Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hongliang Zhu | -- | 3463 | 2023-10-27 09:46:42 | | | |
| 2 | Sirius Huang | Meta information modification | 3463 | 2023-10-27 11:17:34 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cheng, K.; Zhang, C.; Lu, Y.; Li, J.; Tang, H.; Ma, L.; Zhu, H. Roles of GR-RBPs in RNA Metabolism. Encyclopedia. Available online: https://encyclopedia.pub/entry/50865 (accessed on 07 February 2026).
Cheng K, Zhang C, Lu Y, Li J, Tang H, Ma L, et al. Roles of GR-RBPs in RNA Metabolism. Encyclopedia. Available at: https://encyclopedia.pub/entry/50865. Accessed February 07, 2026.
Cheng, Ke, Chunjiao Zhang, Yao Lu, Jinyan Li, Hui Tang, Liqun Ma, Hongliang Zhu. "Roles of GR-RBPs in RNA Metabolism" Encyclopedia, https://encyclopedia.pub/entry/50865 (accessed February 07, 2026).
Cheng, K., Zhang, C., Lu, Y., Li, J., Tang, H., Ma, L., & Zhu, H. (2023, October 27). Roles of GR-RBPs in RNA Metabolism. In Encyclopedia. https://encyclopedia.pub/entry/50865
Cheng, Ke, et al. "Roles of GR-RBPs in RNA Metabolism." Encyclopedia. Web. 27 October, 2023.
Copy Citation
Glycine-rich RNA binding proteins (GR-RBPs), a branch of RNA binding proteins (RBPs), play integral roles in regulating various aspects of RNA metabolism regulation, such as RNA processing, transport, localization, translation, and stability, and ultimately regulate gene expression and cell fate.
glycine-rich RNA-binding protein
post-transcriptional regulation
1. Introduction
Gene expression in plants is typically subject to meticulous regulation at both the transcriptional and post-transcriptional strata. This intricate regulation assumes paramount importance, as it governs the growth and developmental processes of these organisms, while concurrently facilitating their ability to respond to and adapt to a myriad of environmental stimuli. RNA-binding proteins (RBPs) are central regulatory factors controlling post-transcriptional RNA metabolism during plant growth, development, and stress responses [1][2].
RBPs, a general term denoting ubiquitous proteins with the capacity to bind to RNA, interact with RNAs to form dynamic ribonucleoprotein (RNP), thereby assuming a central role in overseeing the destiny and functionality of RNA throughout its intricate life cycle [3]. As such, RBPs serve as pivotal orchestrators in the realm of gene expression by governing various facets, including RNA synthesis, processing (involving capping, splicing, and polyadenylation), editing, transportation, storage, surveillance, quality control, functionality, translation, and eventual RNA turnover. It is pertinent to note that modifications in RNA structure and spatial conformation may induce alterations in the specific RBPs bound to it, consequently giving rise to divergent biological functions [4]. Broadly, RBPs are characterized by the presence of putative RNA-binding domains, including, but not limited to, RNA recognition motifs (RRMs), K homology domains, zinc fingers, DEAD/DEAH boxes, Pumilio/FBF domains, and pentatricopeptide repeat (PPR) domains. Moreover, plant RBPs feature modular auxiliary domains rich in glycine, arginine, and serine residues, presenting diverse configurations [5]. Currently, 1145 RBPs have been captured by RNA interactome capture (RIC), of which 595 were discovered to be novel RBPs candidates [6][7]. RNA-binding proteins are strategically located within various cellular compartments to carry out specific functions, ensuring proper cellular functioning. For instance, AtGRDP2 interacts with proteins involved in RNA processing and translation in different compartments: it associates with PABN3 in the nucleus, EF-1α in the cytosol, and CL15 in chloroplasts [8]. While the functional roles of various RBPs in living organisms have been elucidated over the past few decades, it is evident that the functional roles of RBPs in plants still remain significantly underdeveloped [9].
2. Structural Features of the GR-RBPs
Glycine-rich RNA-binding proteins can be systematically categorized into four discernible classes, denoted as Class I through IV. Class I is characterized by the presence of an RNA recognition motif (RRM) domain in conjunction with a glycine-rich (GR) motif. Class II features a single RRM domain alongside two glycine-rich (GR) motifs, with a Cys3His (CCHC) zinc finger motif positioned between these two glycine-rich domains. Class III stands out due to its unique configuration, where the N-terminal RRM domain is replaced by a cold-shock domain (CSD), followed by two GR-CCHC motifs. Lastly, Class IV is distinguished by the presence of two RRM domains and a GR domain (Figure 1) [10]. These diverse structural domains within GR-RBPs fulfill a spectrum of distinct functional roles.
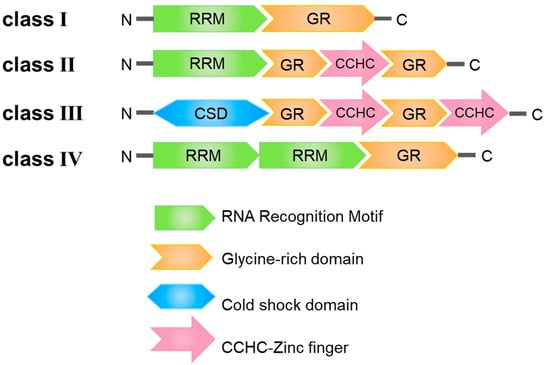
Figure 1. Structural domain characterization of four classes of glycine-rich RNA-binding proteins (GR-RBPs) in plants. RRM: RNA Recognition Motif; GR: Glycine-rich domain; CSD: Cold shock domain; CCHC: CCHC-Zinc finger.
2.1. Glycine-Rich Domain
Glycine-rich proteins were first identified in Petunia in 1986 [11]. They are characterized by glycine motifs consisting of repeating glycine residues, with glycine content ranging from 20% to 70% [10]. Glycine-rich domains are quite prevalent across various organisms, initially leading to the classification of proteins rich in glycine domains into a superfamily [12]. However, some studies have suggested that GRPs may not constitute a distinct protein superfamily, but rather a group of proteins sharing only certain repetitive structural motifs [13]. Within the realm of plants, the diversity in expression patterns, bioregulation, and subcellular localization of such proteins implies that different motif types perform distinct functions. Therefore, multiple GRPs within an organism are likely involved in various physiological and biochemical processes.
GR-RBPs can be distinguished from other GRPs by the presence of nucleic acid binding domains. Furthermore, in addition to glycine, the GR domain also encompasses substantial quantities of aromatic and basic amino acids [14]. The GR domain plays a primary role in mediating interactions between GR-RBPs and other proteins [15]. Meanwhile, the GR domain is an intrinsically disordered region that can drive the onset of phase separation [16]. In Arabidopsis, the glycine-enriched RNA-binding proteins AtRBGD2 and AtRBGD4 can improve the heat tolerance of plants by phase separation [17].
Proteins containing the GR domain have the capacity to induce the formation of discontinuous secondary structures. One is the glycine loop, which forms a nylon clasp-like structure by the interaction between the helices [18]. The other is a structure consisting of β-folds comprising a variable number of anti-parallel chains. The side chains of non-glycine amino acids are oriented toward the same side of the β-fold, resulting in a hydrophobic domain surface that strongly interacts with the hydrophobic regions of its binding partner proteins and macromolecules, thus facilitating the binding of GRP to other proteins [19]. Generally, the GR domain primarily mediates protein–protein interactions. However, it should be noted that the GR domain has also been found to be involved in RNA binding, as seen in tobacco cells where the glycine-rich domain of RZ-1 is implicated in RNA binding [20]. In addition, glycine-rich proteins are structural components of plant cell wall proteins [21]. In rice, OsGRP1 was found to regulate cell elongation of the root [22].
2.2. RNA Recognition Motif
The RNA recognition motif (RRM), also known as the RNA-binding domain (RBD) or ribonucleoprotein domain (RNP), represents the most extensively studied RNA-binding protein motif [23]. RRM was first identified in 1988 [24]. An RRM typically comprises approximately 90 amino acid residues, featuring two conserved sequences referred to as RNP1 and RNP2 [24][25]. RNP1 incorporates eight conserved residues, primarily aromatic and positively charged amino acids, denoted as Lys/Arg-Gly-Phe/Tyr-Gly/Ala-Phe/Tyr-Val/Ile/Leu-X-Phe/Tyr, where X can denote any amino acid. In contrast, the sequence of RNP2 exhibits lower conservation compared to RNP1 and consists of a six-residue sequence Ile/Val/Leu-Phe/Tyr-Ile/Val/Leu-X-Asn-Leu located at the N-terminus of the domain. The typical RRM adopts a structural configuration comprising four anti-parallel beta-strands and two alpha-helices, organized into a β1α1β2β3α2β4 sandwich structure. Notably, RNP1 and RNP2 are situated at β3 and β1, respectively [25].
In eukaryotic proteins, RRMs are frequently encountered in multiple copies within a single protein and may coexist with various other domains for binding single-stranded nucleic acids. Notable examples include zinc fingers of the CCCH and CCHC types, the C-terminal domain of polyadenylate-binding proteins (PABP or PABC), and the WW domain [26][27]. Through their interactions with diverse protein domains, the RRM domain can finely tune its RNA-binding affinity and specificity, thereby diversifying its range of biological functions. Mutations occurring within the RRM domain frequently result in a notable reduction or complete abrogation of RNA binding capability [28]. In addition, the affinity of the RRM for RNA binding is influenced by factors such as the N- and C-terminal regions, interdomain linker, and the secondary structure of RNA [29][30]. Moreover, biochemical and structural studies have demonstrated that the RRM not only participates in RNA recognition but also plays a role in mediating protein–protein interactions. The RNA recognition motif (RRM) domain within the protein eIF3b has the capacity to engage with 69 distinct peptides located at the N-terminus of eIF3j. This interaction assumes a pivotal role in the initiation of protein synthesis within eukaryotic cells by eIF3b [31]. In rice, the RRM domain and GR domain of RBP-P are essential for its association with RBP-L and RBP-208 [15].
2.3. Cold-Shock Domain
The most prominent distinction among the four categorized groups of GR-RBPs is the unique inclusion of the cold-shock domain (CSD), a feature exclusively found in class III. Consequently, this specific class of GR-RBPs is commonly referred to as CSD proteins (CSDPs) due to the presence of the CSD domain [32]. The capacity of the cold-shock domain (CSD) to enhance the binding of CSD proteins to RNA, single-stranded DNA (ssDNA), and double-stranded DNA (dsDNA) bestows upon them a critical role in the regulation of responses to low temperatures, embryogenesis, flowering time, and fruit development [33].
CSD was first identified in Escherichia coli in 1987 [34]. CSD-containing proteins are predominantly observed in bacteria, accounting for 90.9% of occurrences, with 7.3% found in eukaryotes, and plants constituting 0.94% [35]. Eukaryotic CSDs and bacterial CSPs exhibit striking similarity in terms of length, both being approximately 70 amino acids long, and they share conserved sequences, notably RNP1 and RNP2 [36]. In animal and bacterial cells, cold-shock proteins containing CSDs are highly induced in response to cold stress, where they function as RNA chaperone proteins to facilitate efficient translation at low temperatures [37]. In larger eukaryotic proteins, one to five (or even more) CSDs are commonly found, typically associated with naturally unfolded polypeptide regions. A noteworthy observation is that 20% of proteins featuring CSD also possess Zinc Finger CCHC domains, a characteristic shared with class III GR-RBPs. The coexistence of these structural domains may allow CSD-containing proteins to be more diverse in terms of structural domain organization and cellular function [9].
2.4. CCHC Motif
The CCHC motif, also referred to as the zinc knuckle, is present in both class II and class III. It represents a small structural motif characterized by multiple finger-like protrusions that establish tandem contacts with their target molecules. Some of these domains have an affinity for binding metal ions such as zinc and iron [38]. Generally speaking, the CCHC motif shares the consensus sequence CX2CX4HX4C (X for any amino acid, numbers for the number of residues, C and H for cysteine and histidine, respectively) [39]. CHC motifs with a high affinity for DNA and RNA typically consist of a short helix and two short β-strands connected through a zinc knuckle structure. These motifs play crucial roles in regulating the function of nucleic acids and participating in transcriptional or translational regulation by binding to target RNA [40].
Proteins containing the CCHC motif are prevalent in plants, often occurring in tandem with other domains, which could be pertinent to specific biological functions [41]. In the context of cold resistance, the presence of both the CSD at the N-terminus and the CCHC motif at the C-terminus, along with the glycine enrichment domain, collectively form the cold-shock domain proteins in plants. In wheat, for instance, out of the 50 CCHC motif-containing proteins, 28 are tandemly arranged with the RRM domain, and 8 are associated with the CSD domain. Promoter analyses of the TaCCHC-ZFP genes have revealed the presence of 636 cis-elements responsive to environmental stress and phytohormones. This finding strongly suggests that this domain plays a pivotal role in plant development and stress responses mediated by phytohormones [42]. The count of CCHC motifs varies among cold-shock domain proteins (CSDPs), and the organization of zinc finger domains, both in terms of their number and length, has been documented to play a crucial role in the complete functionality of CSDPs during cold adaptation. In the case of cabbage (Brassica oleracea), allelic variation in BoCSDP5 with seven CCHC-type zinc fingers exhibits significantly greater resistance to low temperatures when compared to BoCSDP5 variants containing six CCHC-type zinc fingers [43]. GR-RBPs that incorporate CCHC zinc finger motifs are referred to as zinc finger-containing glycine-rich RNA-binding proteins (RZs). Transcript levels of Arabidopsis AtRZ-1a, AtRZ-1b, and AtRZ-1c were significantly elevated in response to cold stress treatments [44][45]. Furthermore, three members of the RZ family, namely TaRZ1, TaRZ2, and TaRZ3, each harboring CCHC domains, have demonstrated distinctive responsiveness to distinct abiotic stressors [46].
3. Roles of GR-RBPs in RNA Metabolism
The presence of the RRM domain endows RNA-binding proteins with involvement in various facets of RNA processing metabolism. These include 5′-capping, alternative splicing, 3′ polyadenylation, RNA stability, and translation. Ultimately, these RNA-binding proteins play a pivotal role in regulating gene expression as well as plant growth and development [47]. The subsections below provide a detailed description of several RNA processing functions mediated by glycine-rich RNA-binding proteins, emphasizing their significance in plant regulation.
3.1. RNA Alternative Splice and Polyadenylation
Polyadenylation of the 3′-terminus of precursor mRNA plays an important role in eukaryotic gene expression and regulation. This process comprises two closely linked biochemical steps: the initial cleavage at the poly(A) sites (PAS) of the precursor mRNA, followed by the addition of the poly(A) tail [48]. The selection of PAS is variable, a phenomenon known as alternative polyadenylation (APA), which represents a widespread mechanism for regulating gene expression across various species [49]. APA adds complexity to the transcriptome and can regulate the function, stability, localization, and translation efficiency of target RNAs [49].
AtRBGD5, also known as HLP1, plays a pivotal role in the selection of polyadenylation sites at the 3′ end of precursor mRNAs in Arabidopsis (Figure 2) [28]. HLP1 is notably enriched in transcripts associated with RNA metabolism and flowering processes. The homozygous deletion mutant of HLP1, known as hlp1-1, exhibits a late flowering phenotype. CLIP-seq analysis has revealed that HLP1 exhibits a preferential binding affinity for A- and U-rich motifs located in proximity to cleavage and polyadenylation sites. A comprehensive examination of poly(A) site usage has shown that HLP1 deletion mutations lead to the translocation of thousands of poly(A) sites. Notably, the flowering regulator FCA is identified as a direct target of HLP1. HLP1 actively promotes polyadenylation at the distal site of FCA. Consequently, when HLP1 is mutated, there is a shift of the poly(A) site (PSA) from distal to proximal, resulting in the upregulation of FLC and a delay in flowering.
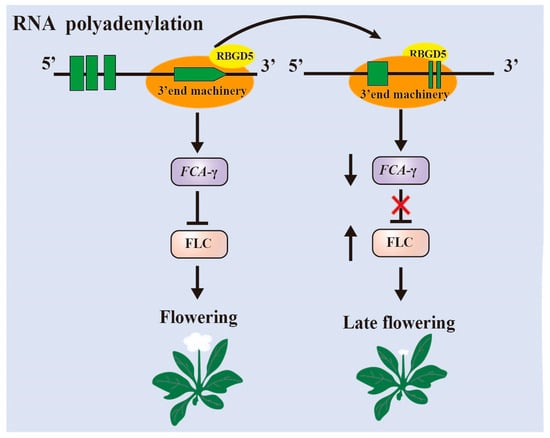
Figure 2. RBGD5 affects the selection of polyadenylation sites at the 3′ end of Arabidopsis FCA precursor mRNAs. The shift of the Poly(A) site from distal to proximal when RBGD5 is mutated results in upregulation of FLC and delayed flowering.
Notably, AtGRP7 has also been documented to bind to antisense precursor mRNAs of FLC and to associate closely with the polyadenylation site (PAS) of the proximal COOLAIR structural type. The loss of AtGRP7 function results in a decrease in the proximal-distal polyadenylation ratio and an increase in the overall abundance of FLC transcripts, ultimately leading to a delay in flowering time [50]. Moreover, AtGRP7 is the first glycine-rich protein in plants known to regulate alternative splicing through direct binding to pre-mRNAs [51]. Furthermore, it has been reported that two functionally redundant RNA-binding proteins, RZ-1B and RZ-1C (collectively referred to as RZ-1) in Arabidopsis, play a vital role in regulating RNA splicing. The loss of RZ-1 function leads to pleiotropic phenotypes, including delayed seed germination, accompanied by the defective splicing of numerous genes and a global perturbation of gene expression. AtRZ-1c, specifically, can bind to FLC, thereby promoting the efficient splicing of FLC introns and repressing FLC transcription, which in turn affects flowering time in Arabidopsis [52].
3.2. miRNA Biogenesis
Post-transcriptional regulation holds a significant role in coordinating gene expression in eukaryotes. Among the crucial players in this RNA-level control are microRNAs (miRNAs). In plants, the significance of miRNAs is underscored by the occurrence of severe developmental defects in mutants with impaired miRNA biogenesis. miRNAs are generated through the endonucleolytic cleavage of long primary microRNAs (pri-miRNAs) featuring internal stem-loop structures. RNA binding proteins (RBPs) play a specific role by interacting with well-defined sequence motifs, thereby exerting control over the generation of miRNAs [5][53].
It has been observed that AtGRP7 exerts an impact on the inventory of miRNAs (Figure 3). The overexpression of AtGRP7 resulted in a significant decrease in the levels of 30 miRNAs and an increase in the levels of 14 miRNAs. Simultaneously, there is an over-accumulation of certain pri-miRNAs, including pri-miR398b, pri-miR398c, pri-miR172b, pri-miR159a, and pri-miR390 upon the upregulation of AtGRP7. Interestingly, the expression levels of their corresponding mature miRNAs are decreased. RNA immunoprecipitation experiments have demonstrated that AtGRP7 directly binds to these pri-miRNAs in vivo, thereby affecting their processing and inhibiting the synthesis of the corresponding mature miRNAs [54]. More recently, researchers have also shown that AtRZ1A is involved in miR398 biogenesis. In atrz-1a mutants, there is a decrease in the steady-state levels of mature miR398, along with a decrease in pri-miR398b levels [55]. This decrease may be attributed to the fact that AtRZ-1a appears to specifically influence the stability of pri-miR398b. Interestingly, these findings contrast with the results observed with AtGRP7, suggesting that GR-RBPs regulate miRNA biogenesis through different mechanisms.
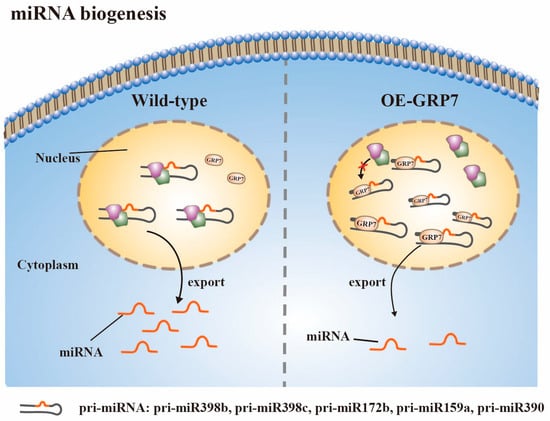
Figure 3. AtGRP7 directly binds pri-miRNAs (pri-miR398b, pri-miR398c, pri-miR172b, pri-miR159a, pri-miR390) in vivo, affecting their processing. Overexpression of AtGRP7 results in an increase in some pri-miRNAs and a decrease in their corresponding mature miRNAs.
The interaction between miRNAs and RBPs offers a broader perspective for studying the mechanisms underlying growth, development, and stress responses in plants. There is still much to uncover about the synthesis, transport, and degradation of GR-RBPs and miRNAs, and these aspects promise to unveil further insights into the intricate processes governing plant biology.
3.3. RNA Assembly by Liquid–Liquid Phase Separation
In cell biology, the concept of liquid–liquid phase separation (LLPS) refers to a state in which biomolecules become spatially concentrated within the cell, leading to the formation of various membraneless cytoplasmic and nuclear compartments. There is a growing body of evidence indicating that LLPS is closely intertwined with several fundamental cellular processes, serving a crucial role in the regulation of gene expression, cell division, signal transduction, stress responses, cytoskeletal dynamics, supramolecular assembly, and more [56]. In plants, LLPS has been reported to play a role in sensing high temperatures, water potential, phytohormones, and responses to pathogens [57]. Currently, significant breakthroughs are being achieved in the functional studies of phase separation in plants, shedding light on its intricate mechanisms [58].
In 2018, it was discovered that AtGRP7 interacts with receptor kinase FERONIA (FER) and undergoes phosphorylation by FER at six serine/tyrosine residues, which enhances the GRP7 protein accumulation in the nucleus and increases the ability of GRP7 to bind its target mRNAs [59]. The FER-dependent phosphorylation of GRP7 enhances its association with the spliceosome component U1-70K, facilitating splice site selection and thereby modulating dynamic alternative splicing (AS). In response to changing external environmental conditions, AtGRP7 acts through the Rapid Alkalinization Factor 1 (RALF1)-FERONIA signaling pathway. It regulates the alternative splicing of its target genes, allowing for adjustments to the transcriptome in response to various stresses in plants. This mechanism highlights the role of AtGRP7 in fine-tuning gene expression in plants under changing environmental conditions.
Recent findings have revealed that FER plays a pivotal role in mediating the liquid–liquid phase separation (LLPS) of GRP7 in response to temperature fluctuations in Arabidopsis (Figure 4) [60]. Under conditions of temperature shifts, GRP7 in the cytoplasm serves as a scaffold protein, recruiting RNA and other molecules, including the RNA chaperones CSP1 and CSP3, as well as the translation-related protein eIF4E. This assembly of molecules undergoes LLPS, leading to the formation of stress granules (SGs). These SGs facilitate the assembly and sequestration of RNA, ultimately blocking translation, thereby ensuring normal root elongation. Conversely, the grp7 mutant was sensitive to fluctuating temperature change, with poor growth and shortened primary root length under the same conditions. Additionally, FER-dependent phosphorylation is crucial for regulating GRP7 phase separation. In fer mutant plants, the LLPS ability of GRP7 is diminished, leading to a phenotype similar to that of the grp7 mutant, which in turn hampers root growth. It is noteworthy that GRP7 in the nucleus also undergoes phase separation, along with a component of the spliceosome, U1-70K. This suggests that phase separation may also be involved in RNA alternative splicing processes.
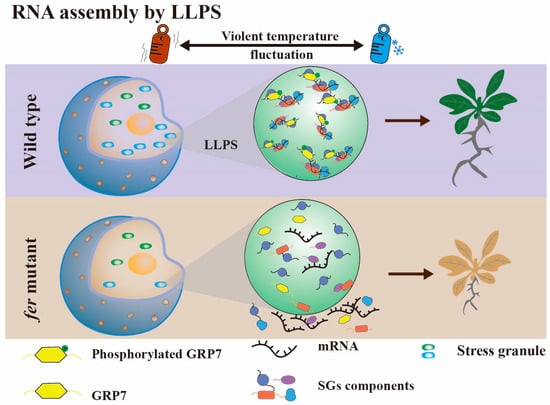
Figure 4. When the temperature changes violently between hot (red thermometer) and cold (blue thermometer), phosphorylated GRP7 in the cytoplasm can be used as a scaffold protein to recruit RNA and other proteins to form SGs by liquid–liquid phase separation, which facilitates RNA assembly and subsequently blocks translation to ensure normal root elongation.
3.4. Translation of RNA
Translation is a fundamental process within the genetic central dogma of molecular biology, playing a crucial role in ensuring the accurate execution of RNA functions. At present, the GR-RBPs that are directly involved in the translation of RNA targets remain largely unknown.
Recent research has highlighted the role of a glycine-rich RNA-binding protein (GR-RBPs), SlRBP1, in the maintenance of chloroplast function in tomato (Figure 5) [61]. SlRBP1 exhibits specific binding to genes associated with photosynthesis and chloroplast function, including β carbonic anhydrase 1 (βCA1), Rubisco activase (RCA), and photosystem I reaction centre subunit II (PsaD). Furthermore, SlRBP1 interacts with SleIF4A2, and their collaboration serves to uphold chloroplast function by enhancing the translatability of transcripts associated with photosynthesis. In cases where SlRBP1 is non-functional, the transcription levels of its target genes remain unchanged, but translation is significantly impaired. Consequently, this leads to structural abnormalities in chloroplasts, a decreased rate of photosynthesis, and the development of dwarf tomato plants with yellow leaves. This research underscores the critical role of SlRBP1 in regulating chloroplast function, and, by extension, plant growth and development.
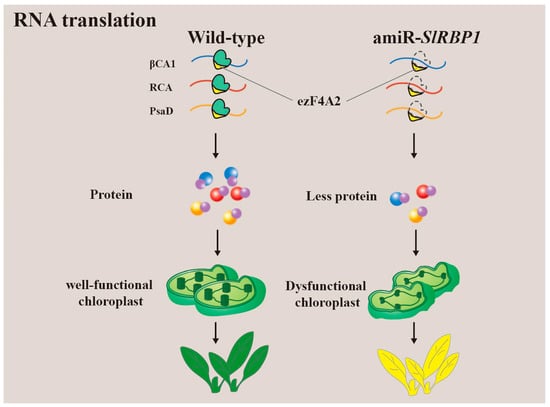
Figure 5. SlRBP1 interacts with SleIF4A2 to together maintain chloroplast function by ensuring translation of photosynthesis-associated transcripts. Loss-of-function of SlRBP1 results in impaired chloroplast ultrastructure, downregulated photosynthesis rate, and dwarf tomato plants with yellow leaves.
References
- Muthusamy, M.; Kim, J.H.; Kim, J.A.; Lee, S.I. Plant RNA Binding Proteins as Critical Modulators in Drought, High Salinity, Heat, and Cold Stress Responses: An Updated Overview. Int. J. Mol. Sci. 2021, 22, 6731.
- Joshna, C.R.; Saha, P.; Atugala, D.; Chua, G.; Muench, D.G. Plant PUF RNA-binding proteins: A wealth of diversity for post-transcriptional gene regulation. Plant Sci. 2020, 297, 110505.
- Hentze, M.W.; Castello, A.; Schwarzl, T.; Preiss, T. A brave new world of RNA-binding proteins. Nat. Rev. Mol. Cell Biol. 2018, 19, 327–341.
- Marondedze, C. The increasing diversity and complexity of the RNA-binding protein repertoire in plants. Proc. Biol. Sci. 2020, 287, 20201397.
- Lorković, Z.J. Role of plant RNA-binding proteins in development, stress response and genome organization. Trends Plant Sci. 2009, 14, 229–236.
- Marondedze, C.; Thomas, L.; Serrano, N.L.; Lilley, K.S.; Gehring, C. The RNA-binding protein repertoire of Arabidopsis thaliana. Sci. Rep. 2016, 6, 29766.
- Bach-Pages, M.; Castello, A.; Preston, G.M. Plant RNA Interactome Capture: Revealing the Plant RBPome. Trends Plant Sci. 2017, 22, 449–451.
- Castro-Bustos, S.; Maruri-Lopez, I.; Ortega-Amaro, M.A.; Serrano, M.; Ovando-Vazquez, C.; Jimenez-Bremont, J.F. An interactome analysis reveals that Arabidopsis thaliana GRDP2 interacts with proteins involved in post-transcriptional processes. Cell Stress Chaperones 2022, 27, 165–176.
- Amir, M.; Kumar, V.; Dohare, R.; Islam, A.; Ahmad, F.; Hassan, M.I. Sequence, structure and evolutionary analysis of cold shock domain proteins, a member of OB fold family. J. Evol. Biol. 2018, 31, 1903–1917.
- Czolpinska, M.; Rurek, M. Plant Glycine-Rich Proteins in Stress Response: An Emerging, Still Prospective Story. Front. Plant Sci. 2018, 9, 302.
- Condit, C.M.; Meagher, R.B. Expression of a gene encoding a glycine-rich protein in petunia. Mol. Cell Biol. 1987, 7, 4273–4279.
- Mangeon, A.; Junqueira, R.M.; Sachetto-Martins, G. Functional diversity of the plant glycine-rich proteins superfamily. Plant Signal. Behav. 2010, 5, 99–104.
- Kar, B.; Nayak, S.; Joshi, R.K. Classification and comparative analysis of Curcuma longa L. expressed sequences tags (ESTs) encoding glycine-rich proteins (GRPs). Bioinformation 2012, 8, 142–146.
- Tang, B.; Lummis, S.C.R. The roles of aromatic residues in the glycine receptor transmembrane domain. BMC Neurosci. 2018, 19, 53.
- Tian, L.; Chou, H.L.; Zhang, L.; Hwang, S.K.; Starkenburg, S.R.; Doroshenk, K.A.; Kumamaru, T.; Okita, T.W. RNA-Binding Protein RBP-P Is Required for Glutelin and Prolamine mRNA Localization in Rice Endosperm Cells. Plant Cell 2018, 30, 2529–2552.
- Lancaster, A.K.; Nutter-Upham, A.; Lindquist, S.; King, O.D. PLAAC: A web and command-line application to identify proteins with prion-like amino acid composition. Bioinformatics 2014, 30, 2501–2502.
- Zhu, S.; Gu, J.; Yao, J.; Li, Y.; Zhang, Z.; Xia, W.; Wang, Z.; Gui, X.; Li, L.; Li, D.; et al. Liquid-liquid phase separation of RBGD2/4 is required for heat stress resistance in Arabidopsis. Dev. Cell 2022, 57, 583–597.
- O’Shea, E.K.; Lumb, K.J.; Kim, P.S. Peptide ‘Velcro’: Design of a heterodimeric coiled coil. Curr. Biol. 1993, 3, 658–667.
- Steinert, P.M.; Mack, J.W.; Korge, B.P.; Gan, S.Q.; Haynes, S.R.; Steven, A.C. Glycine loops in proteins: Their occurrence in certain intermediate filament chains, loricrins and single-stranded RNA binding proteins. Int. J. Biol. Macromol. 1991, 13, 130–139.
- Hanano, S.; Sugita, M.; Sugiura, M. Isolation of a novel RNA-binding protein and its association with a large ribonucleoprotein particle present in the nucleoplasm of tobacco cells. Plant Mol. Biol. 1996, 31, 57–68.
- Ringli, C.; Keller, B.; Ryser, U. Glycine-rich proteins as structural components of plant cell walls. Cell Mol. Life Sci. 2001, 58, 1430–1441.
- Xu, D.; Lei, M.; Wu, R. Expression of the rice Osgrpl promoter-Gus reporter gene is specifically associated with cell elongation/expansion and differentiation. Plant Mol. Biol. 1995, 28, 455–471.
- Clery, A.; Blatter, M.; Allain, F.H. RNA recognition motifs: Boring? Not quite. Curr. Opin. Struct. Biol. 2008, 18, 290–298.
- Dreyfuss, G.; Swanson, M.S.; Piñol-Roma, S. Heterogeneous nuclear ribonucleoprotein particles and the pathway of mRNA formation. Trends Biochem. Sci. 1988, 13, 86–91.
- Maris, C.; Dominguez, C.; Allain, F.H. The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. FEBS J. 2005, 272, 2118–2131.
- Hudson, B.P.; Martinez-Yamout, M.A.; Dyson, H.J.; Wright, P.E. Recognition of the mRNA AU-rich element by the zinc finger domain of TIS11d. Nat. Struct. Mol. Biol. 2004, 11, 257–264.
- Sudol, M.; Sliwa, K.; Russo, T. Functions of WW domains in the nucleus. FEBS Lett. 2001, 490, 190–195.
- Zhang, Y.; Gu, L.; Hou, Y.; Wang, L.; Deng, X.; Hang, R.; Chen, D.; Zhang, X.; Zhang, Y.; Liu, C.; et al. Integrative genome-wide analysis reveals HLP1, a novel RNA-binding protein, regulates plant flowering by targeting alternative polyadenylation. Cell Res. 2015, 25, 864–876.
- Allain, F.H.; Gilbert, D.E.; Bouvet, P.; Feigon, J. Solution structure of the two N-terminal RNA-binding domains of nucleolin and NMR study of the interaction with its RNA target. J. Mol. Biol. 2000, 303, 227–241.
- Varani, L.; Gunderson, S.I.; Mattaj, I.W.; Kay, L.E.; Neuhaus, D.; Varani, G. The NMR structure of the 38 kDa U1A protein—PIE RNA complex reveals the basis of cooperativity in regulation of polyadenylation by human U1A protein. Nat. Struct. Biol. 2000, 7, 329–335.
- ElAntak, L.; Tzakos, A.G.; Locker, N.; Lukavsky, P.J. Structure of eIF3b RNA recognition motif and its interaction with eIF3j: Structural insights into the recruitment of eIF3b to the 40 S ribosomal subunit. J. Biol. Chem. 2007, 282, 8165–8174.
- Chaikam, V.; Karlson, D.T. Comparison of structure, function and regulation of plant cold shock domain proteins to bacterial and animal cold shock domain proteins. BMB Rep. 2010, 43, 1–8.
- Sasaki, K.; Imai, R. Pleiotropic roles of cold shock domain proteins in plants. Front. Plant Sci. 2011, 2, 116.
- Jones, P.G.; VanBogelen, R.A.; Neidhardt, F.C. Induction of proteins in response to low temperature in Escherichia coli. J. Bacteriol. 1987, 169, 2092–2095.
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419.
- Heinemann, U.; Roske, Y. Cold-Shock Domains-Abundance, Structure, Properties, and Nucleic-Acid Binding. Cancers 2021, 13, 190.
- Kim, J.S.; Park, S.J.; Kwak, K.J.; Kim, Y.O.; Kim, J.Y.; Song, J.; Jang, B.; Jung, C.H.; Kang, H. Cold shock domain proteins and glycine-rich RNA-binding proteins from Arabidopsis thaliana can promote the cold adaptation process in Escherichia coli. Nucleic Acids Res. 2007, 35, 506–516.
- Radkova, M.; Revalska, M.; Zhiponova, M.; Iantcheva, A. Evaluation of the role of Medicago truncatula Zn finger CCHC type protein after heterologous expression in Arabidopsis thaliana. Biotechnol. Biotechnol. Equip. 2021, 35, 1686–1695.
- Summers, M.F. Zinc finger motif for single-stranded nucleic acids? Investigations by nuclear magnetic resonance. J. Cell Biochem. 1991, 45, 41–48.
- Wang, Y.; Yu, Y.; Pang, Y.; Yu, H.; Zhang, W.; Zhao, X.; Yu, J. The distinct roles of zinc finger CCHC-type (ZCCHC) superfamily proteins in the regulation of RNA metabolism. RNA Biol. 2021, 18, 2107–2126.
- Han, G.; Qiao, Z.; Li, Y.; Wang, C.; Wang, B. The Roles of CCCH Zinc-Finger Proteins in Plant Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 8327.
- Sun, A.; Li, Y.; He, Y.; Zou, X.; Chen, F.; Ji, R.; You, C.; Yu, K.; Li, Y.; Xiao, W.; et al. Comprehensive Genome-Wide Identification, Characterization, and Expression Analysis of CCHC-Type Zinc Finger Gene Family in Wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 13, 892105.
- Song, H.; Kim, H.; Hwang, B.H.; Yi, H.; Hur, Y. Natural variation in glycine-rich region of Brassica oleracea cold shock domain protein 5 (BoCSDP5) is associated with low temperature tolerance. Genes Genom. 2020, 42, 1407–1417.
- Zhang, J.H.; Zhao, Y.X.; Xiao, H.L.; Zheng, Y.L.; Yue, B. Genome-wide identification, evolution, and expression analysis of RNA-binding glycine-rich protein family in maize. J. Integr. Plant Biol. 2014, 56, 1020–1031.
- Kim, Y.O.; Kim, J.S.; Kang, H. Cold-inducible zinc finger-containing glycine-rich RNA-binding protein contributes to the enhancement of freezing tolerance in Arabidopsis thaliana. Plant J. 2005, 42, 890–900.
- Xu, T.; Gu, L.; Choi, M.J.; Kim, R.J.; Suh, M.C.; Kang, H. Comparative Functional Analysis of Wheat (Triticum aestivum) Zinc Finger-Containing Glycine-Rich RNA-Binding Proteins in Response to Abiotic Stresses. PLoS ONE 2014, 9, e96877.
- Ambrosone, A.; Costa, A.; Leone, A.; Grillo, S. Beyond transcription: RNA-binding proteins as emerging regulators of plant response to environmental constraints. Plant Sci. 2012, 182, 12–18.
- Lutz, C.S.; Moreira, A. Alternative mRNA polyadenylation in eukaryotes: An effective regulator of gene expression. Wiley Interdiscip. Rev. RNA 2011, 2, 22–31.
- Xing, D.; Li, Q.Q. Alternative polyadenylation and gene expression regulation in plants. Wiley Interdiscip. Rev. RNA 2011, 2, 445–458.
- Xiao, J.; Li, C.; Xu, S.; Xing, L.; Xu, Y.; Chong, K. JACALIN-LECTIN LIKE1 Regulates the Nuclear Accumulation of GLYCINE-RICH RNA-BINDING PROTEIN7, Influencing the RNA Processing of FLOWERING LOCUS C Antisense Transcripts and Flowering Time in Arabidopsis. Plant Physiol. 2015, 169, 2102–2117.
- Streitner, C.; Köster, T.; Simpson, C.G.; Shaw, P.; Danisman, S.; Brown, J.W.; Staiger, D. An hnRNP-like RNA-binding protein affects alternative splicing by in vivo interaction with transcripts in Arabidopsis thaliana. Nucleic Acids Res. 2012, 40, 11240–11255.
- Wu, Z.; Zhu, D.; Lin, X.; Miao, J.; Gu, L.; Deng, X.; Yang, Q.; Sun, K.; Zhu, D.; Cao, X.; et al. RNA Binding Proteins RZ-1B and RZ-1C Play Critical Roles in Regulating Pre-mRNA Splicing and Gene Expression during Development in Arabidopsis. Plant Cell 2016, 28, 55–73.
- Terzi, L.C.; Simpson, G.G. Regulation of flowering time by RNA processing. Curr. Top. Microbiol. Immunol. 2008, 326, 201–218.
- Koster, T.; Meyer, K.; Weinholdt, C.; Smith, L.M.; Lummer, M.; Speth, C.; Grosse, I.; Weigel, D.; Staiger, D. Regulation of pri-miRNA processing by the hnRNP-like protein AtGRP7 in Arabidopsis. Nucleic Acids Res. 2014, 42, 9925–9936.
- Luders, J.; Winkel, A.R.; Reichel, M.; Bitterer, V.W.; Scheibe, M.; Widmann, C.; Butter, F.; Koster, T. Identification of Pri-miRNA Stem-Loop Interacting Proteins in Plants Using a Modified Version of the Csy4 CRISPR Endonuclease. Int. J. Mol. Sci. 2022, 23, 8961.
- Wiedner, H.J.; Giudice, J. It’s not just a phase: Function and characteristics of RNA-binding proteins in phase separation. Nat. Struct. Mol. Biol. 2021, 28, 465–473.
- Xu, X.; Zheng, C.; Lu, D.; Song, C.P.; Zhang, L. Phase separation in plants: New insights into cellular compartmentalization. J. Integr. Plant Biol. 2021, 63, 1835–1855.
- Huang, S.; Zhu, S.; Kumar, P.; MacMicking, J.D. A phase-separated nuclear GBPL circuit controls immunity in plants. Nature 2021, 594, 424–429.
- Wang, L.; Yang, T.; Wang, B.; Lin, Q.; Zhu, S.; Li, C.; Ma, Y.; Tang, J.; Xing, J.; Li, X.; et al. RALF1-FERONIA complex affects splicing dynamics to modulate stress responses and growth in plants. Sci. Adv. 2020, 6, eaaz1622.
- Xu, F.; Wang, L.; Li, Y.; Shi, J.; Staiger, D.; Chen, W.; Wang, L.; Yu, F. The Receptor Kinase FER Mediates Phase Separation of Glycine-Rich RNA-Binding Protein 7 to Confer Temperature Resilience in Arabidopsis. bioRxiv 2022, 2022, 483201.
- Ma, L.; Yang, Y.; Wang, Y.; Cheng, K.; Zhou, X.; Li, J.; Zhang, J.; Li, R.; Zhang, L.; Wang, K.; et al. SlRBP1 promotes translational efficiency via SleIF4A2 to maintain chloroplast function in tomato. Plant Cell 2022, 34, 2747–2764.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
976
Revisions:
2 times
(View History)
Update Date:
27 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




