Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Matheus Sewastjanow-Silva | -- | 1840 | 2023-10-30 14:43:29 | | | |
| 2 | Jason Zhu | Meta information modification | 1840 | 2023-11-03 02:30:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jin, J.; Yoshimura, K.; Sewastjanow-Silva, M.; Song, S.; Ajani, J.A. Patient-Derived Xenografts for Cancer Research. Encyclopedia. Available online: https://encyclopedia.pub/entry/50940 (accessed on 07 February 2026).
Jin J, Yoshimura K, Sewastjanow-Silva M, Song S, Ajani JA. Patient-Derived Xenografts for Cancer Research. Encyclopedia. Available at: https://encyclopedia.pub/entry/50940. Accessed February 07, 2026.
Jin, Jiankang, Katsuhiro Yoshimura, Matheus Sewastjanow-Silva, Shumei Song, Jaffer A. Ajani. "Patient-Derived Xenografts for Cancer Research" Encyclopedia, https://encyclopedia.pub/entry/50940 (accessed February 07, 2026).
Jin, J., Yoshimura, K., Sewastjanow-Silva, M., Song, S., & Ajani, J.A. (2023, October 30). Patient-Derived Xenografts for Cancer Research. In Encyclopedia. https://encyclopedia.pub/entry/50940
Jin, Jiankang, et al. "Patient-Derived Xenografts for Cancer Research." Encyclopedia. Web. 30 October, 2023.
Copy Citation
The patient-derived xenograft (PDX) model is the in vivo standard for cancer research as a preclinical platform.
patient-derived xenograft (PDX)
orthotopic PDX (PODX)
humanized mice (HM)
1. Introduction
Cell cultures and in vivo mouse models are the commonly used methods in cancer research. The cell lines are from the in vitro model, primarily used in basic cancer research and drug discovery, providing an indefinite source of biological material for experimental purposes. Cancer cell lines retain many genetic properties of the cancers of origin [1]. The patient-derived xenograft (PDX) model, as a representative of in vivo models, is popular as it allows for the direct assessment of tumor properties using patient specimens. The PDX models are commonly established through the subcutaneous injection of tumor cells, regardless of their origin (heterotopic) or engraftment in the corresponding organs (orthotopic) [2]. This model offers direct means of addressing clinically relevant questions, such as drug screening and evaluating the efficacy of drugs. The PDX models also allow for the study of evolutionary cancer dynamics during progression and drug exposure, as well as the underlying mechanisms of resistance. Although the ability of the PDX models to predict clinical outcomes is not accurate, the addition of new measures, such as the humanized mouse models, can improve predictions and aid in therapeutic decisions. PDXs can also recapitulate the malignant characteristics of different tumors from different patients [3].
2. PDX as the Standard in vivo Model for Cancer Research
The PDX models are the standard platform for translational cancer research, drug screening, and treatment, biomarker development, preclinical evaluation of personalized medicine strategies, and personalized cancer therapy [4]. In 2019, The National Cancer Institute launched a national repository of patient-derived models, including PDXs and in vitro patient-derived cell cultures (https://dtp.cancer.gov/repositories/) (accessed on 23 August 2023). In 2023, the European Molecular Biology Laboratory and the Jackson Laboratory also launched a platform called PDCM Finder (https://www.cancermodels.org) (accessed on 23 August 2023) for patient-derived cancer models (PDCMs). It aggregates clinical, genomic, and functional data obtained from PDXs, organoids, and cell lines. The platform standardized and integrated over 90 million data points from more than 4500 PDX models [5].
The cell-derived xenograft (CDX) and PDX models are popular rodent (typically mice or rats) models for studying human cancers. To create the CDX models, human cancer cell lines are injected into T-cell-deficient nude or severe combined immunodeficient (SCID) mice. In the CDX models, the cells from established cell lines were derived from cancer patients, such as AGS, GT5, KatoIII, MKN45, Snu16, etc., in the context of GAC [6]. For the PDX models, patient-derived tumor fragments, metastasized cells/tissue, circulating free cells, patient-derived malignant ascites, or cancer cells briefly going through in vitro expansion that are xenografted into rodents are termed as the PDX models in a broad sense (PDX sensu lato); however, the PDX models sensu stricto (i.e., in a narrow sense) only include patient-derived tumor fragments and metastasized tissues. Compared with the CDX models, PDX models are more relevant to human cancer biology and are better suited for drug screening, but they are not ideal [7]. A significant characteristic of PDXs sensu stricto is that they are directly implanted into mice without intermediate cell culture, which introduces more complexities.
Tumor fragments or metastasized cells, such as those from malignant ascites cells, are commonly used to create the PDX models by surgically transplanting them into immunodeficient mice. The susceptibility of these models to therapeutic drugs may be closely correlated with data in patients. These models can closely mimic the patient’s tumor, and they are highly useful in predicting the efficacy of drugs. PDXs also allow a “co-clinical trial” approach, where pre-clinical investigations in vivo and clinical trials can be performed, in parallel or sequentially, to assess drug efficacy [8]. Validation studies have shown that the PDX models, mostly, have identical mutational profiles to patient tumors and provide them for drug screening [9]. The PDX models are considered better than cell culture models in recapitulating the histological features and molecular characteristics [10]. For example, in preclinical chemotherapy for breast cancer, 113 tumors were implanted to form PDXs with an overall take rate of 27.4%, and PDXs with the same molecular subtype as the patients were observed in 28 (90.3%) of 31 cases [11]. It was previously shown that PDXs grown in immunodeficient mice closely resemble the original tumors both histologically and genetically [12].
Kopetz et al. (2012) compared PDXs, cancer cells in vitro, and CDXs, and found that PDXs retained key characteristics of tumors from patients, including histologic characteristics, genomic signatures, and the heterogeneity of cancer cells. PDXs even retained stromal and immune cells originating from the patients, making them a more precise model for reproducing the in vivo environment to test drug response [13]. In GAC, Cho et al. (2016) showed good performance of PDXs by testing the combination of the BCL2L1 inhibitor and a cytotoxic drug in BCL2L1-amplified tumors. They observed promising in vivo drug efficacy with significant tumor shrinkage [14]. However, to achieve precision in vivo studies and preclinical testing, PDX tissue banking by cryopreservation has become indispensable to maintain clinical samples’ heterogeneity, vitality, and genetic makeup.
3. Types of PDX Models
Regarding types of PDXs, two types of PDX engraftment are discussed, including heterotopic and orthotopic models. Heterotopic models involve subcutaneous implantation and other subtypes, such as peritoneal injection and tail vein injection. On the other hand, the orthotopic model, the PDOX, involves placing engraftments in the corresponding organs to those in the primary tumors.
Previous reviews have classified PDXs into eight types based on the biomaterial for implantation and the background of the experiment [3][15][16]. Byrne et al. (2017) [3] detailed the eight types of PDXs: the three common PDX types in Table 1 plus the other five less common types of PDXs: ref. [4] metastatic tumor specimens implanted orthotopically at the metastatic site, ref. [5] metastatic tumor specimens implanted subcutaneously, ref. [6] minimal residual disease, ref. [7] clinical trial-associated xenografts, and [8] circulating tumor cell (CTC)-derived PDXs.
Table 1. The three most common types of PDX models for cancer research (modified from Byrne et. al. 2017) [11].
| PDX Models | Advantages | Disadvantages |
|---|---|---|
| (1) Primary tumor specimens implanted subcutaneously (PDX-SC) |
|
|
| (2) Primary tumor specimens implanted orthotopically (PDOX) |
|
|
| (3) Humanized mouse (HM) models |
|
|
Furthermore, PDX was defined as one of six animal models, and it listed retaining heterogeneity and mutations, tumor microenvironment (TME), intact endocrine system, metastasis assessment, and tumor biobank formation as advantages of PDXs [17]. However, all PDX models have limitations, such as being generated in mice with deficient immunity, having different tumorigenesis, and being unsuitable for early-stage cancer.
An illustration of commonly used PDX using GAC primaries and metastatic cells, such as those from malignant ascites, cancer-derived cell lines, and humanized mouse (HM) models, together with syngeneic mouse models.
4. Mouse Host Types for PDX Model
Various types of immunodeficient mice are used in PDX models. It is important to understand the specific immunodeficiency characteristics of each mouse strain. Researchers have summarized the features of the main immunodeficient mouse models used in PDX models in Table 2. The commonly used mouse strains in PDX models include athymic nude mice (Foxn1 null), Rag1/Rag2 mice (Rag1/2 recombinase defects), SCID mice (mutated Prkdc gene), SCID/Beige mice (combined mutated Beige with SCID), NOD/SCID mice (NOD, non-obese diabetic mutation with SCID), NOG and NSG mice (NOD/SCID plus IL2Rγ truncation), as well as NRG mice (NOG with Rag1 mutation, replacing SCID mutation) [17][18]. Cho et al. (2016) listed, in their review, the status of immune cells, such as mature B, mature T, dendritic cells, macrophages, and natural killer (NK) cells in NSG, NOD-SCID, BALB-SCID, B6 Rag1, and nude mice [14].
Table 2. Strains of mouse host types for PDX models, according to Shultz et al. (2007) [18].
| Strain Name | Phenotype | Strain Name | Phenotype |
|---|---|---|---|
| C57BL/6-nu | Nude, athymic, lacks T cells | NOD-Rag1−/− | NOD+ Rag1 mutation leading to lack of mature T and B cells |
| CB17-SCID | Lacks mature T and B cells; radiation sensitive |
NOD-Rag1−/− Prf1−/− | NOD+ Rag1 mutation leading to lack of mature T and B cells; lack of perforin |
| NOD- SCID | No mature T and B cells; radiation sensitive; decreased innate immunity |
NOD-SCID HLA-A2.1-transgenic |
NOD-SCID+ Transgenic expression of human HLA-A2.1 |
| BALB/c-SCID bg | No mature T and B cells; radiation sensitive; decreased NK-cell activity | NOD/LtSz-SCID Il2rg−/− |
No mature T and B cells; radiation sensitive; IL-2Rγ-chain deficiency; reduced multiple cytokine receptors thus many innate immune defects |
| C57BL/6-SCID bg | No mature T and B cells; decreased NK-cell activity | NOD/Shi-SCID Il2rg−/− | Similar to NOD/LtSz-SCID Il2rg−/− mice |
| NOD-SCID B2m−/− |
No mature T and B cells; radiation sensitive; no β2m, leading to lack of MHC class I expression |
BALB/c-Rag2−/− Il2rg−/− | Similar to NOD/LtSz-SCID Il2rg−/− mice |
| NOD-SCID IL-3-, GM-CSF and SCF transgenic |
No mature T and B cells; radiation sensitive; transgenic human cytokine production |
H2d -Rag2−/−Il2rg−/− | Similar to NOD/LtSz-SCID Il2rg−/− mice |
5. Orthotopic PDX Models (PDOXs) as an Emerging Trend
Regarding GAC PDOXs, 10 studies were identified, of which 70% were CDXs. In 90% of these studies, implantation was performed in the subserosal layer of the stomach wall. Tumor engraftment success rates varied widely, ranging from 0 to 100%. In studies utilizing either cell suspension or tumor fragments, metastases were observed in 40% of PDOXs implanted into the subserosal layer. However, there is insufficient evidence to determine whether the submucosal site is more effective than the subserosal layer or whether tissue fragments are more successful than cell suspensions for engraftment and metastases.
This usual injection method involves the submucosal injection of 0.1 million tumor cell suspensions with 10 μL PBS using a microsyringe that enables precise volume injection. It is crucial to ensure that there is only one bubble-like spot without any leakage on the stomach body wall after injection (Figure 1A). The tumor cells are supposed to be injected into submucosal and muscularis propria layers. Researchers routinely monitor the tumor burden using a bioluminescence system, and after 2 weeks of injection, most mice show abdominal localized spotty signals (Figure 1B).
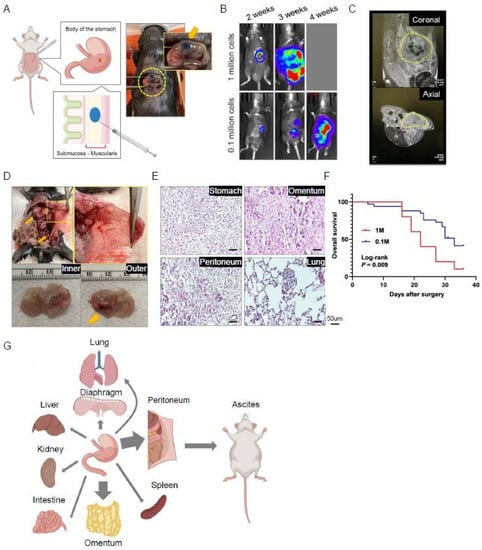
Figure 1. Procedure and characteristics of an orthotopic model of gastric cancer. Yellow cycles highlight the focus of the photos. (A) The illustration depicts the orthotopic injection of tumor cells into the stomach wall. (B) Representative bioluminescence images of injected mice. (C) Representative MRI images of injected mice taken 3 weeks after injection. (D) Representative macroscopic images of the abdominal cavity and peritoneal membrane of injected mice, as well as resected stomach and the developed primary tumor (arrow). (E) Representative hematoxylin and eosin (H&E)-stained images of tumoral tissues in multiple structures. (F) Survival curve of orthotopic mice injected with 0.1 million (n = 21) or 1 million (n = 12) tumor cells. (G) Graphical illustration of the orthotopic gastric cancer model and its progression into various organs.
References
- Mirabelli, P.; Coppola, L.; Salvatore, M. Cancer Cell Lines Are Useful Model Systems for Medical Research. Cancers 2019, 11, 1098.
- Lee, M.W.; Miljanic, M.; Triplett, T.; Ramirez, C.; Aung, K.L.; Eckhardt, S.G.; Capasso, A. Current methods in translational cancer research. Cancer Metastasis Rev. 2021, 40, 7–30.
- Byrne, A.T.; Alférez, D.G.; Amant, F.; Annibali, D.; Arribas, J.; Biankin, A.V.; Bruna, A.; Budinská, E.; Caldas, C.; Chang, D.K.; et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat. Rev. Cancer 2017, 17, 254–268.
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinská, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Mælandsmo, C.M.; et al. Patient-derived xenograft models: An emerging platform for translational cancer research. Cancer Discov. 2014, 4, 998–1013.
- Perova, Z.; Martinez, M.; Mandloi, T.; Gomez, F.L.; Halmagyi, C.; Follette, A.; Mason, J.; Newhauser, S.; Begley, D.A.; Krupke, D.M.; et al. PDCM Finder: An open global research platform for patient-derived cancer models. Nucleic Acids Res. 2023, 51, D1360–D1366.
- Jin, J.; Xu, Y.; Huo, L.; Ma, L.; Scott, A.W.; Pizzi, M.P.; Li, Y.; Wang, Y.; Yao, X.; Song, S.; et al. An improved strategy for CRISPR/Cas9 gene knockout and subsequent wildtype and mutant gene rescue. PLoS ONE 2020, 15, e0228910.
- Tian, H.; Lyu, Y.; Yang, Y.-G.; Hu, Z. Humanized Rodent Models for Cancer Research. Front. Oncol. 2020, 10, 1696.
- Yoshida, G.J. Applications of patient-derived tumor xenograft models and tumor organoids. J. Hematol. Oncol. 2020, 13, 4.
- Rivera, M.; Fichtner, I.; Wulf-Goldenberg, A.; Sers, C.; Merk, J.; Patone, G.; Alp, K.M.; Kanashova, T.; Mertins, P.; Hoffmann, J.; et al. Patient-derived xenograft (PDX) models of colorectal carcinoma (CRC) as a platform for chemosensitivity and biomarker analysis in personalized medicine. Neoplasia 2021, 23, 21–35.
- Shi, J.; Li, Y.; Jia, R.; Fan, X. The fidelity of cancer cells in PDX models: Characteristics, mechanism and clinical significance. Int. J. Cancer 2020, 146, 2078–2088.
- Yu, J.; Qin, B.; Moyer, A.M.; Sinnwell, J.P.; Thompson, K.J.; Copland, J.A.; Marlow, L.A.; Miller, J.M.; Yin, P.; Gao, B.; et al. Establishing and characterizing patient- derived xenografts using pre-chemotherapy percutaneous biopsy and post-chemotherapy surgical samples from a prospective neoadjuvant breast cancer study. Breast Cancer Res. 2017, 19, 130.
- Hylander, B.L.; Punt, N.; Tang, H.; Hillman, J.; Vaughan, M.; Bshara, W.; Pitoniak, R.; Repasky, A.E. Origin of the vasculature supporting growth of primary patient tumor xenografts. J. Transl. Med. 2013, 11, 110.
- Kopetz, S.; Lemos, R.; Powis, G. The promise of patient-derived xenografts: The best laid plans of mice and men. Clin. Cancer Res. 2012, 18, 5160–5162.
- Cho, S.-Y.; Kang, W.; Han, J.Y.; Min, S.; Kang, J.; Lee, A.; Kwon, J.Y.; Lee, C.; Park, H. An Integrative Approach to Precision Cancer Medicine Using Patient-Derived Xenografts. Mol. Cells 2016, 39, 77–86.
- Murayama, T.; Gotoh, N. Patient-Derived Xenograft Models of Breast Cancer and Their Application. Cells 2019, 8, 621.
- Pan, B.; Wei, X.; Xu, X. Patient-derived xenograft models in hepatopancreatobiliary cancer. Cancer Cell Int. 2022, 22, 41.
- Abdolahi, S.; Ghazvinian, Z.; Muhammadnejad, S.; Saleh, M.; Aghdaei, H.A.; Baghaei, K. Patient-derived xenograft (PDX) models, applications and challenges in cancer research. J. Transl. Med. 2022, 20, 206.
- Shultz, L.D.; Ishikawa, F.; Greiner, D.L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 2007, 7, 118–130.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
611
Revisions:
2 times
(View History)
Update Date:
03 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




