Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aysel Aslanli | -- | 5101 | 2023-10-30 10:10:00 | | | |
| 2 | Jessie Wu | -6 word(s) | 5095 | 2023-10-31 03:17:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Efremenko, E.; Aslanli, A.; Stepanov, N.; Senko, O.; Maslova, O. Biomimetics as Antifungals. Encyclopedia. Available online: https://encyclopedia.pub/entry/50922 (accessed on 07 February 2026).
Efremenko E, Aslanli A, Stepanov N, Senko O, Maslova O. Biomimetics as Antifungals. Encyclopedia. Available at: https://encyclopedia.pub/entry/50922. Accessed February 07, 2026.
Efremenko, Elena, Aysel Aslanli, Nikolay Stepanov, Olga Senko, Olga Maslova. "Biomimetics as Antifungals" Encyclopedia, https://encyclopedia.pub/entry/50922 (accessed February 07, 2026).
Efremenko, E., Aslanli, A., Stepanov, N., Senko, O., & Maslova, O. (2023, October 30). Biomimetics as Antifungals. In Encyclopedia. https://encyclopedia.pub/entry/50922
Efremenko, Elena, et al. "Biomimetics as Antifungals." Encyclopedia. Web. 30 October, 2023.
Copy Citation
Biomimetics, which are similar to natural compounds that play an important role in the metabolism, manifestation of functional activity and reproduction of various fungi, have a pronounced attraction in the current search for new effective antifungals. Actual trends in the development of this area of research indicate that unnatural amino acids can be used as such biomimetics, including those containing halogen atoms; compounds similar to nitrogenous bases embedded in the nucleic acids synthesized by fungi; peptides imitating fungal analogs; molecules similar to natural substrates of numerous fungal enzymes and quorum-sensing signaling molecules of fungi and yeast, etc.
antifungal peptides
enzyme-like activity
growth inhibition
antifungal activity
fungal metabolites
2. Antifungal Peptides (AFPs)
Antimicrobial peptides (AMPs) produced by living organisms attract particular interest as new antimicrobial agents with their wide range of biotargets (bacteria, fungi, parasites) capable of replacing blockbuster antibiotics [1]. To date, there are a number of databases for documenting AMPs [2]. In this research, researchers considered information from four of them: the Antimicrobial Peptides Database (APD3), the Collection of Antimicrobial Peptides (CAMPR3), the Database of Antimicrobial Activity and Structure of Peptides (DBAASP), and the Database of Antimicrobial Peptides (dbAMPs) [3][4][5][6] (Figure 1).
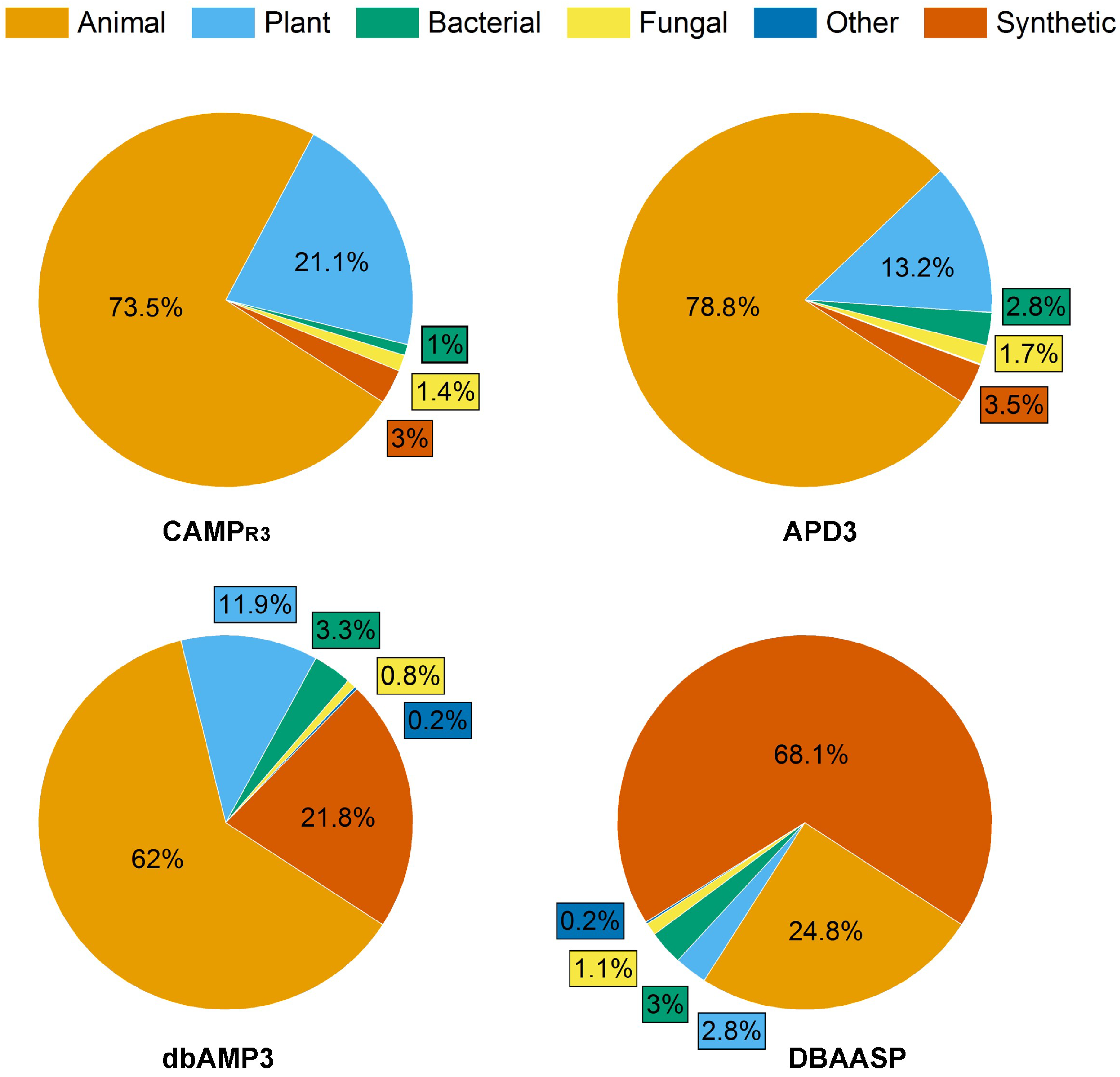
Figure 1. Composition of AMP databases according to the source of peptides with antifungal activity. Total number of AFPs in each database was taken as 100%: 1311, 1144, 5968 and 5540 for APD3, CAMPR3, DBAASP and dbAMPs, respectively.
APD3 is a system dedicated to the discovery timeline, glossary, nomenclature, classification, structure, information search, prediction, design, statistics and tools of AMPs and beyond. As of August 2023, the APD3 contains a total of 3569 AMPs.
The dbAMP database, created by Dr. Lee’s team, contains information about 28,709 different types of AMPs in 3044 organisms, including experimentally verified AMPs. The information from such protein databases as UniProt, NCBI and Protein Data Bank was used for the construction of dbAMP.
The DBAASP database contains 20,876 AMPs and information about their chemical structure, amino acid sequences, target species and object, and hemolytic and cytotoxic activities of peptides. In addition, DBAASP provides a tool for the in silico prediction/design of new AMPs.
CAMPR3 contains information on the AMP sequences, protein definition, accession numbers, activity, source organism, target organisms, protein family descriptions and N and C terminal modifications. Currently, the database contains 8164 peptide sequences and also provides tools for sequence alignment, pattern creation and pattern and HMM-based searches.
The peptides in these databases can be classified into four categories: (i) natural AMPs; (ii) predicted peptides, which are predicted by machine learning or other technologies and tested to be active; (iii) synthetic peptides, derived from natural AMPs; and (iv) patented AMPs. Thus, the described AMP databases are an effective tool for the analysis, prediction and design of new peptides with desired properties, particularly AFPs.
As of August 2023, the proportion of AFPs among all AMPs reported in the APD3, CAMPR3, DBAASP and dbAMPs databases was 36.7%, 14%, 28.6% and 19.3%, respectively (Figure 1).
Interestingly, the ratio of synthetic AFPs (SAFPs) to natural AFPs varied depending on the database under consideration and was the maximum (68.1%) in the DBAASP database.
Thus, it is obvious that covering, in one article, the huge number of peptides known is a nearly impossible task. When selecting substances for discussion, researchers were guided by the number of studies conducted with each of the peptides, the presence of a known structure, and the presence of synthetic analogs in the case of the natural peptides under consideration. At the same time, the most recent and relevant synthetic peptides obtained by prediction and studied against various pathogens were also considered.
According to the data of Figure 1 and Table 1 [7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23]), a number of AMPs with antifungal activity have already been identified, which can be obtained from various natural sources. Due to the possibility of obtaining SAFPs capable of imitating various natural peptides, such biomimetics can find effective application in practice and are notable objects for current developments and investigations (Table 2 [9][14][19][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43]).
Table 1. Natural AMPs with antifungal activity.
| # | Antifungal Peptide | Origin | Molecular Weight [Reference] | Antifungal Effect |
|---|---|---|---|---|
| Mechanism of action: inhibition of chitin biosynthesis | ||||
| 1 | Nikkomycin Z | fungi | 495.4 Da [7] | 0.5–64 mg/L (yeasts, fungi) a |
| Mechanism of action: destabilization of plasma membrane, pore formation, cell wall damage | ||||
| 2 | Polymyxin B | bacteria | 1301.6 Da [8] | 16–256 mg/L (multi-drug-resistant fungal strains) a |
| 3 | Colistin | bacteria | 1155.4 Da [8] | |
| 4 | Lactoferampin B | bovine | 2389.8 Da [9] | 0.7–39 µM (C. albicans) c |
| 5 | Lactoferricin B | bovine | 3125.8 Da [9] | 0.31–400 mg/L (yeasts) a 4–32 µM (fungi) a |
| 6 | Lactoferricin H | human | 5513 Da [9] | 10 mg/L (C. albicans) a |
| 7 | Halictine Hal-2 | sweat bee | 1452.85 Da [10] | 1.6–25 µM (Candida spp.) a |
| 8 | Halocidin | ascidian | 3445.1 Da [11] | Effect was not estimated |
| 9 | Magainin-2 | frog | 2466.9 Da [12][13] | 6.25 µM (Saccharomyces cerevisiae, Trichosporon beigelii, Candida albicans) a 60-100 mg/L (Penicillium digitatum; Alternaria solani; Phytophthora infestans) a |
| 10 | Defensin DefMT3 | ticks | 1613.1 Da [14] | 4 µM (Fusarium culmorum; F. graminearum) b |
| 11 | Neosartorya fischeri antifungal protein (NFAP) | ascomycete | 6600 Da [15] | 12.5–100 mg/L (fungi) a |
| 12 | Indolicidin | bovine | 1906.3 Da [16] | 12.5–50 mg/L (C. albicans) a |
| 13 | Leg2 | chickpea legumin hydrolysates | 2157.6 Da [17] | 125–250 µM (S. cerevisiae, Zygosaccharomyces bailii) a |
| 14 | LL-37 | human | 4493.3 Da [18] | 4–64 µM (Candida spp.) a |
| Mechanism of action: cell/spore lysis, cell wall perturbations | ||||
| 15 | Cecropin B | silkworm | 3835.7 Da [19] | 0.9 mg/L (C. albicans) a 160–320 mg/L (F. solani) a |
| 16 | Osmotin | plant | 24285.3 Da [20] | 4–25 mg/L (fungi) a |
| 17 | Stomoxyn | stable fly | 4474.2 Da [21] | 0.8–50 µM (yeasts); 0.4–7 µM (fungi) a 50–100 µM (A. fumigatus) a |
| 18 | Temporin B | frog | 1391.8 Da [22] | 1.4–4 µM (Candida spp.) a |
| 19 | Temporin G | 1457.8 Da [23] | 8–128 µM (yeasts/fungi) a | |
a MIC, minimal inhibiting concentration; b IC50, half maximal inhibitory concentration; c LC50, 50% lethal concentration.
Table 2. Synthetic/(semi)synthetic antimicrobial peptides with antifungal activity.
| # | Antifungal Peptide | Molecular Weight; [Reference] | Antifungal Effect |
|---|---|---|---|
| Mechanism of action: inhibition of 1,3-β-d-glucan synthase | |||
| 20 | Anidulafungin | 1140.2 Da [24][25][26] | 0.06–0.25 mg/L (Candida spp.) a 0.015–32 mg/L (fungi) a |
| 21 | Caspofungin | 1093.3 Da [24][25][26] | 0.25–4 mg/L (Candida spp.) a |
| 22 | Micafungin | 1270.3 Da [24][25][26] | 0.015–4 mg/L (Candida spp.) a |
| 23 | CGA-N12-0801 | 993.2 [27] | 3.5–31.4 mg/L (C. tropicalis) a |
| Mechanism of action: destabilization of plasma membrane, pore formation, cell wall damage | |||
| 24 | Lf(1-11) H | 1317.5 Da [19] | >12.5 mg/L (Candida spp.); 80–160 mg/L (F. solani), 4.3 µM (A. fumigatus) a |
| 25 | Lfchimera (bLfcin/Lfampin) | 4422 Da [9] | 6.25 mg/L (C. parapsilosis) a |
| 26 | PPD1 | <1000 Da [28][29] | ~4.9 mg/L (A. flavus) a |
| 27 | 66-10 | ~4 mg/L (A. flavus) a | |
| 28 | 77-3 | 994.2 Da [28][29] | 3.5–5 mg/L (A. flavus, A. parasiticus) a |
| 29 | D4E1 | 2079.4 Da [28] | 7.75 µM (A. flavus); 0.60 µM (V. dahliae) b 13.02 µM (C. destructivum) b |
| 30 | KK14 | 144.2 Da [30] | 6.25–100 mg/L (fungi) a |
| 31 | PAF26 | 991.2 Da [31] | 4–6 µM (P. digitatum) a |
| 32 | C12O3TR | n.d. [32] | 3.12–25 mg/L (fungi, yeasts) a |
| 33 | Trp-His[1-(3,5-di-tert-butylbenzyl)]-NHBn | n.d. [33] | 3.81 mg/L (C. neoformans) a |
| 34 | Halictine Hal-2 derivatives | 1471 Da [34] | 0.5–1 µM (Candida spp., S. cerevisiae) a |
| 35 | di-K19Hc | 4115.1 Da [35] | <4 mg/L (C. albicans); <16 mg/L (Aspergillus sp.) a |
| 36 | Pexiganan/MSI-78 | 2478.2 Da [19] | 10–80 mg/L (F. solani) a |
| 37 | γ-core DefMT3 | 1611.8 Da [14] | 1–2 µM (F. culmorum; F. graminearum) b |
| 38 | γNFAP, γNFAP-opt | 1700 Da [15] | 12.5–200 mg/L (fungi) a |
| 39 | PepGAT | 1044.18 Da [36][37][38] | 40–80 mg/L (Candida spp., P. digitatum) a |
| 40 | PepKAA | 1238.44 Da [36][37][38] | |
| 41 | RcAlb-PepII | 637.77 Da [36][39] | 17–250 µM (Candida spp.) a 0.04 mg/L (Cryptococcus neoformans) a |
| Mechanism of action: production of reactive oxygen species, cell wall degradation | |||
| 42 | Mo-CBP3-PepI | 893.12 Da [40] | 2.2 µM (C. albicans) c |
| 43 | Mo-CBP3-PepII | 1031.30 [40] | 17.5 µM (C. albicans) c |
| 44 | Mo-CBP3-PepIII | 692.85 [41] | |
| 45 | Octominin | 2652.2 Da [42] | 50 mg/L (C. albicans) a |
| Mechanism of action: Cell/spore lysis, cell wall perturbations | |||
| 46 | Osm-pepA | 3050.5 Da [43] | 40 µM (S. cerevisiae) a 20 µM (Pichia pastoris) a |
a MIC, minimal inhibiting concentration; b IC50, half maximal inhibitory concentration; c IC90, concentration that inhibited yeast growth by 90%.
It should be noted that AFPs can be classified by their total charge, secondary structure, mechanism of action, etc. When discussing AFPs, the main attention was paid to their origin (natural, semi-synthetic or synthetic), the object of their influence, the mechanism of their influence on fungal cells and the level of antifungal effect. Such information was summarized in Table 1 and Table 2.
Despite the fact that the mechanism of action of AMPs in relation to fungal cells is not as well studied as in relation to bacteria, the basic principle of their antifungal action is similar to the antibacterial one and most often consists in violation of the functions and integrity of the cell wall and plasma membrane. Due to this principle of action of AMPs, the development of resistance to them in microorganisms is considered to be almost impossible. AFPs, in addition to the mentioned effect on fungal cells, are able to specifically inhibit membrane proteins, β-1,3-glucan and chitin synthases, thereby contributing to the formation of defects in the cell wall, or inhibit H+-ATPase, causing an apoptosis-like process. Some AFPs can also induce intracellular generation of ROS, destroying various biomolecules (lipids, proteins, nucleic acids, etc.) by their active oxidation [44][45][46][47] (Table 1).
Semi-synthetic AFPs are chemically modified natural peptides while preserving the active centers of the origin molecule in order to achieve optimal properties [48][49]. Echinocandins (anidulafungin (#20), caspofungin (#21), micafungin (#22)), having a lipopeptide nature, are members of the “youngest” clinically used group of semi-synthetic AFPs. Echinocandins consist of a cyclic hexapeptide nucleus with a lipid side chain. The peptide lipid tail anchors the lipopeptide in the cell membrane next to the target enzyme. These peptides act as non-competitive inhibitors of a key enzyme in the synthesis of β-1,3-glucan [49]. Unlike semi-synthetic AFPs, SAFPs are obtained entirely by chemical synthesis. Most often, a solid-phase method is used for the synthesis of SAFPs, based on the addition of one amino acid at one step of the synthesis, which allows investigators to study the role of each amino acid in the synthesized sequence.
In addition to the methods of chemical modification and synthesis of AMP molecules, the use of methods of computer molecular design played a significant role in obtaining synthetic AMPs with the desired characteristics. This made it possible to combine information about the chemical properties and biological activity acquired by new peptides and the amino acid sequences present in them. This, in turn, made it possible to develop methods for predicting and evaluating the antifungal potential of synthesized sequences in silico [50].
Examples of Synthetic Analogs of the Natural Antifungal Peptides
When analyzing examples of currently created semi-synthetic and synthetic AFPs, the result of studying their properties and mechanisms of action, as well as comparing them with natural analogs, is always interesting and useful. Therefore, the main idea of the analysis was to show how appropriate it is to obtain certain synthetic analogs of natural peptides from the point of view of influencing the peptide properties (antimicrobial efficiency, toxicity, stability, mechanism of action, etc.). It appears that the targets of action of AFPs can be different, and this is due to their different chemical structures.
An important role in studying the antimicrobial properties of protein/peptide molecules is played by the study of their three-dimensional structures. The study of the active sites of protein/peptide molecules and mechanisms of action is an important tool in the search for effective therapeutic compounds. However, it should be taken into account that, unlike proteins, peptides can take on multiple conformations, and their 3D structures can change due to intermolecular interactions with solvent molecules and biomolecules (Figure 2) [51].
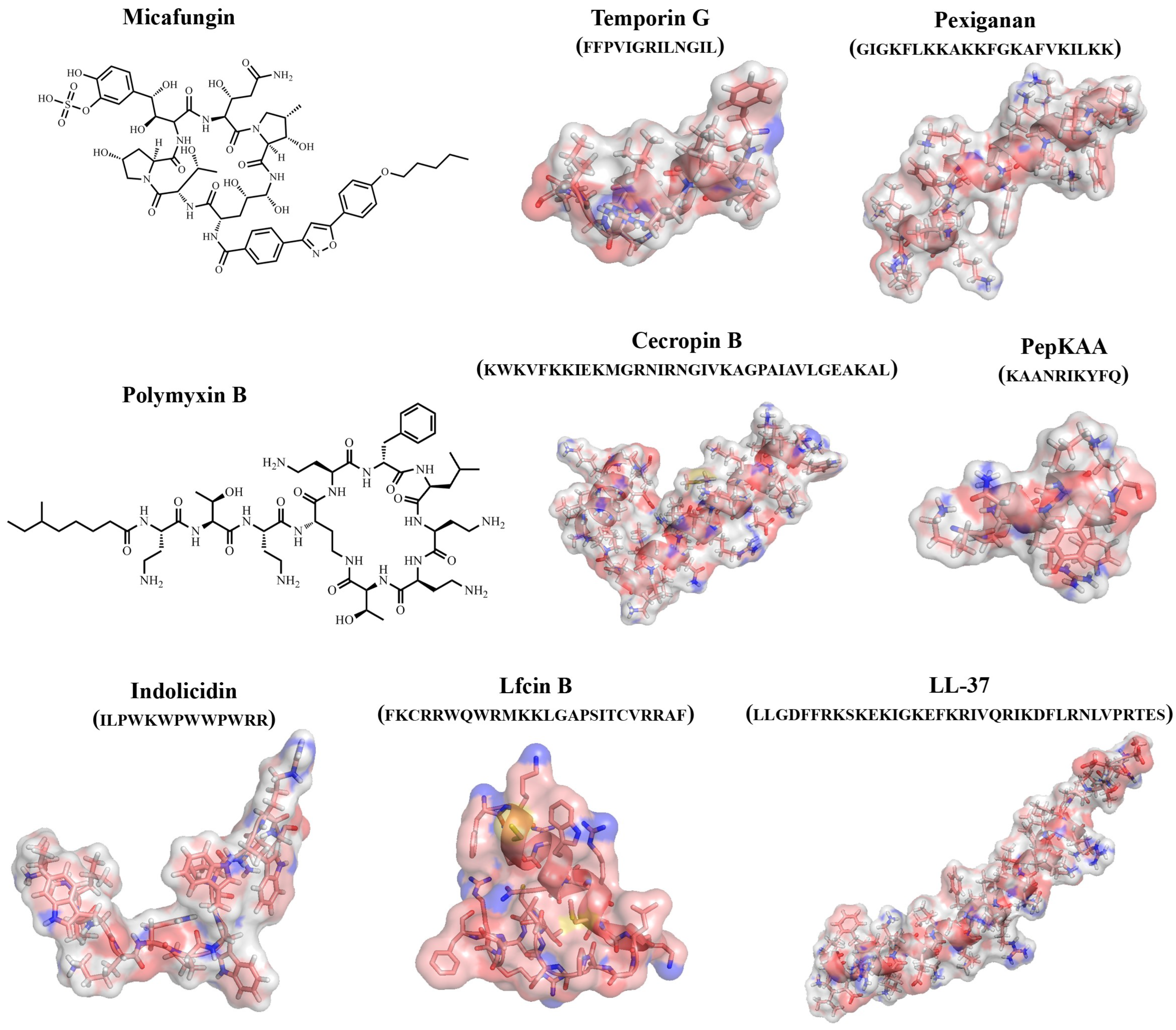
Figure 2. Examples of structures of natural and synthetic AFPs.
Antimicrobial peptides polymyxin B (#2) and colistin (polymyxin E) (#3) exhibited antifungal activity against 11 MDR yeast and filamentous fungal strains, including strains belonging to the Candida, Cryptococcus and Rhodotorula yeast genera, along with others belonging to Aspergillus, Fusarium, Scedosporium, Lichtheimia and Rhizopus, with MICs ranging from 16 to 128 μg/mL, except for the Aspergillus species [8].
It was shown that peptides naturally derived from milk protein lactoferrin, such as lactoferricin (Lfcin) (#5,6), lactoferrampin (Lfampin) (#4) and Lf(1-11) (#24) and some modified or synthesized peptides (#25) could also exhibit strong antifungal activity. In particular, the antifungal activity of bovine lactoferrin-derived Lfcin (#5) was investigated against a wide range of fungal species, and it was shown that bLfcin (#5) demonstrates strongly enhanced antimicrobial activity compared to lactoferrin [9][19][52][53][54].
Using a simple and reliable method, a set of anti-Candida peptide CGA-N12 (#23) analogs was rationally designed, and seven CGA-N12 analogs with significantly improved antifungal activity against C. tropicalis were screened [27].
An investigation of the effect of four antimicrobial peptides—PPD1 (FRLHF) (#26), 66-10 (FRLKFH) (#27), 77-3 (FRLKFHF) (#28) and D4E1 (FKLRAKIKVRLRAKIKL) (#29)—on the aflatoxin production by A. flavus and A. parasiticus suggested that AMPs at the near minimum inhibitory concentrations (MIC) were effectively inhibiting aflatoxins without hindering the growth of the fungi. At higher concentrations, these peptides exerted fungicidal action on A. flavus [28][29].
A C14-residue peptide named KK14 (#30), with the sequence KKFFRAWWAPRFLK-NH2, was designed and inhibited the conidial germination and fungal growth of food contaminants. The substitution of a Pro residue with Arg increased the helical content of the peptide, not only with its antifungal activity but also its cytotoxicity. The insertion of an unnatural bulky residue, β-diphenylalanine, or a full denantiomerization, overall increased the antifungal potency [30].
The small antimicrobial peptide PAF26 (Ac-RKKWFW-NH2) (#31) demonstrated multiple detrimental effects on the filamentous fungi Penicillium digitatum, which ultimately resulted in the permeation and killing of the growing cells [31].
Ultrashort peptide H-Orn-Orn-Trp-Trp-NH2 (O3TR) (#32) inhibited the growth of the filamentous fungi Fusarium culmorum, Penicillium expansum and A. niger, and the yeasts Saccharomyces cerevisiae, Zygosaccharomyces bailii, Z. rouxii, Debaryomyces hansenii and Kluyveromyces lactis. The addition of a C12 fatty-acid chain tail at the N-terminus of these peptides improved its antifungal activity by 2–8-fold in relation to different fungi [32].
The rationally designed and synthesized new structural class of dipeptides Trp-His(1-Bn)-OMe/NHBn and tripeptides His(1-Bn)-Trp-His(1-Bn)-OMe/NHBn, particularly Trp-His[1-(3,5-di-tert-butylbenzyl)]-NHBn (#33), possessing modified amphiphilic histidine along with hydrophobic tryptophan residues, demonstrated promising antifungal activity with membrane lytic action against C. neoformans [33].
The mechanism of action and activity of a series of synthetic analogs (#34) of the halictine HAL-2 peptide (#7) (from the venom of the wild bee Halictus sexcinctus) was investigated in relation to cells of Candida spp. It was found that halictines can rapidly permeabilize cell membranes and cause the leakage of cytosolic components and that their mode of action is likely to depend on the plasma-membrane sterols. Pre-treatment with the inhibitors of sterol synthesis (terbinafine and fluconazole) resulted in a significant reduction in peptide efficacy, while their killing efficacy increased when combined with amphotericin B [10][34].
A synthetic analog of antimicrobial peptide halocidin (#8), di-K19Hc (#35), has exhibited improved antifungal activity against a panel of fungi, including several strains of Aspergillus and Candida [11][35].
MSI-78 (pexiganan) (#36) is a synthetic peptide derived from naturally occurring magainin-2 (#9) produced by Xenopus laevis. An in vitro evaluation of the antifungal activity of MSI-78 against clinical isolates of F. solani demonstrated that MICs of this peptide can vary between 10 and 80 mg/L [12][13][19].
An investigation of the antifungal activity of Ixodes ricinus defensins (DefMT3, DefMT6 and DefMT7) (#10) and their γ-core motifs (#37) against Fusarium species demonstrated that the antifungal activity of the γ-core of selected peptides, particularly DefMT3 (#37), was higher than the full peptides [14].
It has been shown that a number of AFPs can exhibit not only antifungal activity but also inhibit the production of mycotoxins synthesized by fungi as molecules for their self-defense [28][44]. It turned out that, in some cases, individual AFPs may lose their ability to inhibit the growth of fungi but retain their effectiveness in inhibiting the biosynthesis of mycotoxins by fungi. For instance, the treatment of F. graminearum cells with the reduced form of the γ-core of the tick defensin DefMT3 (TickCore3, TC3) decreases the growth of the fungal cells and abrogates mycotoxin (trichothecene B) production. The oxidation of TC3 leads to the loss of its growth-inhibitory activity, while the anti-mycotoxin activity is retained [55].
The full-length Neosartorya (Aspergillus) fischeri AMPs (#11) and novel rationally designed γ-core peptide derivatives γNFAP-opt and γNFAP-optGZ (#38) exhibited high efficacy by inhibiting the growth of the agriculturally relevant filamentous ascomycetes in vitro [15].
Using an easy step-by-step way to choose, characterize and test potential sequences to be assayed as synthetic AMPs, two peptides (PepGAT (#39) and PepKAA (#40)) with antimicrobial potential against Candida spp., including activity against biofilms and without any hemolytic effects, were identified and characterized [37]. SAFPs PepGAT (#39) and PepKAA (#40) demonstrated strong inhibition of P. digitatum growth. All peptides targeted the fungal membrane, leading to pore formation, loss of internal content and death. The induction of high levels of ROS was also a mechanism employed by some peptides [36][37][38].
A small peptide, RcAlb-PepII (#41), designed based on the primary structure of Rc-2S-Alb, a 2S albumin from the seed cake of Ricinus communis, strongly inhibited the growth of Klebsiella pneumoniae and Candida parapsilosis, and induced morphological alterations in their cell surface. The peptide also degraded and reduced the biofilm formation in C. parapsilosis and K. pneumonia cells [39].
The antifungal activity of three peptides, called Mo-CBP3-PepI (CPIAQRCC) (#42), Mo-CBP3-PepII (NIQPPCRCC) (#43) and Mo-CBP3-PepIII (AIQRCC) (#44), designed based on the structure of Mo-CBP3, a chitin-binding protein purified from Moringa oleifera seeds, was evaluated against C. albicans and C. parapsilosis biofilms [40][41][56]. Eight SAMPs were tested regarding their antifungal potential against C. neoformans, and five SAMPs showed an inhibitory effect on C. neoformans growth at low concentrations. Peptides induced many morphological alterations, such as in the cell membrane, wall damage and loss of internal content in C. neoformans cells [36].
A designed and synthesized synthetic peptide consisting of 23 amino acids, named Octominin (1GWLIRGAIHAGKAIHGLIHRRRH23) (#45), from a defense protein 3 cDNA sequence of Octopus minor showed an inhibitory effect against Candida albicans by causing ultrastructural cell wall deformities [42].
Plants’ multifunctional protein osmotin plays an important role in plant immune systems, inducing abiotic stress tolerance and providing protection from fungal infections [26]. The cacao osmotin (#16)-like protein (TcOsm1)-derived peptides, named Osm-pepA (#46) and Osm-pepB, inhibited the growth of yeasts (Saccharomyces cerevisiae S288C and Pichia pastoris X-33) and spore germination of the phytopathogenic fungi Fusarium f. sp. glycines and Colletotrichum gossypi. Osm-pepA was more efficient than Osm-pepB [43].
Inspired by antimicrobial peptides, different side chain methionine, leucine, and tyrosine-based polymethacrylates (#51) (pH-responsive cationic biocompatible polymers) have been designed, and their fungicidal activities have been investigated against A. niger, demonstrating the effective inhibition of hyphal growth and distortion of conidiophores [57].
The antifungal activity of a series of peptides with a varying number of lysine and tryptophan residue repeats (KWn-NH2) was confirmed against C. albicans [58].
Summarizing the information presented, it can be concluded that various methods of the chemical modification of natural peptides, including phosphorylation, cyclization, halogenation, etc. [45], can be used to obtain semi-synthetic AFPs (Figure 3).
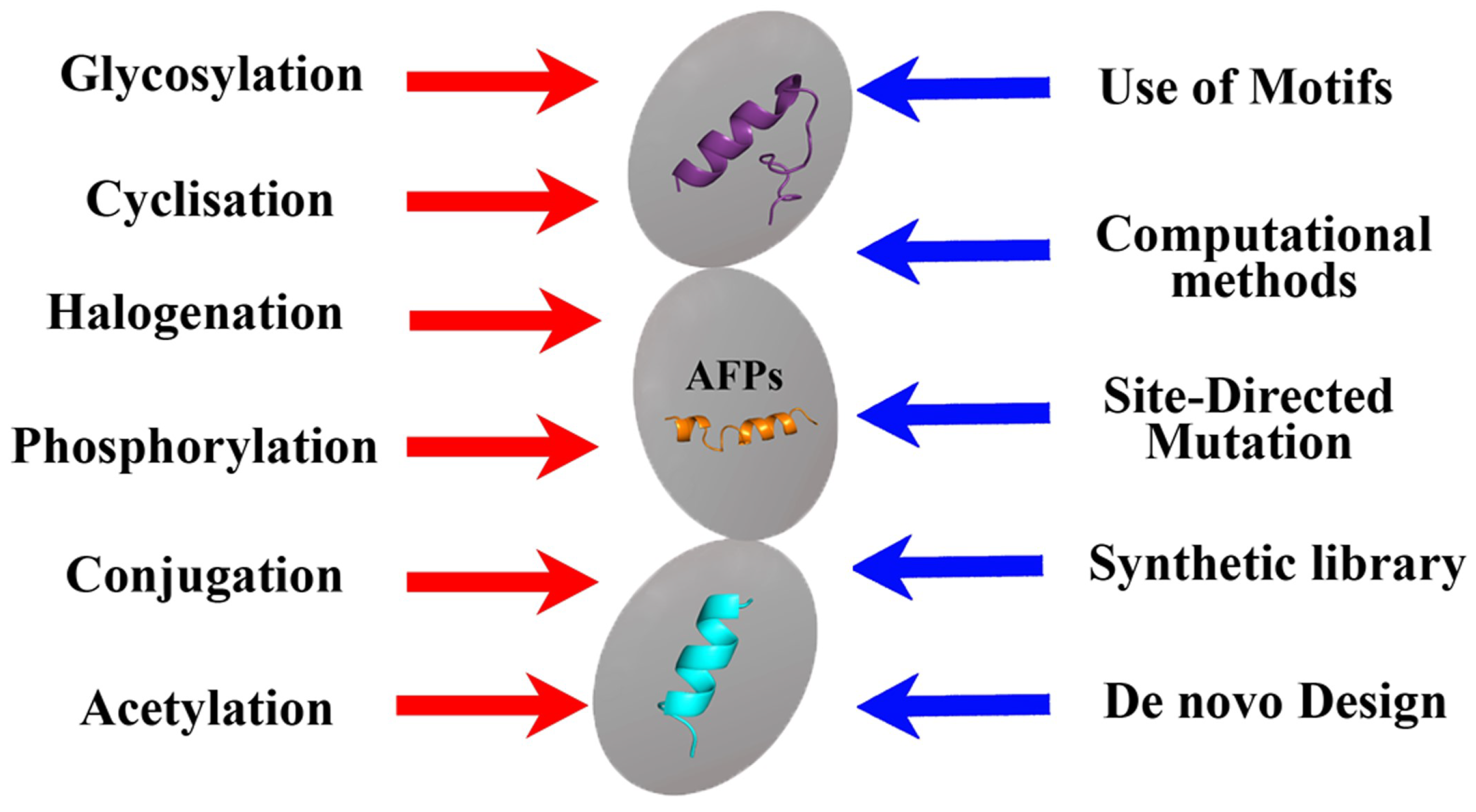
Figure 3. Approaches used in the development of biomimetic AFPs.
However, in addition to the same methods, computer molecular analysis and the design of the peptides being created play an important role in the design of synthetic AFPs, which makes it possible to proceed not from the natural basis that can only be modified, but from obtaining the synthesis of new compounds with the desired properties and taking into account the use of key amino acid motifs (Figure 3). At the same time, of course, all new AFPs must be evaluated not only for the effectiveness of the antifungal effect obtained from them but also for toxicity, including prion properties [59].
Thus, the use of various AFPs for their modifications described in this section is an effective approach to solving the problem of pathogenicity and resistance of fungi. The fact that these peptides, as natural molecules, make it possible to create a number of different biomimetics/peptidomimetics based upon them, which can be used as antifungals, allows researchers to conclude that there is a need for significant development of rational design methods today.
The use of organic synthesis methods makes it possible to construct SAFPs with the most effective amino acid sequences and desired characteristics, as well as changed mechanisms (targets) of action.
4. Combination of Antifungal Peptides with Each Other and/or with Antifungal Drugs
Despite expectations of the absence of resistance formation in fungi to the effects of new antifungals, including AFPs [60][61], azoles [62] and 2-DG [63], researchers note such cases. One of the effective solutions to this problem today is the combined use of various antifungals. Such possible combinations can include various traditional antifungal agents and AFPs (Table 3) [19][39][56][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79]. Such combinations make it possible to join different mechanisms (targets) of the effects of antifungals and significantly decrease their toxicity by improving the effectiveness of the action and reducing the doses applied in comparison with individual substances.
Table 3. Combination of AFPs with different antifungal agents.
| Components of Combined Antifungals | Target [Reference] | Antifungal Effect | |
|---|---|---|---|
| (#) AFP as the first component | (#) Second component | ||
| (#42) Mo-CBP3-PepI | (#75) Nystatin | C. albicans [39] | 0.13 a |
| (#43) Mo-CBP3-PepII | |||
| (#44) Mo-CBP3-PepIII | C. parapsilosis [56] | 82% b | |
| (#48) Itraconazole | 96% b | ||
| (#76) RcAlb-PepIII | C. neoformans [66] | 84.1% b | |
| (#77) l-His(2-adamantyl)-l-Trp-l-His(2-phenyl)-OMe | (#47) Amphotericin B | C. neoformans [65] | 0.28 a |
| (#78) l-Trp-l-His(1-biphenyl)-NHBzl | C. neoformans [67] | 0.28 a | |
| (#79) Fluconazole | 1.04 a | ||
| (#80) l-His[1-(4-n-butylphenyl)]-l-Trp-l-His[1-(4-n-butylphenyl)]-NHBzl | (#47) Amphotericin B | 0.31 a | |
| (#79) Fluconazole | 0.75 a | ||
| (#81) Lactofungin | (#47) Amphotericin B | C. albicans, C. glabrata, C. neoformans, C. deuterogattii [68] |
0.16–0.28 a |
| (#11) Neosartorya fischeri AFPs (NFAP) | (#82) NFAP2 | Botrytis cinerea, Cladosporium herbarum [69] | 1.25 a |
| (#11) NFAP | (#38) γNFAP-opt | 0.28–1.50 a | |
| (#82) NFAP2 | (#38) γNFAP-opt | 0.31–1.5 a | |
| (#36) MSI-78 | (#83) Voriconazole | Fusarium solani [19] | 0.34 a |
| (#24) hLf(1-11) | 0.21 a | ||
| (#15) Cecropin B | 0.17 a | ||
| (#36) MSI-78 | (#47) Amphotericin B | 0.37 a | |
| (#24) hLf(1-11) | 0.31 a | ||
| (#15) Cecropin B | 0.28 a | ||
| (#84) Brilacidin (non-peptide mimetic of host defense peptides) | (#21) Caspofungin | Aspergillus fumigatus [70] | 0.39 a |
| (#83) Voriconazole | 1.0 a | ||
| (#85) Geldanamycin | 0.64 a | ||
| (#86) DP-23 peptoid | (#79) Fluconazole | A. flavus, A. niger [64] | 0.16–0.38 a |
| (#87) SPO peptoid | |||
| (#88) P256 and P256 | (#47) Amphotericin B | C. albicans [71] | 0.28 a |
| (#89) γ-AA peptide MW5 | (#79) Fluconazole | C. albicans [72] | ≤0.5 a |
| (#90) 14-helical β-peptide | (#91) Isoamyl alcohol | C. albicans [73] | 4 mg/L d |
| (#92) TP10-NH2 (analog of transporan 10) | (#93) Ciprofloxacin or Levofloxacin | Candida spp. [74] | 6.3–100 μM c |
| (#94) HLopt2 (mimic of human lactoferrin) | (#79) Fluconazole | Candida spp. [75] | 2–125 mg/L c |
| (#95) ToAP2 | (#96) NDBP-5.7 | C. albicans [76] | 0.75 a |
| (#95) ToAP2 | (#47) Amphotericin B | 0.18 a | |
| (#96) NDBP-5.7 | 0.18 a | ||
| (#95) ToAP2 | (#79) Fluconazole | 0.5 a | |
| (#96) NDBP-5.7 | 0.56 a | ||
| (#97) KW-23 | C. albicans [77] | 0.37–0.60 a | |
| (#98) gH625M | C. albicans [78] | 0.30 a | |
| (#99) Flucytosine | 0.20 a | ||
| (#98) gH625M | (#47) Amphotericin B | 0.5–0.8 a | |
| (#100) Fengycin | (#101) Surfactin | Rhizopus solonifer [79] | 5 a |
a Fractional inhibitory concentration index (FICI). FICI values suggest antagonistic (>4.0), indifferent (>1 to <4), additive (>0.5–1) and synergistic (≤0.5) effects. b Percent of biofilm and growth inhibition; c MIC/MIC50, minimum inhibitory concentration (complete inhibition/at least 50% inhibition); d minimal biofilm prevention concentrations (MBPC).
The synergistic effect of combined antifungals makes it possible to effectively combat yeast biofilms of the genus Candida, both at the stage of their formation and already for the degradation of mature biofilms. A combination of AFPs Mo-CBP3-PepI (#42) and Mo-CBP3-PepIII (#44) with nystatin (#75) and itraconazole (#48) against Candida species biofilms resulted in a 2- to 4-fold improvement of antibiofilm activity of antifungals [39][56]. The combination of Mo-CBP3-PepIII (44) and RcAlb-PepIII (#76) synthetic peptides with itraconazole (#48) enhanced the activity of the latter by 10-fold against C. neoformans [66]. Likewise, synthetic histidine-containing amphipathic peptides (#78,80) enhanced the activity of amphotericin B (#47) against Cryptococcus neoformans by 4- to 16-fold [65][67]. When combined with lactoferrin-derived synthetic peptide lactofungin (#81), the minimum inhibitory concentration of amphotericin B (#47) for Candida spp. and C. neoformans decreased by 4 times [68].
The antifungal activity of two N. fischeri peptides, NFAP (#11) and NFAP2 (#82), and their γ-core peptide derivatives (γNFAP-opt, γNFAP2-opt) (#38), was tested in vitro against Botrytis, Cladosporium and Fusarium spp. A synergistic mechanism of action was observed when NFAP (11) or NFAP2 (#82) was applied in combination with γNFAP-opt (#38). The investigated proteins and peptides did not show any toxicity to tomato plant leaves, except for γNFAP2-opt [69].
Fusarium infections have been associated with high mortality rates due to the lack of effective treatment strategies. The in vitro activity of AFPs MSI-78 (#36), hLf(1-11) (#24) and cecropin B (#15) combined with amphotericin B (#47) or voriconazole (#83) were tested against ten Fusarium solani strains. All AFPs demonstrated a synergistic mechanism of action when combined with conventional antifungals [19].
The combination of brilacidin (a synthetic, nonpeptidic, small molecule mimetic of defensin) (#84) with caspofungin (#21) has a synergism that is able to affect A. fumigatus viability through multiple mechanisms of action, encompassing functional changes/depolarization of the microorganism cell membrane, interference to calcineurin signaling, and misexpression of the cell wall integrity pathway, and prevents β-1,3-glucan biosynthesis [70].
The use of mimetic peptides DP-23 (short lipopeptide) (#86) and SPO (N-substituted polyglycine) (#87) in combination with fluconazole (#79) provided a synergistic effect on A. niger and A. flavus cells [80].
Peptides P255 and P256 (#88), obtained from hexapeptide PDF 26, were combined with amphotericin B (#47) and investigated against C. albicans. Both peptides showed a synergistic effect with a polyene antibiotic, while P256 showed a stronger antifungal effect than P255. Peptides violated the integrity of the cell wall, increased membrane permeability, disrupted cell morphology and caused intracellular changes: they affected the expression of genes for replication and repair of fungal DNA, the biosynthesis of cell wall components and ergosterol. They also increased the production of ROS in cells and bound to the genomic DNA of fungi [71].
γ-AA peptides are a class of peptidomimetics with a definite folded structure and resistance to proteolytic hydrolysis. It has been shown that lipo-γ-AA peptide MW5 (#89) significantly increases the efficacy of fluconazole (#79) action against azole-resistant C. albicans CARG5 cells. The peptide destroys the cell membrane and provokes the production of ROS [72].
Synthetic peptidomimetics of antimicrobial α-peptides exhibit fungicidal activity as a result of the hydrophobic and electrostatic interactions with cell membranes, which lead to permeabilization and subsequent cell death. These peptidomimetics were more stable than their natural counterparts and were not degraded by proteases. β-peptides (#90) were obtained as structurally constructed on the basis of natural α-helical AMPs. These mimetics (#90) possessed a fungicidal effect against C. albicans cells and inhibited the formation of yeast biofilms. The hydrophobicity of the β-peptides directly correlates with their antifungal properties and a narrow range of concentrations at which β-peptides effectively kill C. albicans cells without lysis of erythrocytes. The combination of these peptides (#90) with isoamyl alcohol (#91) reduced their MIC for inhibiting biofilms by 4 times [73].
Conjugates of fluoroquinolone antibiotics (ciprofloxacin or levofloxacin) (#93) with the TP10-NH2 peptide (#92) penetrating into yeast cells showed an antifungal effect against different strains of genus Candida. At the same time, the synergistic effect of these conjugates was not revealed in the experiments on C. glabrata cells [74].
Antifungal effect against Candida spp. cells was investigated for conjugates obtained on the basis of a modified fragment of lactoferrin HLopt2 (#94) and ciprofloxacin, levofloxacin (#93) and fluconazole (#79). Three different nutrient media were used for these purposes. There was no activity against four different Candida species for the substances studied on the RPMI (mimics physiological conditions) medium. The use of BP (1% peptone) and BHI1/100 (0.034% brain-heart infusion) media resulted in the inhibition of cell growth, especially C. tropicalis, whereas C. glabrata cells were the most resistant among all tested strains [75].
The antifungal activity of cationic antimicrobial peptides ToAP2 (#95) (from a cDNA library of the scorpion Tityus obscurus venom gland) and NDBP-5.7 (#96) (from a cDNA library of the scorpion Opisthacanthus cayaporum venom gland) was demonstrated against C. albicans cells. Both peptides affected membrane permeability and caused such changes in the morphology of fungal cells as cell wall deformations and disruption of ultrastructural cell organization. Both peptides showed synergism with amphotericin B (#47) and demonstrated synergistic and additive effects in combination with fluconazole (#79), TopAP2 (#95) and NBDP-5.7 (#96), respectively [76].
The unique ultra-short peptide KW23 (#97) had a positive effect on standard and resistant Candida species, showing powerful synergistic antimicrobial activity in combination with fluconazole (#79). Interestingly, was the fact that the effect of this combination was additive with respect to the resistant strain. In addition, the peptide showed a low enough toxicity to human erythrocytes [77].
Persistent cells that can tolerate lethal concentrations of antimicrobials and grow again after their removal are found in different microbial populations. It is assumed that the appearance of persistent yeast cells involves different regulations of genes correlating with the pathways of ergosterol biosynthesis (ERG1 and ERG25) and β-1,6-glucan (SKN1 and KRE1). Special studies have shown that membranes of cells in biofilms contain a lower concentration of ergosterol, mainly in the deepest layers of the biofilm, in comparison with planktonic yeast cells. It is likely that cells from mature biofilms require less ergosterol to maintain membrane fluidity, and this is also confirmed by the limited effectiveness of biomimetics aimed at inhibiting ergosterol biosynthesis. In this regard, the possibility of destroying resistant persistent-derived biofilms of C. albicans with gH625-M peptide (#98), which is an analog of the viral membranotropic peptide gH625, was investigated [78]. The combination of gH625-M (#98) with various antifungals (fluconazole (#79), 5-flucytosine (#99) and amphotericin B (#47)) demonstrated a synergistic effect when acting on persistent cells in biofilms using relatively low doses of the antifungals (Table 3).
Among the antifungals that can be used in effective combination with AFPs, metal-containing compounds and enzymes are discussed [81][82]. The main mechanism of the antifungal action of metals is the triggering of the generation and accumulation of ROS. Enzymes are of special interest due to their wide range of substrate specificity of action and various mechanisms of antifungal actions.
The combination of metalloenzymes, such as hexahistidine-containing organophosphorus hydrolase (His6-OPH), capable of hydrolyzing signal molecules of yeast QS, with AFPs (polymyxins (#2,3), bacitracin, Lfcin (#5)) resulted in a notable improvement in the antimicrobial efficiency of action of the AFPs (up to 8.5 times) against different yeast species, such as Saccharomyces cerevisiae, Candida sp., Trichosporon beigeii, etc. It is interesting that His6-OPH had an increased catalytic efficiency of action in the hydrolysis of its substrates (QS molecules) when it was introduced in combination with AFPs [83][84]. Thus, the combination of the AFPs with enzymes possessing antifungal activity is a perspective trend in the development of efficient antifungals.
To determine the effect of combining two antifungals and interpreting the results obtained, a fractional inhibitory concentration index (FICI) range (from 0.5 to 4) is usually applied. The synergistic effect corresponds to FICI values of < 0.5 and is achieved through a combination of different mechanisms of action on antifungals. From the studies analyzed in this section, it follows that only in the case of a combination of fengycin (#100) with surfactin (#101) that an antagonistic effect was revealed (FICI = 4), which is an indicator of the incompatibility of the mechanisms of action of these compounds with each other [79].
In the case of a combination of brilacidin (84) with conventional fungicides, for AFPs of Neosartorya fischeri (#11,38,82), among themselves and histidine-containing amphipathic peptides (#78,80) with fluconazole (#79), an additive effect (FICI > 1) was marked, which means that these antifungals do not enhance the effectiveness of each other’s actions [67][69][70]. In the case of all the other analyzed combinations, a synergistic effect was revealed. The most effective variants were obtained in the case of combinations of synthetic peptides Mo-CBP3-PepI (#42) and Mo-CBP3-PepII (#43) with nystatin (#75) (FICI = 0.13) [39][56]. It is interesting to note that when combining two synthetic peptides, MSI-78 (#36) and hLf(1-11) (#24), with a natural peptide, Cecropin B (#15), as well as with voriconazole (#83) or with amphotericin B (#47), the strongest synergistic effect was achieved with a natural peptide; however, not with considered successful antifungals like azole and polyene antibiotic [19].
When developing biomimetics and some combinations with them, special attention is paid to minimizing their toxicity (or its complete absence) in relation to human/animal/plant cells, as well as to the type of antifungal effect (mechanism of action) on cells in order to avoid the development of resistance [85][86]. At the initial stage of such investigations, toxicity is assessed using modern computer modeling methods [87]. Erythrocytes are used as experimental models both in vitro and in vivo [88].
Taking into account the fact that a minimal number of cases of fungal resistance have been detected with respect to SAFPs, as well as a minimum level of toxicity shown in these peptides for other cells, they seem promising candidates in the development of modern antifungals.
References
- Konakbayeva, D.; Karlsson, A.J. Strategies and opportunities for engineering antifungal peptides for therapeutic applications. Curr. Opin. Biotechnol. 2023, 81, 102926.
- Ramazi, S.; Mohammadi, N.; Allahverdi, A.; Khalili, E.; Abdolmaleki, P. A review on antimicrobial peptides databases and the computational tools. Database 2022, 2022, baac011.
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093.
- Waghu, F.H.; Barai, R.S.; Gurung, P.; Idicula-Thomas, S. CAMPR3: A database on sequences, structures and signatures of antimicrobial peptides. Nucleic Acids Res. 2016, 44, D1094–D1097.
- Pirtskhalava, M.; Amstrong, A.A.; Grigolava, M.; Chubinidze, M.; Alimbarashvili, E.; Vishnepolsky, B.; Gabrielian, A.; Rosenthal, A.; Hurt, D.E.; Tartakovsky, M. DBAASP v3: Database of antimicrobial/cytotoxic activity and structure of peptides as a resource for development of new therapeutics. Nucleic Acids Res. 2021, 49, D288–D297.
- Jhong, J.H.; Yao, L.; Pang, Y.; Li, Z.; Chung, C.R.; Wang, R.; Li, S.; Li, W.; Luo, M.; Ma, R.; et al. dbAMP 2.0: Updated resource for antimicrobial peptides with an enhanced scanning method for genomic and proteomic data. Nucleic Acids Res. 2022, 50, D460–D470.
- Bentz, M.L.; Nunnally, N.; Lockhart, S.R.; Sexton, D.J.; Berkow, E.L. Antifungal activity of nikkomycin Z against Candida auris. J. Antimicrob. Chemother. 2021, 76, 1495–1497.
- Yousfi, H.; Ranque, S.; Rolain, J.M.; Bittar, F. In vitro polymyxin activity against clinical multidrug-resistant fungi. Antimicrob. Resist. Infect. Control 2019, 8, 66.
- Fernandes, K.E.; Carter, D.A. The antifungal activity of lactoferrin and its derived peptides: Mechanisms of action and synergy with drugs against fungal pathogens. Front. Microbiol. 2017, 8, 2.
- Kočendová, J.; Vaňková, E.; Volejníková, A.; Nešuta, O.; Buděšínský, M.; Socha, O.; Hájek, M.; Hadravová, R.; Čeřovský, V. Antifungal activity of analogues of antimicrobial peptides isolated from bee venoms against vulvovaginal Candida spp. FEMS Yeast Res. 2019, 19, foz013.
- Jang, W.S.; Kim, K.N.; Lee, Y.S.; Nam, M.H.; Lee, I.H. Halocidin: A new antimicrobial peptide from hemocytes of the solitary tunicate, Halocynthia aurantium. FEBS Lett. 2002, 521, 81–86.
- Park, Y.; Lee, D.G.; Hahm, K.S. HP (2–9)-magainin 2 (1–12), a synthetic hybrid peptide, exerts its antifungal effect on Candida albicans by damaging the plasma membrane. J. Pept. Sci. 2004, 10, 204–209.
- Alan, A.R. Sensitivity of bacterial and fungal plant pathogens to the lytic peptides, MSI-99, magainin II, and cecropin B. Mol. Plant-Microbe Interact. 2002, 15, 701–708.
- Tonk, M.; Cabezas-Cruz, A.; Valdés, J.J.; Rego, R.O.; Grubhoffer, L.; Estrada-Peña, A.; Vilcinskas, A.; Kotsyfakis, M.; Rahnamaeian, M. Ixodes ricinus defensins attack distantly-related pathogens. Dev. Comp. Immunol. 2015, 53, 358–365.
- Tóth, L.; Váradi, G.; Boros, É.; Borics, A.; Ficze, H.; Nagy, I.; Tóth, G.K.; Rákhely, G.; Marx, F.; Galgóczy, L. Biofungicidal potential of Neosartorya (Aspergillus) fischeri antifungal protein NFAP and novel synthetic-core peptides. Front. Microbiol. 2020, 11, 820.
- Lee, D.G.; Kim, H.K.; Am Kim, S.; Park, Y.; Park, S.C.; Jang, S.H.; Hahm, K.S. Fungicidal effect of indolicidin and its interaction with phospholipid membranes. Biochem. Biophys. Res. Commun. 2003, 305, 305–310.
- Heymich, M.-L.; Nißl, L.; Hahn, D.; Noll, M.; Pischetsrieder, M. Antioxidative, antifungal and additive activity of the antimicrobial peptides Leg1 and Leg2 from Chickpea. Foods 2021, 10, 585.
- Rather, I.A.; Sabir, J.S.M.; Asseri, A.H.; Ali, S. Antifungal activity of human cathelicidin LL-37, a membrane disrupting peptide, by triggering oxidative stress and cell cycle arrest in Candida auris. J. Fungi 2022, 8, 204.
- Denardi, L.B.; Weiblen, C.; Ianiski, L.B.; Stibbe, P.C.; Santurio, J.M. Activity of MSI-78, h-Lf1-11 and cecropin B antimicrobial peptides alone and in combination with voriconazole and amphotericin B against clinical isolates of Fusarium solani. J. Med. Mycol. 2021, 31, 101119.
- de Freitas, C.D.T.; de Souza Lopes, J.L.; Beltramini, L.M.; de Oliveira, R.S.B.; Oliveira, J.T.A.; Ramos, M.V. Osmotin from Calotropis procera latex: New insights into structure and antifungal properties. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2501–2507.
- Landon, C.; Meudal, H.; Boulanger, N.; Bulet, P.; Vovelle, F. Solution structures of stomoxyn and spinigerin, two insect antimicrobial peptides with an α-helical conformation. Biopolymers 2006, 81, 92–103.
- Kakar, A.; Holzknecht, J.; Dubrac, S.; Gelmi, M.L.; Romanelli, A.; Marx, F. New perspectives in the antimicrobial activity of the amphibian temporin B: Peptide analogs are effective inhibitors of Candida albicans growth. J. Fungi 2021, 7, 457.
- D’Auria, F.D.; Casciaro, B.; De Angelis, M.; Marcocci, M.E.; Palamara, A.T.; Nencioni, L.; Mangoni, M.L. Antifungal activity of the frog skin peptide temporin G and its effect on Candida albicans virulence factors. Int. J. Mol. Sci. 2022, 23, 6345.
- Mroczyńska, M.; Brillowska-Dąbrowska, A. Review on current status of echinocandins use. Antibiotics 2020, 9, 227.
- Antachopoulos, C.; Meletiadis, J.; Sein, T.; Roilides, E.; Walsh, T.J. Comparative in vitro pharmacodynamics of caspofungin, micafungin, and anidulafungin against germinated and nongerminated Aspergillus conidia. Antimicrob. Agents Chemother. 2008, 52, 321–328.
- Kovács, R.; Tóth, Z.; Locke, J.B.; Forgács, L.; Kardos, G.; Nagy, F.; Borman, A.M.; Majoros, L. Comparison of in vitro killing activity of rezafungin, anidulafungin, caspofungin, and micafungin against four Candida auris clades in RPMI-1640 in the absence and presence of human serum. Microorganisms 2021, 9, 863.
- Li, R.; Wu, J.; He, F.; Xu, Q.; Yin, K.; Li, S.; Li, W.; Wei, A.; Zhang, L.; Zhang, X.-H.; et al. Rational design, synthesis, antifungal evaluation and docking studies of antifungal peptide CGA-N12 analogues based on the target CtKRE9. Bioorg. Chem. 2023, 132, 106355.
- Devi, M.S.; Sashidhar, R.B. Antiaflatoxigenic effects of selected antifungal peptides. Peptides 2019, 115, 15–26.
- Devi, S.M.; Raj, N.; Sashidhar, R.B. Efficacy of short-synthetic antifungal peptides on pathogenic Aspergillus flavus. Pestic. Biochem. Physiol. 2021, 174, 104810.
- Thery, T.; Shwaiki, L.N.; O’Callaghan, Y.C.; O’Brien, N.M.; Arendt, E.K. Antifungal activity of a de novo synthetic peptide and derivatives against fungal food contaminants. J. Pept. Sci. 2019, 25, e3137.
- Munoz, A.; López-García, B.; Marcos, J.F. Studies on the mode of action of the antifungal hexapeptide PAF26. Antimicrob. Agents Chemother. 2006, 50, 3847–3855.
- Thery, T.; O’Callaghan, Y.; O’Brien, N.; Arendt, E.K. Optimisation of the antifungal potency of the amidated peptide H-Orn-Orn-Trp-Trp-NH2 against food contaminants. Int. J. Food Microbiol. 2018, 265, 40–48.
- Sharma, K.; Aaghaz, S.; Maurya, I.K.; Rudramurthy, S.M.; Singh, S.; Kumar, V.; Tikoo, K.; Jain, R. Antifungal evaluation and mechanistic investigations of membrane active short synthetic peptides-based amphiphiles. Bioorg. Chem. 2022, 127, 106002.
- Kodedová, M.; Sychrová, H. Synthetic antimicrobial peptides of the halictines family disturb the membrane integrity of Candida cells. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1851–1858.
- Jang, W.S.; Kim, H.K.; Lee, K.Y.; Am Kim, S.; Han, Y.S.; Lee, I.H. Antifungal activity of synthetic peptide derived from halocidin, antimicrobial peptide from the tunicate, Halocynthia aurantium. FEBS Lett. 2006, 580, 1490–1496.
- Aguiar, T.K.B.; Neto, N.A.S.; Freitas, C.D.T.; Silva, A.F.B.; Bezerra, L.P.; Malveira, E.A.; Branco, L.A.C.; Mesquita, F.P.; Goldman, G.H.; Alencar, L.M.R.; et al. Antifungal potential of synthetic peptides against Cryptococcus neoformans: Mechanism of action studies reveal synthetic peptides induce membrane–pore formation, DNA degradation, and apoptosis. Pharmaceutics 2022, 14, 1678.
- Souza, P.F.; Marques, L.S.; Oliveira, J.T.; Lima, P.G.; Dias, L.P.; Neto, N.A.; Lopes, F.E.S.; Sousa, J.S.; Silva, A.F.B.; Caneiro, R.F.; et al. Synthetic antimicrobial peptides: From choice of the best sequences to action mechanisms. Biochimie 2020, 175, 132–145.
- Lima, P.G.; Freitas, C.D.; Oliveira, J.T.; Neto, N.A.; Amaral, J.L.; Silva, A.F.; Sousa, J.S.; Franco, O.L.; Souza, P.F.N. Synthetic antimicrobial peptides control Penicillium digitatum infection in orange fruits. Food Res. Int. 2021, 147, 110582.
- Dias, L.P.; Souza, P.F.; Oliveira, J.T.; Vasconcelos, I.M.; Araújo, N.M.; Tilburg, M.F.; Guedes, M.I.F.; Carneiro, R.F.; Lopes, J.L.S.; Sousa, D.O. RcAlb-PepII, a synthetic small peptide bioinspired in the 2S albumin from the seed cake of Ricinus communis, is a potent antimicrobial agent against Klebsiella pneumoniae and Candida parapsilosis. Biochim. Biophys. Acta Biomembr 2020, 1862, 183092.
- Lima, P.G.; Souza, P.F.N.; Freitas, C.D.T.; Oliveira, J.T.A.; Dias, L.P.; Neto, J.X.S.; Vasconcelos, I.M.; Lopes, J.L.S.; Sousa, D.O.B. Anticandidal activity of synthetic peptides: Mechanism of action revealed by scanning electron and fluorescence microscopies and synergism effect with nystatin. J. Pept. Sci. 2020, 26, e3249.
- Oliveira, J.T.; Souza, P.F.; Vasconcelos, I.M.; Dias, L.P.; Martins, T.F.; Van Tilburg, M.F.; Guedes, M.I.F.; Sousa, D.O. Mo-CBP3-PepI, Mo-CBP3-PepII, and Mo-CBP3-PepIII are synthetic antimicrobial peptides active against human pathogens by stimulating ROS generation and increasing plasma membrane permeability. Biochimie 2019, 157, 10–21.
- Nikapitiya, C.; Dananjaya, S.H.S.; Chandrarathna, H.P.S.U.; De Zoysa, M.; Whang, I. Octominin: A novel synthetic anticandidal peptide derived from defense protein of Octopus minor. Mar. Drugs 2020, 18, 56.
- Falcao, L.L.; Silva-Werneck, J.O.; Ramos, A.D.R.; Martins, N.F.; Bresso, E.; Rodrigues, M.A.; Bemquerer, M.P.; Marcellino, L.H. Antimicrobial properties of two novel peptides derived from Theobroma cacao osmotin. Peptides 2016, 79, 75–82.
- Martínez-Culebras, P.V.; Gandía, M.; Garrigues, S.; Marcos, J.F.; Manzanares, P. Antifungal peptides and proteins to control toxigenic fungi and mycotoxin biosynthesis. Int. J. Mol. Sci. 2021, 22, 13261.
- Struyfs, C.; Cammue, B.P.A.; Thevissen, K. Membrane-interacting antifungal peptides. Front. Cell Dev. Biol. 2021, 9, 649875.
- Rautenbach, M.; Troskie, A.M.; Vosloo, J.A. Antifungal peptides: To be or not to be membrane active. Biochimie 2016, 130, 132–145.
- Li, T.; Li, L.; Du, F.; Sun, L.; Shi, J.; Long, M.; Chen, Z. Activity and Mechanism of Action of Antifungal Peptides from Microorganisms: A Review. Molecules 2021, 26, 3438.
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 582779.
- Helmy, N.M.; Parang, K. Cyclic Peptides with Antifungal Properties Derived from Bacteria, Fungi, Plants, and Synthetic Sources. Pharmaceuticals 2023, 16, 892.
- Cardoso, M.H.; Orozco, R.Q.; Rezende, S.B.; Rodrigues, G.; Oshiro, K.G.N.; Cândido, E.S.; Franco, O.L. Computer-aided design of antimicrobial peptides: Are we generating effective drug candidates? Front. Microbiol. 2020, 10, 3097.
- Masuda, Y. Bioactive 3D structures of naturally occurring peptides and their application in drug design. Biosci. Biotechnol. Biochem. 2021, 85, 24–32.
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596.
- Andrés, M.T.; Acosta-Zaldívar, M.; Fierro, J.F. Antifungal mechanism of action of lactoferrin: Identification of H+-ATPase (P3A-type) as a new apoptotic-cell membrane receptor. Antimicrob. Agents Chemother. 2016, 60, 4206–4216.
- Gruden, Š.; Poklar Ulrih, N. Diverse mechanisms of antimicrobial activities of lactoferrins, lactoferricins, and other lactoferrin-derived peptides. Int. J. Mol. Sci. 2021, 22, 11264.
- Leannec-Rialland, V.; Cabezas-Cruz, A.; Atanasova, V.; Chereau, S.; Ponts, N.; Tonk, M.; Vivinskas, A.; Ferrer, N.; Valdes, J.J.; Richard-Forget, F. Tick defensin γ-core reduces Fusarium graminearum growth and abrogates mycotoxins production with high efficiency. Sci. Rep. 2021, 11, 7962.
- Bezerra, L.P.; Freitas, C.D.T.; Silva, A.F.B.; Amaral, J.L.; Neto, N.A.S.; Silva, R.G.G.; Parra, A.L.C.; Goldman, G.H.; Oliveira, J.T.A.; Mesquita, F.P.; et al. Synergistic antifungal activity of synthetic peptides and antifungal drugs against Candida albicans and C. parapsilosis biofilms. Antibiotics 2022, 11, 553.
- Datta, L.P.; Mukherjee, T.; Das, T.K. Biomimetic pH responsive amphiphilic polymers: Solution property dependent antifungal mechanism. React. Funct. Polym. 2021, 165, 104937.
- Ramamourthy, G.; Park, J.; Seo, C.J.; Vogel, H.; Park, Y. Antifungal and antibiofilm activities and the mechanism of action of repeating lysine-tryptophan peptides against Candida albicans. Microorganisms 2020, 8, 758.
- Efremenko, E.; Aslanli, A.; Lyagin, I. Advanced situation with recombinant toxins: Diversity, production and application purposes. Int. J. Mol. Sci. 2023, 24, 4630.
- Jung, S.I.; Finkel, J.S.; Solis, N.V.; Chaili, S.; Mitchell, A.P.; Yeaman, M.R.; Filler, S.G. Bcr1 functions downstream of Ssd1 to mediate antimicrobial peptide resistance in Candida albicans. Eukaryot. Cell. 2013, 12, 411–419.
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers. 2018, 4, 18026.
- Pimienta, D.A.; Cruz Mosquera, F.E.; Palacios Velasco, I.; Giraldo Rodas, M.; Oñate-Garzón, J.; Liscano, Y. Specific focus on antifungal peptides against azole resistant Aspergillus fumigatus: Current status, challenges, and future perspectives. J. Fungi 2023, 9, 42.
- Laussel, C.; Léon, S. Cellular toxicity of the metabolic inhibitor 2-deoxyglucose and associated resistance mechanisms. Biochem. Pharmacol. 2020, 182, 114213.
- Sharma, L.; Bisht, G.S. Synergistic effects of short peptides and antibiotics against bacterial and fungal strains. J. Pept. Sci. 2023, 29, e3446.
- Aaghaz, S.; Sharma, K.; Maurya, I.K.; Rudramurthy, S.M.; Singh, S.; Kumar, V.; Tikoo, K.; Jain, R. Anticryptococcal activity and mechanistic studies of short amphipathic peptides. Arch. Pharm. 2023, 356, 2200576.
- Aguiar, T.K.B.; Feitosa, R.M.; Neto, N.A.S.; Malveira, E.A.; Gomes, F.I.R.; Costa, A.C.M.; Freitas, C.D.T.; Mesquita, F.P.; Souza, P.F.N. Giving a hand: Synthetic peptides boost the antifungal activity of itraconazole against Cryptococcus neoformans. Antibiotics 2023, 12, 256.
- Sharma, K.; Aaghaz, S.; Maurya, I.K.; Sharma, K.K.; Singh, S.; Rudramurthy, S.M.; Kumar, V.; Tikoo, K.; Jain, R. Synthetic amino acids-derived peptides target Cryptococcus neoformans by inducing cell membrane disruption. Bioorg. Chem. 2023, 130, 106252.
- Fernandes, K.E.; Payne, R.J.; Carter, D.A. Lactoferrin-derived peptide lactofungin is potently synergistic with amphotericin B. Antimicrob. Agents Chemother. 2020, 64, 10–1128.
- Tóth, L.; Poór, P.; Ördög, A.; Váradi, G.; Farkas, A.; Papp, C.; Bende, G.; . Tóth, G.K.; Rákhely, G.; Marx, F.; et al. The combination of Neosartorya (Aspergillus) fischeri antifungal proteins with rationally designed γ-core peptide derivatives is effective for plant and crop protection. BioControl 2022, 67, 249–262.
- Dos Reis, T.F.; de Castro, P.A.; Bastos, R.W.; Pinzan, C.F.; Souza, P.F.; Ackloo, S.; Hossain, M.A.; Drewry, D.H.; Alkhazraji, S.; Ibrahim, A.S.; et al. A host defense peptide mimetic, brilacidin, potentiates caspofungin antifungal activity against human pathogenic fungi. Nat. Commun. 2023, 14, 2052.
- Ding, K.; Shen, P.; Xie, Z.; Wang, L.; Dang, X. In vitro and in vivo antifungal activity of two peptides with the same composition and different distribution. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 252, 109243.
- Zhang, X.; Wang, M.; Zhu, X.; Peng, Y.; Fu, T.; Hu, C.H.; Cai, J.; Liao, G. Development of Lipo-γ-AA Peptides as potent antifungal agents. J. Med. Chem. 2022, 65, 8029–8039.
- Rodríguez-López, A.D.L.; Lee, M.R.; Wang, N.B.; Dunn, K.K.; Sanchez, H.; Raman, N.; Andes, D.R.; Lynn, D.M.; Palecek, S.P. Small-molecule morphogenesis modulators enhance the ability of 14-helical β-peptides to prevent Candida albicans biofilm formation. Antimicrob. Agents Chemother. 2019, 63, 10–1128.
- Ptaszyńska, N.; Gucwa, K.; Olkiewicz, K.; Heldt, M.; Serocki, M.; Stupak, A.; Martynow, D.; Dębowski, D.; Gitlin-Domagalska, A.; Lica, J.; et al. Conjugates of ciprofloxacin and levofloxacin with cell-penetrating peptide exhibit antifungal activity and mammalian cytotoxicity. Int. J. Mol. Sci. 2020, 21, 4696.
- Ptaszyńska, N.; Gucwa, K.; Olkiewicz, K.; Łȩgowska, A.; Okońska, J.; Ruczyński, J.; Gitlin-Domagalska, A.; Dȩbowski, D.; Milewski, S.; Rolka, K. Antibiotic-based conjugates containing antimicrobial HLopt2 peptide: Design, synthesis, antimicrobial and cytotoxic activities. ACS Chem. Biol. 2019, 14, 2233–2242.
- do Nascimento Dias, J.; de Souza Silva, C.; de Araújo, A.R.; Souza, J.M.T.; de Holanda Veloso Junior, P.H.; Cabral, W.F.; da Glória da Silva, M.; Eaton, P.; de Souza de Almeida Leite, J.R.; Nicola, A.M.; et al. Mechanisms of action of antimicrobial peptides ToAP2 and NDBP-5.7 against Candida albicans planktonic and biofilm cells. Sci. Rep. 2020, 10, 10327.
- Darwish, R.M.; Salama, A.H. A pilot study on ultrashort peptide with fluconazole: A promising novel anticandidal combination. Vet. World 2023, 16, 1284–1288.
- Galdiero, E.; de Alteriis, E.; De Natale, A.; D’Alterio, A.; Siciliano, A.; Guida, M.; Lombardi, L.; Falanga, A.; Galdiero, S. Eradication of Candida albicans persister cell biofilm by the membranotropic peptide gH625. Sci. Rep. 2020, 10, 5780.
- Tao, Y.; Bie, X.M.; Lv, F.X.; Zhao, H.Z.; Lu, Z.X. Antifungal activity and mechanism of fengycin in the presence and absence of commercial surfactin against Rhizopus stolonifer. J. Microbiol. 2011, 49, 146–150.
- Ai, L.; Fu, S.; Li, Y.; Zuo, M.; Huang, W.; Huang, J.; Jin, Z.; Chen, Y. Natural products-based: Synthesis and antifungal activity evaluation of novel L-pyroglutamic acid analogues. Front. Plant Sci. 2022, 13, 1102411.
- Lyagin, I.; Aslanli, A.; Domnin, M.; Stepanov, N.; Senko, O.; Maslova, O.; Efremenko, E. Metal nanomaterials and hydrolytic enzyme-based formulations for improved antifungal activity. Int. J. Mol. Sci. 2023, 24, 11359.
- Efremenko, E.; Stepanov, N.; Aslanli, A.; Lyagin, I.; Senko, O.; Maslova, O. Combination of enzymes with materials to give them antimicrobial features: Modern trends and perspectives. J. Funct. Biomater. 2023, 14, 64.
- Aslanli, A.; Domnin, M.; Stepanov, N.; Efremenko, E. “Universal” Antimicrobial combination of bacitracin and His6-OPH with lactonase activity, acting against various bacterial and yeast cells. Int. J. Mol. Sci. 2022, 23, 9400.
- Aslanli, A.; Domnin, M.; Stepanov, N.; Efremenko, E. Synergistic antimicrobial action of lactoferrin-derived peptides and quorum quenching enzymes. Int. J. Mol. Sci. 2023, 24, 3566.
- Tevyashova, A.; Efimova, S.; Alexandrov, A.; Omelchuk, O.; Ghazy, E.; Bychkova, E.; Zatonsky, G.; Grammatikova, N.; Dezhenkova, L.; Solovieva, S.; et al. Semisynthetic amides of amphotericin B and nystatin A1: A comparative study of in vitro activity/toxicity ratio in relation to selectivity to ergosterol membranes. Antibiotics 2023, 12, 151.
- Bezerra, L.P.; Silva, A.F.; Santos-Oliveira, R.; Alencar, L.M.; Amaral, J.L.; Neto, N.A.; Silva, R.G.; Belém, M.O.; de Andrade, C.R.; Oliveira, J.T.; et al. Combined antibiofilm activity of synthetic peptides and antifungal drugs against Candida spp. Future Microbiol. 2022, 17, 1133–1146.
- Khabbaz, H.; Karimi-Jafari, M.H.; Saboury, A.A.; BabaAli, B. Prediction of antimicrobial peptides toxicity based on their physico-chemical properties using machine learning techniques. BMC Bioinform. 2021, 22, 549.
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Joakim Hakansson, J.; Hansen, P.R.; Svenson, J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206.
More
Information
Subjects:
Mycology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
626
Revisions:
2 times
(View History)
Update Date:
31 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




