplemented with Ascophyllum nodosum L. (Fucaceae, Phaeophyceae). J. Appl. Phycol. 2019, 31, 3275–3281.
- Pinna, C.; Vecchiato, C.G.; Grandi, M.; Stefanelli, C.; Zannoni, A.; Biagi, G. Seaweed Supplementation Failed to Affect Fecal Microbiota and Metabolome as Well as Fecal IgA and Apparent Nutrient Digestibility in Adult Dogs. Animals 2021, 11, 2234.
- Zhang, M.; Mo, R.; Li, M.; Qu, Y.; Wang, H.; Liu, T.; Liu, P.; Wu, Y. Comparison of the Effects of Enzymolysis Seaweed Powder and Saccharomyces boulardii on Intestinal Health and Microbiota Composition in Kittens. Metabolites 2023, 13, 637.
- Abu Hafsa, S.H.; Khalel, M.S.; El-Gindy, Y.M.; Hassan, A.A. Nutritional Potential of Marine and Freshwater Algae as Dietary Supplements for Growing Rabbits. Ital. J. Anim. Sci. 2021, 20, 784–793.
- Knizatova, N.; Massanyi, M.; Rossi, R.; Ondruska, L.; Kovacik, A.; Tokarova, K.; Gren, A.; Formicki, G.; Binkowski, L.; Halo, M.; et al. The Effect of Brown Seaweed and Polyphenol Supplementation in Male Rabbits on the Blood Profile and Antioxidant Markers. Vet. Med. 2022, 67, 527–537.
- Vizzarri, F.; Chiapparini, S.; Corino, C.; Casamassima, D.; Palazzo, M.; Parkanyi, V.; Ondruska, L.; Rossi, R. Dietary Supplementation with Natural Extracts Mixture: Effects on Reproductive Performances, Blood Biochemical and Antioxidant Parameters in Rabbit Does. Ann. Anim. Sci. 2020, 20, 565–578.
- Vizzari, F.; Massányi, M.; Knížatová, N.; Corino, C.; Rossi, R.; Ondruška, Ľ.; Tirpák, F.; Halo, M.; Massányi, P. Effects of Dietary Plant Polyphenols and Seaweed Extract Mixture on Male-Rabbit Semen: Quality Traits and Antioxidant Markers. Saudi J. Biol. Sci. 2021, 28, 1017–1025.
- Rossi, R.; Vizzarri, F.; Ratti, S.; Palazzo, M.; Casamassima, D.; Corino, C. Effects of Long-Term Supplementation with Brown Seaweeds and Polyphenols in Rabbit on Meat Quality Parameters. Animals 2020, 10, 2443.
- Rossi, R.; Vizzarri, F.; Chiapparini, S.; Ratti, S.; Casamassima, D.; Palazzo, M.; Corino, C. Effects of Dietary Levels of Brown Seaweeds and Plant Polyphenols on Growth and Meat Quality Parameters in Growing Rabbit. Meat Sci. 2020, 161, 107987.
- Al-Soufi, S.; García, J.; Muíños, A.; López-Alonso, M. Marine Macroalgae in Rabbit Nutrition—A Valuable Feed in Sustainable Farming. Animals 2022, 12, 2346.




