Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giovanna Della Porta | -- | 2896 | 2023-10-31 10:56:21 | | | |
| 2 | Rita Xu | -1 word(s) | 2895 | 2023-11-01 02:30:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lamparelli, E.P.; Marino, M.; Szychlinska, M.A.; Della Rocca, N.; Ciardulli, M.C.; Scala, P.; D'auria, R.; Testa, A.; Viggiano, A.; Cappello, F.; et al. Bioplastic-Based Nanoparticles for Drug Delivery Systems. Encyclopedia. Available online: https://encyclopedia.pub/entry/50974 (accessed on 03 February 2026).
Lamparelli EP, Marino M, Szychlinska MA, Della Rocca N, Ciardulli MC, Scala P, et al. Bioplastic-Based Nanoparticles for Drug Delivery Systems. Encyclopedia. Available at: https://encyclopedia.pub/entry/50974. Accessed February 03, 2026.
Lamparelli, Erwin Pavel, Marianna Marino, Marta Anna Szychlinska, Natalia Della Rocca, Maria Camilla Ciardulli, Pasqualina Scala, Raffaella D'auria, Antonino Testa, Andrea Viggiano, Francesco Cappello, et al. "Bioplastic-Based Nanoparticles for Drug Delivery Systems" Encyclopedia, https://encyclopedia.pub/entry/50974 (accessed February 03, 2026).
Lamparelli, E.P., Marino, M., Szychlinska, M.A., Della Rocca, N., Ciardulli, M.C., Scala, P., D'auria, R., Testa, A., Viggiano, A., Cappello, F., Meccariello, R., Della Porta, G., & Santoro, A. (2023, October 31). Bioplastic-Based Nanoparticles for Drug Delivery Systems. In Encyclopedia. https://encyclopedia.pub/entry/50974
Lamparelli, Erwin Pavel, et al. "Bioplastic-Based Nanoparticles for Drug Delivery Systems." Encyclopedia. Web. 31 October, 2023.
Copy Citation
Plastics have changed human lives, finding a broad range of applications from packaging to medical devices. Biodegradable plastic materials have been introduced on the market. These polymers are biodegradable but also bioresorbable and, indeed, are fundamental tools for drug formulations, thanks to their transient ability to pass through biological barriers and concentrate in specific tissues.
bioplastics
nanoparticles
drug delivery systems
1. Introduction
Plastic diffusion is deemed a significant indicator of the onset of the Anthropocene, [1] an era in which humans altered and dominated the Earth and its ecosystems [2]. Despite the convenience aspects, the widespread use of plastics and their uncontrolled waste has resulted in negative impacts on the environment and human health [3]. In addition, most plastic products are added with various chemical compounds to improve functional properties, such as plasticizers (phthalates), flame retardants, antioxidants (i.e., IRGAFOS-168), acid scavengers, stabilizers (e.g., bisphenol A, BPA), and pigments [4]. Plastic degradation produces microplastics (MPs, particle size lower than 5 mm) and nanoplastics (NPs, size less than 1 µm) [5] that cross biological barriers and accumulate in the food chain [6].
More recently, “environmentally sustainable” plastic materials fabricated adopting biodegradable polymers, such as polylactic acid (PLA) and poly-lactic-co-glycolic acid (PLGA), have been introduced on the market. These bioplastics undergo a more rapid degradation in the environment; indeed, these biopolymers have been developed for pharmaceutical and biomedical applications in order to have a transient polymer (bioresorbable) but with mechanical properties similar to the non-biodegradable ones [7]. However, the recent widespread use of bioplastic-based materials needs a critical approach because the polymer’s faster biodegradation poses an issue for a more rapid accumulation in the form of particles in living tissues.
This issue is confirmed by the fact that these polymers are properly adopted in the form of micro- and nanoparticles as drug carriers in pharmaceutical formulations. For example, bioplastic-based NPs can be targeted and accumulated (depot systems) in a given tissue in order to achieve a proper sustained release of the loaded drug [8][9]. Bioplastic NPs can also prolong the therapeutic effect of a given drug, improving its efficacy [9]. Recent studies also suggest that specific concentrations of bioplastic NPs in the central nervous system (CNS) promote their passage through the blood–brain barrier (BBB) or blood–cerebrospinal fluid barrier (BCSFB). Indeed, bioplastic NPs seemed able to cross the BBB through transcytosis pathways and proper surface modifications can allow their passage through the BBB via receptor-mediated endocytosis or to deeply diffuse in the brain parenchyma [10]. This behavior, while extremely interesting for the development of new drug formulations for the CNS, poses significant challenges in terms of cost, failure, and clinical implementation; on the other hand, it may also raise public health concerns.
2. Bioplastics: Definition, Chemical Properties, and Applications
Bioplastics can be divided into two categories: biodegradable and biobased [11][12][13]. Biobased plastics are entirely or partially made from biological resources and are not necessarily biodegradable. Plastics’ biodegradability is determined by the chemical composition of the polymer and environmental conditions [14]. On the other hand, biotic degradation is a process in which microorganisms, such as fungi or bacteria, reduce polymeric structure into smaller molecules that are utilized as a source of carbon or energy [15]. Photodegradation and hydrolysis consist of chemical processes in which high-energy radiations (UV) and water molecules induce polymer chain degradation [16][17][18]. In polymer degradation, biotic and abiotic factors can sometimes act together. Typically, abiotic degradation produces small fragments of plastic, which are subsequently degraded by microorganisms [18]. However, this process inevitably leads to the formation of small plastic particles with different characteristics and size [11][19].
Non-biodegradable plastics or petroleum-based plastics (conventional plastics) include polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), and polystyrene (PS), which belong to the polyolefin class [20]. They are thermoplastic polymers in which olefin monomer units like ethylene, styrene, and vinyl chloride are combined to form long chains [21][22] (Figure 1). Polyolefins represent the leading industrial polymers due to their remarkable chemical stability and mechanical characteristics [23]. The manufacturing processes of these plastics and applications have been well described elsewhere [24][25][26][27][28][29][30][31][32][33][34]. The so-called “biobased” plastics, such as bio-PE obtained from sugar cane [35] and bio-PET produced by the oxidization of bio-ethylene derived from the fermentation of glucose [36], find many applications in the packaging sector, particularly for drinking bottles and textile industries; however, they are non-biodegradable [37].
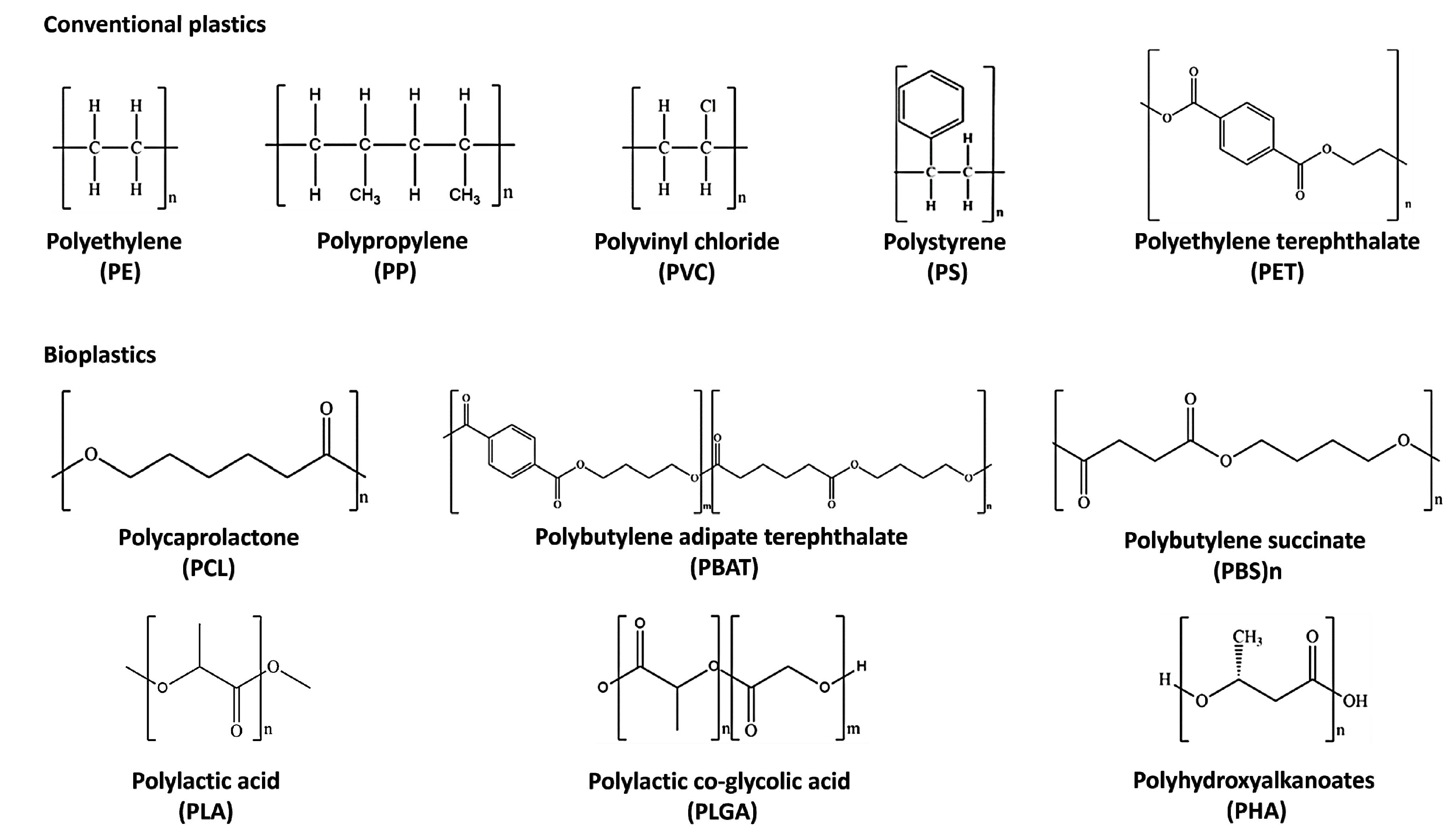
Figure 1. Chemical structures of the most diffused conventional plastics and bioplastics.
Biodegradable plastics comprise poly-caprolactone (PCL), poly-butylene succinate (PBS), poly-butylene adipate terephthalate (PBAT), poly-lactic acid (PLA), poly-lactic-co-glycolic acid (PLGA), and poly-hydroxy-alkanoate (PHA) [14] (Figure 1). PCL is suitable for a wide range of medical applications such as implantable biomedical devices, sutures, and tissue engineering scaffolds due to its biocompatibility and slow degradation [38][39][40]. It is used for the synthesis of green polyurethane [41] and commonly blended with biobased biodegradable plastics [42] to improve its thermal and mechanical properties [43]. On the contrary, PBS has a relatively slow biodegradation rate and biocompatibility [43].
PLA is made from 100% bioresources and is totally biodegradable and recyclable [44]. It is produced by a combination of lactic acid monomers derived from the fermentation of sugars obtained by sugar cane, potatoes, and corn [45][46]. PLA degrades in 6 to 24 months in the environment, depending on various factors, such as temperature, product size and shape, and isomer ratio. Despite some inherent weaknesses like brittleness and moisture uptake, it exhibits good thermomechanical properties like the traditional plastics PET and PP and has been extensively applied in different fields, ranging from packaging applications, bowls, films, and bottles, to clothes, textile furniture, hygiene products, and mulch films for agriculture [47][48][49]. Furthermore, it can be also copolymerized with polyethylene glycol (PEG) to enhance its hydrophilic and biocompatibility properties, making it suitable for drug delivery systems [50]. However, despite its eco-friendly characteristics, the commercial production of PLA is hindered by the high cost of raw materials and the lack of composting infrastructure in most markets. Implementing composting infrastructure would enable the widespread use of PLA and would reduce the environmental impact of traditional plastics [51].
PLGA is another bioplastic component frequently used as a copolymer of polyglycolic acid (PGA) and PLA. In fact, it is frequently employed in biomedical applications, due to its biocompatibility and fast biodegradation. PLGA, like PLA, can be produced by polycondensation or ring-opening polymerization, varying its molecular weights and monomer ratios to ameliorate its degradation rate [52].
PHAs are aliphatic polyesters synthesized through the polymerization of b-, g-, and d-hydroxyalkanoic acids obtained from the fermentation of sugars and lipids from various feedstocks [53]. PHAs are polymerized by bacteria, which can synthesize them under stressful conditions as a carbon and energy reserve [54]. Large-scale production of PHAs is expensive, requiring fermentation, isolation, and purification processes that limit their widespread use [55][56]. Nonetheless, PHAs are driving the growth of the biodegradable bioplastics market, with production capacity expected to triple in the next five years [57]. Poly-4-hydroxybutyrate (PHB) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) are the most commonly used PHAs: PHB has a high elastic modulus and better barrier properties than PLA. However, it is brittle and has lower thermal stability. PHBV has PP-like properties and is commercially available added with a hydroxyvalerate (HV) that is able to confer more flexibility than PHB [58]. Like PLA, PHAs find various applications in the packaging and biomedical fields as single-use items. PHB and PHBV have been investigated for their potential use as bioresorbable materials for surgical sutures, wound dressings, tissue scaffolds, bone fracture fixation plates, and porous sheets for tissue regeneration in injured soft tissues [53]. The biodegradability of PHAs depends on factors, such as chain configuration, crystallinity, and processing conditions. Another important advantage of PHAs is their high degradation rate in marine environments [59].
3. Bioplastic-Based Polymeric NPs for Drug Delivery in the Brain
3.1. Bioplastic MPs/NPs as Drug Delivery Systems
Significant advancements in nanomedicine have led to the development of drug delivery systems based on bioplastic MPs/NPs. These systems offer several advantages, including increased drug shelf-life, targeting to the specific organ/tissue, reduced therapeutic dose administrations, improving drug effectiveness, and minimizing side effects. Among drug delivery systems, the PLA and PLGA-NPs are the most extensively studied [60], because of their high drug-loading capacity, good biocompatibility, biodegradability, and tunable release properties. PLA/PLGA pharmaceutical formulations were developed for sustained delivery of several drugs used in cancer treatment, anti-inflammatory compounds, and drugs for neurological disease [61][62], while many others are still in phase II or III of clinical trials [63] (see Table 1).
In the field of treatment of neurodegenerative diseases, pharmaceutical formulations have been often unsuccessful due to the BBB that is very difficult to cross [64]. Instead, PLA/PLGA NPs have emerged as a promising solution for delivering drugs to the brain, as they can overcome the physiological barrier through different strategies, such as transcytosis pathways (Trojan horse strategy) or by escaping the efflux pumps by bearing specific ligands onto the particle surface [10]. In general, the smaller size coupled with surface modifications improved formulation pharmacokinetics, enhancing cell uptake and, consequently, drug absorption [65][66].
Table 1. Bioplastic MPs/NPs-based drug delivery systems approved by the FDA for disease treatments.
| Name | Bioplastic | Loaded Drug | Therapeutic Application | Company | FDA Approval (Date) | Ref. |
|---|---|---|---|---|---|---|
| Lupron Depot® | PLGA | Leuprolide acetate | Prostate cancer, endometriosis | Takeda–Abbott Products (Osaka, Japan) | 1989 | [67] |
| Atridox® | PLA | Doxycycline hyclate | Chronic adult periodontitis | Tolmar (Fort Collins, CO, USA) | 1998 | [61] |
| Sandostatin Lar® | PLGA | Octreotide acetate | Acromegaly | Novartis (Mulgrave, VIC, Australia) | 1998 | [61] |
| Trelstar® | PLGA | Triptoreline pamoate | Advanced prostate cancer | Allergan (Gordon, NSW, Australia) |
2001 | [61] |
| Risperdal Consta® | PLGA | Risperidone | Schizophrenia, bipolar I disorder | Janssen (Beerse, Belgium) | 2003 | [68] |
| Vivitrol® | PLGA | Naltrexone | Alcohol dependence | Alkermes (Waltham, MA, USA) | 2006 | [62] |
| Signifor Lar® | PLGA | Pasireotide pamoate | Acromegaly | Novartis (Mulgrave, VIC, Australia) | 2014 | [61] |
| Sublocade® | PLGA | Buprenorphine | Opioid disorder | Indivior (Richmond, VA, USA) | 2017 | [61] |
| Triptodur Kit® | PLGA | Triptorelin pamoate | Central precocious puberty | Arbor (Mulgrave, VIC, Australia) | 2017 | [61] |
| Scenesse® | PLGA | Afamelanotide | Prevention of phototoxicity in erythropoietic protoporphyria | Clinuvel (Melbourne, VIC, Australia) | 2019 | [69] |
| Durysta® | PLA/PLGA | Bimatoprost | Glaucoma, open-angle, intraocular hypertension | Allergan (Gordon, NSW, Australia) | 2020 | [69] |
3.2. Techniques for Fabricating Bioplastic MPs/NPs
Several technologies have been described for the fabrication of bioplastic MPs/NPs, such as nanoprecipitation, solvent evaporation or extraction, spray drying, and supercritical fluids [70][71]. All described technologies are schematically illustrated in Figure 2, and examples of MPs/NPs’ characterizations by laser scattering (size distribution curve) or SEM/TEM (micrographs) are shown in Figure 3.
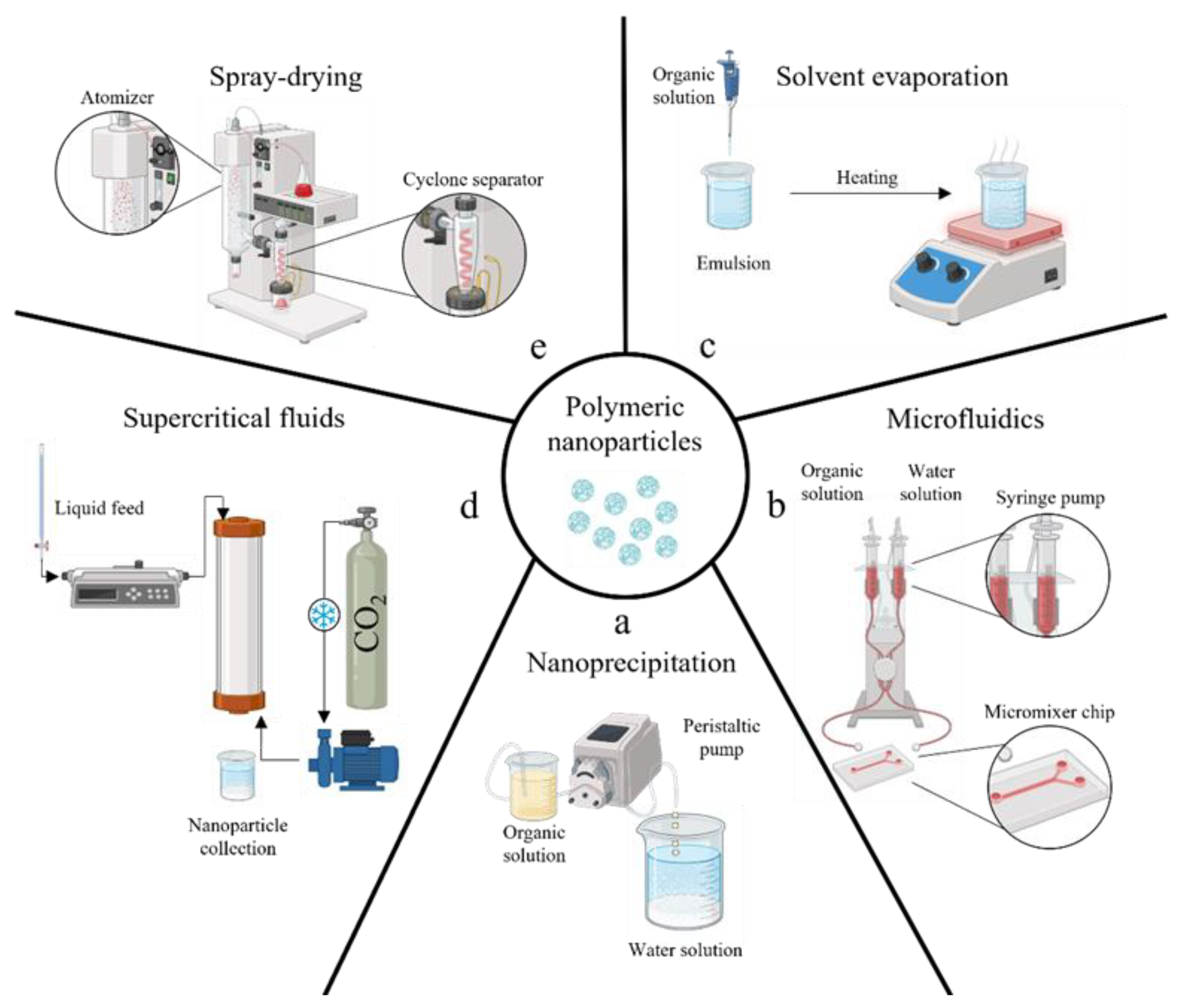
Figure 2. A schematic illustration of the principal manufacturing methods to produce polymeric micro/nano-particles: conventional nanoprecipitation by anti-solvent effect (a), nanoprecipitation enhanced by adopting microfluidics channel (b), solvent extraction/evaporation of emulsion (c), supercritical fluids emulsion extraction (d), spray drying (e).
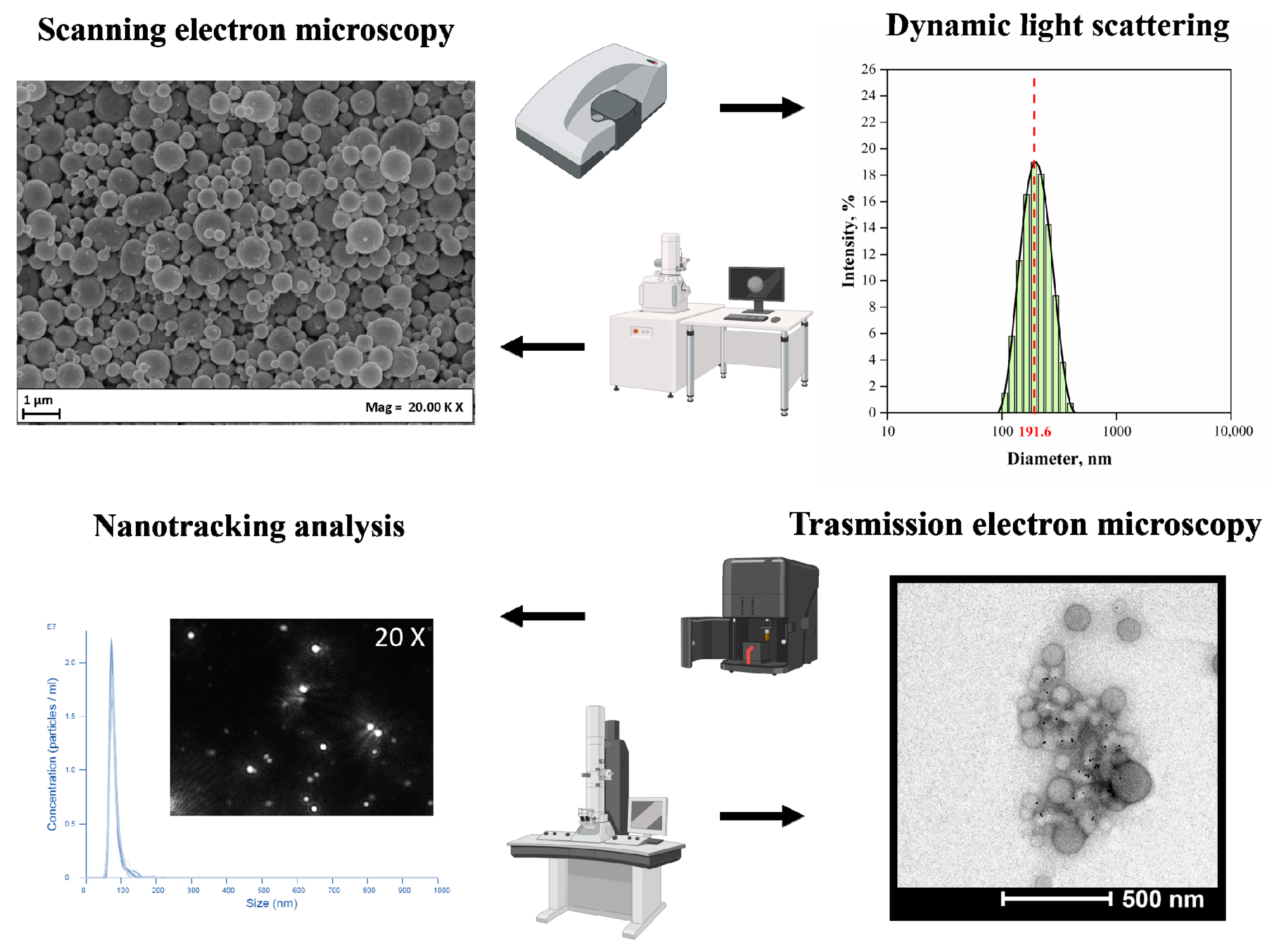
Figure 3. Chemical and physical characterization of the polymeric nanoparticles.
The nanoprecipitation process involves the precipitation of a polymer from a water-miscible organic solvent by mixing it with an aqueous medium, acting as an anti-solvent (see Figure 2). The size can be controlled by adjusting different parameters, such as organic solvent, polymer concentration, and surfactant amount in the water [72][73][74]. Since proper mixing is required between the two fluids, microfluidic systems have been recently described as promising tools for this task [75]. Indeed, the nanoprecipitation within micrometering channels assures strict control over the particle size by varying pump flow rates and micromixer geometry [76][77]; however, the method is unsuitable for encapsulating hydrophilic drugs into the NPs [74] (Figure 2b).
Solvent evaporation from emulsion is also widely used; that is, when emulsions undergo evaporation or extraction, the dispersed oily droplets within the surrounding water phase can solidify due to organic solvent removal, developing micro/nanocarriers (Figure 2c) [78][79][80]. Physical properties of resulting particles can be modulated by varying surfactant type and concentration, stirring rate, and solvent evaporation conditions; the method can encapsulate hydrophobic and hydrophilic drugs depending on the use of single or multiple emulsions; however, the evaporation step demonstrates fluctuations in reproducibility from one batch to another [81][82]. At the same time, extraction requires comparatively large amounts of a second solvent, with the related issue of further solvent recovery. Both processes require processing times of several hours that can promote aggregation phenomena between the droplets, producing carriers with a larger polydispersity [83]. It should also be noted that, despite the widespread use of solvent evaporation/extraction processes to prepare polymeric carriers, there are no established standard protocols, and each preparation follows its own set of procedures. Finally, this may not be suitable for temperature-sensitive drugs due to the risk of degradation during the solvent evaporation step as well as poor encapsulation efficiency of high water-soluble compounds reported [84].
Dense carbon dioxide technologies have been proposed to produce bioplastic MPs/NPs for different drug delivery purposes [85][86][87][88] or tissue engineering [89][90]. Supercritical emulsion extraction (SEE) technology operating in a continuous layout using a counter-current packed tower [91][92] was described for both PLA and PLGA carriers’ fabrication. In detail, an emulsion that contains polymer undergoes solvent removal using supercritical carbon dioxide as an extraction fluid (Figure 2d). The dense-gas extraction technology ensured improved performances, such as a better batch-to-batch reproducibility [93], more accurate carrier size control—thanks to a fixed droplet shrinkage without aggregation phenomena [94], lower solvent residue, and better-controlled encapsulation efficiency [84][89][95].
Nano spray drying is a relatively recent technique enabling the single-step fabrication of bioplastic MPs/NPs starting from a small sample volume. In this process, a liquid solution containing both the polymer and the drug is transformed into tiny droplets through atomization by a spray nozzle. Subsequently, these droplets undergo rapid drying as they are exposed to a stream of hot air or inert gas. As a result, solid particles are formed through the evaporation of the solvent (Figure 2e). In this case, the particle size and especially the size distribution may be impacted by electrospray process parameters, such as nozzle diameter, spraying rate, and drying temperature [96]. This method can yield nanoparticles with a narrow size distribution and high drug-loading capacity [97]. In contrast, conventional spray drying can produce carriers with lower encapsulation efficiency and larger size and granulometry.
To sum up, the choice of suitable technology for the production of bioplastic MPs/NPs for drug delivery will depend on several factors, including polymer or drug solubility, desired carrier size, distribution, and shape or surface charge.
3.3. Bioplastic MPs/NPs Delivery to the Brain
In general, a drug delivery system facilitates the attainment of the desired therapeutic response of an active substance by enhancing its bioavailability at the target site while ensuring optimal effectiveness and safety [98]. Due to the presence of the BBB, which limits the entrance of external substances into the brain, many efforts are being made to use bioplastic NPs as drug carriers to the brain. Although parenteral administration is the prevalent route for bioplastic MPs/NPs, its effectiveness for brain drug delivery is still in development. Intranasal administration is an alternative route to bypass the BBB as it allows direct access to the brain, even though its clinical application is hindered by the limited knowledge of nanoparticle deposition and absorption in this anatomical site [99]. Intracranial administration, by bioplastic MPs/NPs’ injection directly into the brain tissue or cerebrospinal fluid, is a direct and effective route for brain drug delivery using bioplastic MPs/NPs. However, this route is invasive and may cause tissue damage or inflammation [100]. Intrathecal administration is another direct route for brain drug delivery that involves the injection of bioplastic MPs/NPs into the spinal cord or cerebrospinal fluid [101]. This route accounts for the targeted delivery of NPs to the brain and has shown to be the most promising for brain tumors and neurodegenerative disease treatment [101]. Overall, the intravenous route remains the preferred choice as those mentioned before are too invasive. As a result, several strategies have been devised to overcome the BBB and improve the delivery of bioplastic MPs/NPs to the brain. One strategy is to modify the surface of bioplastic MPs/NPs with BBB-penetrating molecules, such as peptides, antibodies, or aptamers. These modifications can increase the affinity of NPs to the BBB and enhance their transport across the barrier [102]. Another strategy involves employing ultrasound or magnetic fields to increase BBB permeability and enhance the transport of bioplastic MPs/NPs’ carriers [103].
Recently bioplastic MPs/NPs have been engineered to overcome the BBB or target specific cell types in the brain, such as neurons or glial cells. These targeted nanoparticles can enhance drug delivery to specific regions of the brain and reduce off-target effects [104]. Essentially, there are three distinct strategies to accomplish this objective: adsorptive-mediated transcytosis, transporter-mediated transcytosis, and receptor-mediated transcytosis. The first mechanism can be facilitated through electrostatic interactions between the negatively charged components present on the luminal surface of cerebral endothelial cells and the cationic groups or conjugating specific compounds, such as lectins, cardiolipin, and heparin to the NPs surface [105]. Another approach for delivering drugs to the brain is utilizing transporter-mediated transcytosis. Indeed, it is feasible to synthesize nanoparticles with surface-conjugated molecules (glucose and its analogs, glutathione, and amino acids) that exhibit strong recognition by the transporters that are overexpressed in brain endothelial cells [106]. The last approach to access brain tissue involves the use of receptors overexpressed within the BBB. In more detail, transferrin, lactoferrin, low-density lipoproteins, and nicotinic acetylcholine receptors are commonly employed receptors to achieve receptor-mediated transcytosis across the BBB [104].
Finally, the use of prodrug strategies can also enhance the delivery of bioplastic MPs/NPs to the brain. Prodrugs are inactive precursors of active drugs that can be activated within the brain by specific enzymes. In fact, hydrophilic and high-molecular-weight compounds cannot traverse the BBB or the blood–cerebrospinal fluid barrier (BCSFB) through paracellular pathways. In contrast, lipophilic solutes can passively permeate these barriers [107]; hence, hydrophilic drugs can be chemically modified into lipophilic prodrugs by concealing polar functional groups. This approach can increase the brain concentration of active drugs while reducing their systemic toxicity [108].
Altogether, the described strategies offer promising solutions to overcome the BBB and enhance the delivery of bioplastic NPs to the brain.
References
- Crutzen, P.J. The “Anthropocene”. In Earth System Science in the Anthropocene; Ehlers, E., Krafft, T., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2006; pp. 13–18. ISBN 978-3-540-26588-7.
- Zalasiewicz, J.; Waters, C.N.; Ivar Do Sul, J.A.; Corcoran, P.L.; Barnosky, A.D.; Cearreta, A.; Edgeworth, M.; Gałuszka, A.; Jeandel, C.; Leinfelder, R.; et al. The Geological Cycle of Plastics and Their Use as a Stratigraphic Indicator of the Anthropocene. Anthropocene 2016, 13, 4–17.
- Minter, A. Junkyard Planet: Travels in the Billion-Dollar Trash Trade, 1st ed.; Bloomsbury Press: New York, NY, USA, 2013; ISBN 978-1-60819-791-0.
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. J. Hazard. Mater. 2018, 344, 179–199.
- Sangkham, S.; Faikhaw, O.; Munkong, N.; Sakunkoo, P.; Arunlertaree, C.; Chavali, M.; Mousazadeh, M.; Tiwari, A. A Review on Microplastics and Nanoplastics in the Environment: Their Occurrence, Exposure Routes, Toxic Studies, and Potential Effects on Human Health. Mar. Pollut. Bull. 2022, 181, 113832.
- Riechers, M.; Fanini, L.; Apicella, A.; Galván, C.B.; Blondel, E.; Espiña, B.; Kefer, S.; Keroullé, T.; Klun, K.; Pereira, T.R.; et al. Plastics in Our Ocean as Transdisciplinary Challenge. Mar. Pollut. Bull. 2021, 164, 112051.
- Narancic, T.; Cerrone, F.; Beagan, N.; O’Connor, K.E. Recent Advances in Bioplastics: Application and Biodegradation. Polymers 2020, 12, 920.
- Prajapati, V.D.; Jani, G.K.; Kapadia, J.R. Current Knowledge on Biodegradable Microspheres in Drug Delivery. Expert Opin. Drug Deliv. 2015, 12, 1283–1299.
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-Based Biodegradable Microspheres in Drug Delivery: Recent Advances in Research and Application. Drug Deliv. 2021, 28, 1397–1418.
- Annu; Sartaj, A.; Qamar, Z.; Md, S.; Alhakamy, N.A.; Baboota, S.; Ali, J. An Insight to Brain Targeting Utilizing Polymeric Nanoparticles: Effective Treatment Modalities for Neurological Disorders and Brain Tumor. Front. Bioeng. Biotechnol. 2022, 10, 788128.
- Filiciotto, L.; Rothenberg, G. Biodegradable Plastics: Standards, Policies, and Impacts. ChemSusChem 2021, 14, 56–72.
- Wu, Z.; Shi, W.; Valencak, T.G.; Zhang, Y.; Liu, G.; Ren, D. Biodegradation of Conventional Plastics: Candidate Organisms and Potential Mechanisms. Sci. Total Environ. 2023, 885, 163908.
- Zimmermann, L.; Dombrowski, A.; Völker, C.; Wagner, M. Are Bioplastics and Plant-Based Materials Safer than Conventional Plastics? In Vitro Toxicity and Chemical Composition. Environ. Int. 2020, 145, 106066.
- Rujnić-Sokele, M.; Pilipović, A. Challenges and Opportunities of Biodegradable Plastics: A Mini Review. Waste Manag. Res. 2017, 35, 132–140.
- Chandra, R. (Ed.) Environmental Waste Management; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4987-2475-3.
- Ghosh, S.K.; Pal, S.; Ray, S. Study of Microbes Having Potentiality for Biodegradation of Plastics. Environ. Sci. Pollut. Res. 2013, 20, 4339–4355.
- Zhang, X.; Xia, M.; Su, X.; Yuan, P.; Li, X.; Zhou, C.; Wan, Z.; Zou, W. Photolytic Degradation Elevated the Toxicity of Polylactic Acid Microplastics to Developing Zebrafish by Triggering Mitochondrial Dysfunction and Apoptosis. J. Hazard. Mater. 2021, 413, 125321.
- Silva, R.R.A.; Marques, C.S.; Arruda, T.R.; Teixeira, S.C.; De Oliveira, T.V. Biodegradation of Polymers: Stages, Measurement, Standards and Prospects. Macromol 2023, 3, 371–399.
- Andrady, A.L. The Plastic in Microplastics: A Review. Mar. Pollut. Bull. 2017, 119, 12–22.
- Bher, A.; Mayekar, P.C.; Auras, R.A.; Schvezov, C.E. Biodegradation of Biodegradable Polymers in Mesophilic Aerobic Environments. Int. J. Mol. Sci. 2022, 23, 12165.
- Lee, W.-T.; Van Muyden, A.; Bobbink, F.D.; Mensi, M.D.; Carullo, J.R.; Dyson, P.J. Mechanistic Classification and Benchmarking of Polyolefin Depolymerization over Silica-Alumina-Based Catalysts. Nat. Commun. 2022, 13, 4850.
- Elgharbawy, A.S.; Ali, R.M. A Comprehensive Review of the Polyolefin Composites and Their Properties. Heliyon 2022, 8, e09932.
- Hees, T.; Zhong, F.; Stürzel, M.; Mülhaupt, R. Tailoring Hydrocarbon Polymers and All-Hydrocarbon Composites for Circular Economy. Macromol. Rapid Commun. 2019, 40, 1800608.
- Yao, Z.; Seong, H.J.; Jang, Y.-S. Environmental Toxicity and Decomposition of Polyethylene. Ecotoxicol. Environ. Saf. 2022, 242, 113933.
- Paxton, N.C.; Allenby, M.C.; Lewis, P.M.; Woodruff, M.A. Biomedical Applications of Polyethylene. Eur. Polym. J. 2019, 118, 412–428.
- Li, W.C.; Tse, H.F.; Fok, L. Plastic Waste in the Marine Environment: A Review of Sources, Occurrence and Effects. Sci. Total Environ. 2016, 566–567, 333–349.
- Galloway, T.S. Micro- and Nano-Plastics and Human Health. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 343–366. ISBN 978-3-319-16509-7.
- Li, X.; Meng, L.; Zhang, Y.; Qin, Z.; Meng, L.; Li, C.; Liu, M. Research and Application of Polypropylene Carbonate Composite Materials: A Review. Polymers 2022, 14, 2159.
- Lewandowski, K.; Skórczewska, K. A Brief Review of Poly(Vinyl Chloride) (PVC) Recycling. Polymers 2022, 14, 3035.
- Wypych, G. Chemical Structure of PVC. In PVC Degradation and Stabilization; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–23. ISBN 978-1-895198-85-0.
- Abdel-Monem, R.A.; Rabie, S.T.; El-Liethy, M.A.; Hemdan, B.A.; El-Nazer, H.A.; Gaballah, S.T. Chitosan- PVC Conjugates/Metal Nanoparticles for Biomedical Applications. Polym. Adv. Tech. 2022, 33, 514–523.
- Wünsch, J.R. Polystyrene: Synthesis, Production and Applications; Rapra Review Reports; Rapra Technology Ltd.: Shawbury, UK, 2000; ISBN 978-1-85957-191-0.
- Kik, K.; Bukowska, B.; Sicińska, P. Polystyrene Nanoparticles: Sources, Occurrence in the Environment, Distribution in Tissues, Accumulation and Toxicity to Various Organisms. Environ. Pollut. 2020, 262, 114297.
- Nisticò, R. Polyethylene Terephthalate (PET) in the Packaging Industry. Polym. Test. 2020, 90, 106707.
- Rahman, M.H.; Bhoi, P.R. An Overview of Non-Biodegradable Bioplastics. J. Clean. Prod. 2021, 294, 126218.
- Ferreira, F.V.; Cividanes, L.S.; Gouveia, R.F.; Lona, L.M.F. An Overview on Properties and Applications of Poly(Butylene Adipate-co-Terephthalate)-PBAT Based Composites. Polym. Eng. Sci. 2019, 59, E7–E15.
- Welle, F. Twenty Years of PET Bottle to Bottle Recycling—An Overview. Resour. Conserv. Recycl. 2011, 55, 865–875.
- Chan, D.S.; Fnais, N.; Ibrahim, I.; Daniel, S.J.; Manoukian, J. Exploring Polycaprolactone in Tracheal Surgery: A Scoping Review of in-Vivo Studies. Int. J. Pediatr. Otorhinolaryngol. 2019, 123, 38–42.
- Chen, T.; Cai, T.; Jin, Q.; Ji, J. Design and Fabrication of Functional Polycaprolactone. e-Polymers 2015, 15, 3–13.
- Hajiali, F.; Tajbakhsh, S.; Shojaei, A. Fabrication and Properties of Polycaprolactone Composites Containing Calcium Phosphate-Based Ceramics and Bioactive Glasses in Bone Tissue Engineering: A Review. Polym. Rev. 2018, 58, 164–207.
- Džunuzović, J.V.; Stefanović, I.S.; Džunuzović, E.S.; Dapčević, A.; Šešlija, S.I.; Balanč, B.D.; Lama, G.C. Polyurethane Networks Based on Polycaprolactone and Hyperbranched Polyester: Structural, Thermal and Mechanical Investigation. Prog. Org. Coat. 2019, 137, 105305.
- Sadeghi, A.; Mousavi, S.M.; Saljoughi, E.; Kiani, S. Biodegradable Membrane Based on Polycaprolactone/Polybutylene Succinate: Characterization and Performance Evaluation in Wastewater Treatment. J. Appl. Polym. Sci. 2021, 138, 50332.
- Lule, Z.C.; Wondu Shiferaw, E.; Kim, J. Thermomechanical Properties of SiC-Filled Polybutylene Succinate Composite Fabricated via Melt Extrusion. Polymers 2020, 12, 418.
- Wu, Y.; Gao, X.; Wu, J.; Zhou, T.; Nguyen, T.T.; Wang, Y. Biodegradable Polylactic Acid and Its Composites: Characteristics, Processing, and Sustainable Applications in Sports. Polymers 2023, 15, 3096.
- Van Beilen, J.B.; Poirier, Y. Production of Renewable Polymers from Crop Plants. Plant J. 2008, 54, 684–701.
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-Lactic Acid Synthesis for Application in Biomedical Devices—A Review. Biotechnol. Adv. 2012, 30, 321–328.
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84.
- Tait, M.; Pegoretti, A.; Dorigato, A.; Kalaitzidou, K. The Effect of Filler Type and Content and the Manufacturing Process on the Performance of Multifunctional Carbon/Poly-Lactide Composites. Carbon 2011, 49, 4280–4290.
- Fambri, L.; Dorigato, A.; Pegoretti, A. Role of Surface-Treated Silica Nanoparticles on the Thermo-Mechanical Behavior of Poly(Lactide). Appl. Sci. 2020, 10, 6731.
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic Acid (PLA) Synthesis and Modifications: A Review. Front. Chem. China 2009, 4, 259–264.
- Ahvenainen, R. (Ed.) Novel Food Packaging Techniques; Woodhead Publishing in Food Science and Technology; Woodhead Publishing: Cambridge, UK, 2003; ISBN 978-1-85573-675-7.
- Jem, K.J.; Tan, B. The Development and Challenges of Poly (Lactic Acid) and Poly (Glycolic Acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70.
- Bhatia, S.K.; Otari, S.V.; Jeon, J.-M.; Gurav, R.; Choi, Y.-K.; Bhatia, R.K.; Pugazhendhi, A.; Kumar, V.; Rajesh Banu, J.; Yoon, J.-J.; et al. Biowaste-to-Bioplastic (Polyhydroxyalkanoates): Conversion Technologies, Strategies, Challenges, and Perspective. Bioresour. Technol. 2021, 326, 124733.
- Koller, M. Biodegradable and Biocompatible Polyhydroxy-Alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molecules 2018, 23, 362.
- Kalia, V.C.; Singh Patel, S.K.; Shanmugam, R.; Lee, J.-K. Polyhydroxyalkanoates: Trends and Advances toward Biotechnological Applications. Bioresour. Technol. 2021, 326, 124737.
- Silva, J.B.; Pereira, J.R.; Marreiros, B.C.; Reis, M.A.M.; Freitas, F. Microbial Production of Medium-Chain Length Polyhydroxyalkanoates. Process Biochem. 2021, 102, 393–407.
- Döhler, N.; Wellenreuther, C.; Wolf, A. Market Dynamics of Biodegradable Bio-Based Plastics: Projections and Linkages to European Policies. EFB Bioecon. J. 2022, 2, 100028.
- Fredi, G.; Dorigato, A. Recycling of Bioplastic Waste: A Review. Adv. Ind. Eng. Polym. Res. 2021, 4, 159–177.
- Lamberti, F.M.; Román-Ramírez, L.A.; Wood, J. Recycling of Bioplastics: Routes and Benefits. J. Polym. Environ. 2020, 28, 2551–2571.
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18.
- Zhong, H.; Chan, G.; Hu, Y.; Hu, H.; Ouyang, D. A Comprehensive Map of FDA-Approved Pharmaceutical Products. Pharmaceutics 2018, 10, 263.
- Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of Hybrid PLGA Nanoparticles: Future of Smart Drug Delivery and Theranostics Medicine. Mater. Des. 2020, 193, 108805.
- Alsaab, H.O.; Alharbi, F.D.; Alhibs, A.S.; Alanazi, N.B.; Alshehri, B.Y.; Saleh, M.A.; Alshehri, F.S.; Algarni, M.A.; Almugaiteeb, T.; Uddin, M.N.; et al. PLGA-Based Nanomedicine: History of Advancement and Development in Clinical Applications of Multiple Diseases. Pharmaceutics 2022, 14, 2728.
- Akhtar, A.; Andleeb, A.; Waris, T.S.; Bazzar, M.; Moradi, A.-R.; Awan, N.R.; Yar, M. Neurodegenerative Diseases and Effective Drug Delivery: A Review of Challenges and Novel Therapeutics. J. Control. Release 2021, 330, 1152–1167.
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for Biorelated Polymers and Applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410.
- Faiyaz, M. Nanomaterials in Alzheimer’s Disease Treatment: A Comprehensive Review. Front. Biosci. 2021, 26, 851.
- Anselmo, A.C.; Mitragotri, S. An Overview of Clinical and Commercial Impact of Drug Delivery Systems. J. Control. Release 2014, 190, 15–28.
- Molavi, F.; Barzegar-Jalali, M.; Hamishehkar, H. Polyester Based Polymeric Nano and Microparticles for Pharmaceutical Purposes: A Review on Formulation Approaches. J. Control. Release 2020, 320, 265–282.
- Tawde, V.; Chaurasia, S.; Gupta, S.; Rastogi, R.; Dantuluri, A.K.; Liu, W.; Mcmahon, S.; Seo, D.-W. Smart Bioreso Rbable Polymers for Pharmaceuticals and Medical Devices. Yakhak Hoeji 2022, 66, 1–6.
- Mahalingam, M.; Krishnamoorthy, K. Selection of a Suitable Method for the Preparation of Polymeric Nanoparticles: Multi-Criteria Decision Making Approach. Adv. Pharm. Bull. 2015, 5, 57.
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731.
- Iván Martínez-Muñoz, O.; Fernando Ospina-Giraldo, L.; Elizabeth Mora-Huertas, C. Nanoprecipitation: Applications for Entrapping Active Molecules of Interest in Pharmaceutics. In Nano- and Microencapsulation—Techniques and Applications; Abu-Thabit, N., Ed.; IntechOpen: London, UK, 2021; ISBN 978-1-83968-348-0.
- Bilati, U.; Allémann, E.; Doelker, E. Development of a Nanoprecipitation Method Intended for the Entrapment of Hydrophilic Drugs into Nanoparticles. Eur. J. Pharm. Sci. 2005, 24, 67–75.
- Barichello, J.M.; Morishita, M.; Takayama, K.; Nagai, T. Encapsulation of Hydrophilic and Lipophilic Drugs in PLGA Nanoparticles by the Nanoprecipitation Method. Drug Dev. Ind. Pharm. 1999, 25, 471–476.
- Leung, M.H.M.; Shen, A.Q. Microfluidic Assisted Nanoprecipitation of PLGA Nanoparticles for Curcumin Delivery to Leukemia Jurkat Cells. Langmuir 2018, 34, 3961–3970.
- Lamparelli, E.P.; Ciardulli, M.C.; Scala, P.; Scognamiglio, M.; Charlier, B.; Di Pietro, P.; Izzo, V.; Vecchione, C.; Maffulli, N.; Della Porta, G. Lipid Nano-Vesicles for Thyroid Hormone Encapsulation: A Comparison between Different Fabrication Technologies, Drug Loading, and an in Vitro Delivery to Human Tendon Stem/Progenitor Cells in 2D and 3D Culture. Int. J. Pharm. 2022, 624, 122007.
- Zhang, H.; Yang, J.; Sun, R.; Han, S.; Yang, Z.; Teng, L. Microfluidics for Nano-Drug Delivery Systems: From Fundamentals to Industrialization. Acta Pharm. Sin. B 2023, 13, 3277–3299.
- Freitas, S.; Merkle, H.P.; Gander, B. Microencapsulation by Solvent Extraction/Evaporation: Reviewing the State of the Art of Microsphere Preparation Process Technology. J. Control. Release 2005, 102, 313–332.
- Katou, H.; Wandrey, A.J.; Gander, B. Kinetics of Solvent Extraction/Evaporation Process for PLGA Microparticle Fabrication. Int. J. Pharm. 2008, 364, 45–53.
- Nag, D.; Nath, B. Nath Review on Solvent Evaporation Technique: A Promising Method for Microencapsulation. World J. Pharm. Res. 2018, 7, 356–372.
- Meng, F.T.; Ma, G.H.; Liu, Y.D.; Qiu, W.; Su, Z.G. Microencapsulation of Bovine Hemoglobin with High Bio-Activity and High Entrapment Efficiency Using a W/O/W Double Emulsion Technique. Colloids Surf. B Biointerfaces 2004, 33, 177–183.
- Miyazaki, Y.; Onuki, Y.; Yakou, S.; Takayama, K. Effect of Temperature-Increase Rate on Drug Release Characteristics of Dextran Microspheres Prepared by Emulsion Solvent Evaporation Process. Int. J. Pharm. 2006, 324, 144–151.
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A Review of Microencapsulation Methods for Food Antioxidants: Principles, Advantages, Drawbacks and Applications. Food Chem. 2019, 272, 494–506.
- Govoni, M.; Lamparelli, E.P.; Ciardulli, M.C.; Santoro, A.; Oliviero, A.; Palazzo, I.; Reverchon, E.; Vivarelli, L.; Maso, A.; Storni, E.; et al. Demineralized Bone Matrix Paste Formulated with Biomimetic PLGA Microcarriers for the Vancomycin Hydrochloride Controlled Delivery: Release Profile, Citotoxicity and Efficacy against S. Aureus. Int. J. Pharm. 2020, 582, 119322.
- Della Porta, G.; Adami, R.; Gaudio, P.D.; Prota, L.; Aquino, R.; Reverchon, E. Albumin/Gentamicin Microspheres Produced by Supercritical Assisted Atomization: Optimization of Size, Drug Loading and Release. J. Pharm. Sci. 2010, 99, 4720–4729.
- Della Porta, G.; De Vittori, C.; Reverchon, E. Supercritical Assisted Atomization: A Novel Technology for Microparticles Preparation of an Asthma-Controlling Drug. AAPS PharmSciTech 2005, 6, E421–E428.
- Campardelli, R.; Della Porta, G.; Reverchon, E. Solvent Elimination from Polymer Nanoparticle Suspensions by Continuous Supercritical Extraction. J. Supercrit. Fluids 2012, 70, 100–105.
- Campardelli, R.; Adami, R.; Della Porta, G.; Reverchon, E. Nanoparticle Precipitation by Supercritical Assisted Injection in a Liquid Antisolvent. Chem. Eng. J. 2012, 192, 246–251.
- Palazzo, I.; Lamparelli, E.P.; Ciardulli, M.C.; Scala, P.; Reverchon, E.; Forsyth, N.; Maffulli, N.; Santoro, A.; Della Porta, G. Supercritical Emulsion Extraction Fabricated PLA/PLGA Micro/Nano Carriers for Growth Factor Delivery: Release Profiles and Cytotoxicity. Int. J. Pharm. 2021, 592, 120108.
- Trucillo, E.; Bisceglia, B.; Valdrè, G.; Giordano, E.; Reverchon, E.; Maffulli, N.; Della Porta, G. Growth Factor Sustained Delivery from Poly-lactic-co-glycolic Acid Microcarriers and Its Mass Transfer Modeling by Finite Element in a Dynamic and Static Three-dimensional Environment Bioengineered with Stem Cells. Biotech. Bioeng. 2019, 116, 1777–1794.
- Della Porta, G.; Falco, N.; Giordano, E.; Reverchon, E. PLGA Microspheres by Supercritical Emulsion Extraction: A Study on Insulin Release in Myoblast Culture. J. Biomater. Sci. Polym. Ed. 2013, 24, 1831–1847.
- Della Porta, G.; Campardelli, R.; Falco, N.; Reverchon, E. PLGA Microdevices for Retinoids Sustained Release Produced by Supercritical Emulsion Extraction: Continuous versus Batch Operation Layouts. J. Pharm. Sci. 2011, 100, 4357–4367.
- Falco, N.; Reverchon, E.; Della Porta, G. Injectable PLGA/Hydrocortisone Formulation Produced by Continuous Supercritical Emulsion Extraction. Int. J. Pharm. 2013, 441, 589–597.
- Cricchio, V.; Best, M.; Reverchon, E.; Maffulli, N.; Phillips, G.; Santin, M.; Della Porta, G. Novel Superparamagnetic Microdevices Based on Magnetized PLGA/PLA Microparticles Obtained by Supercritical Fluid Emulsion and Coating by Carboxybetaine-Functionalized Chitosan Allowing the Tuneable Release of Therapeutics. J. Pharm. Sci. 2017, 106, 2097–2105.
- Palazzo, I.; Raimondo, M.; Della Porta, G.; Guadagno, L.; Reverchon, E. Encapsulation of Health-Monitoring Agent in Poly-Methyl-Methacrylate Microcapsules Using Supercritical Emulsion Extraction. J. Ind. Eng. Chem. 2020, 90, 287–299.
- Morais, A.Í.S.; Vieira, E.G.; Afewerki, S.; Sousa, R.B.; Honorio, L.M.C.; Cambrussi, A.N.C.O.; Santos, J.A.; Bezerra, R.D.S.; Furtini, J.A.O.; Silva-Filho, E.C.; et al. Fabrication of Polymeric Microparticles by Electrospray: The Impact of Experimental Parameters. J. Funct. Biomater. 2020, 11, 4.
- Strojewski, D.; Krupa, A. Spray Drying and Nano Spray Drying as Manufacturing Methods of Drug-Loaded Polymeric Particles. Polim. Med. 2022, 52, 101–111.
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905.
- Hanson, L.R.; Frey, W.H. Intranasal Delivery Bypasses the Blood-Brain Barrier to Target Therapeutic Agents to the Central Nervous System and Treat Neurodegenerative Disease. BMC Neurosci. 2008, 9, S5.
- Patel, T.; Zhou, J.; Piepmeier, J.M.; Saltzman, W.M. Polymeric Nanoparticles for Drug Delivery to the Central Nervous System. Adv. Drug Deliv. Rev. 2012, 64, 701–705.
- Fowler, M.J.; Cotter, J.D.; Knight, B.E.; Sevick-Muraca, E.M.; Sandberg, D.I.; Sirianni, R.W. Intrathecal Drug Delivery in the Era of Nanomedicine. Adv. Drug Deliv. Rev. 2020, 165–166, 77–95.
- Bukari, B.; Samarasinghe, R.M.; Noibanchong, J.; Shigdar, S.L. Non-Invasive Delivery of Therapeutics into the Brain: The Potential of Aptamers for Targeted Delivery. Biomedicines 2020, 8, 120.
- Burgess, A.; Shah, K.; Hough, O.; Hynynen, K. Focused Ultrasound-Mediated Drug Delivery through the Blood–Brain Barrier. Expert Rev. Neurother. 2015, 15, 477–491.
- Pinheiro, R.G.R.; Coutinho, A.J.; Pinheiro, M.; Neves, A.R. Nanoparticles for Targeted Brain Drug Delivery: What Do We Know? Int. J. Mol. Sci. 2021, 22, 11654.
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci. 2022, 23, 4153.
- Kou, L.; Bhutia, Y.D.; Yao, Q.; He, Z.; Sun, J.; Ganapathy, V. Transporter-Guided Delivery of Nanoparticles to Improve Drug Permeation across Cellular Barriers and Drug Exposure to Selective Cell Types. Front. Pharmacol. 2018, 9, 27.
- Zhang, S.; Gan, L.; Cao, F.; Wang, H.; Gong, P.; Ma, C.; Ren, L.; Lin, Y.; Lin, X. The Barrier and Interface Mechanisms of the Brain Barrier, and Brain Drug Delivery. Brain Res. Bull. 2022, 190, 69–83.
- Botti, G.; Dalpiaz, A.; Pavan, B. Targeting Systems to the Brain Obtained by Merging Prodrugs, Nanoparticles, and Nasal Administration. Pharmaceutics 2021, 13, 1144.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
01 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




