Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nasko Nachev | -- | 4068 | 2023-10-24 13:38:29 | | | |
| 2 | Jason Zhu | -1 word(s) | 4067 | 2023-10-25 03:25:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Stoyanova, N.; Nachev, N.; Spasova, M. Electrospinning Method for Plant Extracts. Encyclopedia. Available online: https://encyclopedia.pub/entry/50739 (accessed on 02 March 2026).
Stoyanova N, Nachev N, Spasova M. Electrospinning Method for Plant Extracts. Encyclopedia. Available at: https://encyclopedia.pub/entry/50739. Accessed March 02, 2026.
Stoyanova, Nikoleta, Nasko Nachev, Mariya Spasova. "Electrospinning Method for Plant Extracts" Encyclopedia, https://encyclopedia.pub/entry/50739 (accessed March 02, 2026).
Stoyanova, N., Nachev, N., & Spasova, M. (2023, October 24). Electrospinning Method for Plant Extracts. In Encyclopedia. https://encyclopedia.pub/entry/50739
Stoyanova, Nikoleta, et al. "Electrospinning Method for Plant Extracts." Encyclopedia. Web. 24 October, 2023.
Copy Citation
Since antiquity, humans have known about plants as a medicinal cure. Plant extracts are attracting more attention as a result of their natural origin and wide range of desirable features. Nanotechnology’s progress and innovations enable the production of novel materials with enhanced properties for a broad range of applications. Electrospinning is a cutting-edge, flexible and economical technique that allows the creation of continuous nano- and microfibrous membranes with tunable structure, characteristics and functionalities.
medicinal and aromatic plant extracts
electrospinning
bioactive nanofibrous materials
1. Electrospinning Method
An increasing number of scientific studies have recently focused on the production of micro- and nanofibrous materials. There are several basic methods for obtaining polymer micro- and nanofibers including the method of self-organization [1], the template method [2], by phase separation [3], drawing [4], blowing (extruding) from melt [5] as well as by electrospinning [6][7]. Among these methods electrospinning is the only method that is relatively easy, efficient and enables the production of fibrous materials of the desired design and morphology [6][8][9]. In recent years, electrospinning has been one of the most dynamic developing nanotechnologies worldwide. Moreover, it is extremely attractive due to the possibility of the industrial production of non-woven fabrics. The two main processes differ depending on the solution viscosity: electrospraying or electrospinning [10]. When dilute polymer solutions are used, electrospraying occurs resulting in micro- or nano-sized particle production. A fiber fabrication process begins when concentrated polymer solutions are subjected to an electric field. This process is known as electrospinning, and it allows the production of irregularly deposited or oriented fibers, possessing remarkable properties, with diverse morphology, large specific surface area and porous structure. Fibrous mats can be obtained from solution or melt and may find potential application in medicine (mats with antitumor, antibacterial or hemostatic activity), in the pharmacy (drug carriers), filtration materials or photocatalytic removal of organic pollutants in the purification of water, for the immobilization of enzymes or for the preparation of fibrous materials with magnetic properties [11][12][13][14][15].
1.1. Schematic Representation of the Electrospinning/Electrospraying Set-Up
The schematic representation of the typical electrospinning/electrospraying set-up is shown in Figure 1. The equipment for this process consists of three basic elements: a high-voltage power supply, a reservoir with a capillary (needle) where the polymer solution is placed and a metal collector (static or rotating) on which the fibers/particles are deposited (Figure 1). The presence of pumps allows the solutions to be fed at a controlled flow rate. Moreover, the jet trajectory can be controlled through the use of additional focusing devices. Once a voltage is applied, the electrostatic forces deform the droplet formed at the end of the capillary/needle into a cone shape called a Taylor cone. When the electrostatic forces overcome the surface tension, a jet is ejected from the tip of the cone. Initially, the jet moves in a straight line, but after a while it begins to perform whip-like movements due to the high charge density causing extensive plastic deformation. Meanwhile, during the jet’s flight the solvent evaporates, and dry nano- and microfibers are deposited on the collector.
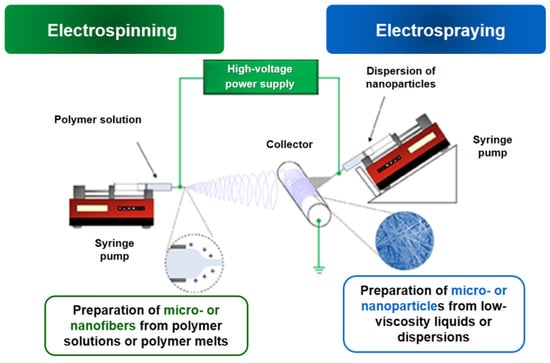
Figure 1. Schematic representation of the electrospinning/electrospraying set-up.
1.2. Factors Influencing the Process
The formation of fibers by electrospinning is a process that is influenced by many factors, including the parameters of both the spinning solution and the electrospinning process. The polymer solution must have a sufficiently high viscosity for the conduction of electrospinning. Lower viscosity values result in preparation of defective fibers, while higher values result in cylindrical fibers with a larger diameter [16]. It is well known that electrospinning occurs when, under the action of electrostatic forces of the applied field from a polymer solution or melt in the presence of a sufficient number of chain entanglements, an electrically charged jet is drawn, leading to the formation of fibers. The following equation can be used to calculate the number of chain entanglements during the process of fiber formation.
where Mw—weight average molar mass, φp—volume fraction of the polymer in the solution, and Me—molar mass of chain entanglement in melt [8]. The authors observed that electrospinning produced fibers with defects at values of (ne)soln = 2 and defect-free fibers at (ne)soln > 3.5. The analysis performed by Shenoy and co-authors showed that fibers begin to form in the presence of chain entanglements. The validity of Equation (1) has been confirmed for solutions of polystyrene, poly (lactic acid), polyethylene oxide (PEO) and polyvinylpyrrolidone (PVP). It should be noted that this model is only valid for polymer solutions dissolved in good solvents, and in some cases deviations from Shenoy’s theory have been observed when working with highly volatile solvents.
Depending on the concentration, the polymer solutions could be divided into dilute, semi-dilute without entanglement of the polymer chains, semi-diluted with entanglement of the polymer chains and concentrated. A schematic representation of the modes is shown in Figure 2 (inset figures). SEM micrographs of particles, fibers with defects, and defect-free fibers obtained at the different concentration regimes are shown as well. In dilute solutions, the polymer chains interact mainly with the solvent molecules (Figure 2a). In semi-dilute solutions without chain entanglement (Figure 2b), the concentration has not reached the required level for an optimal number of entanglements between the polymer chains. In semi-diluted solutions with entanglement of the polymer chains, a certain concentration is reached, which is necessary for sufficient entanglements. Typically, the number of entanglements in the solution must be above two to initiate fiber formation (Figure 2c) [17][18].
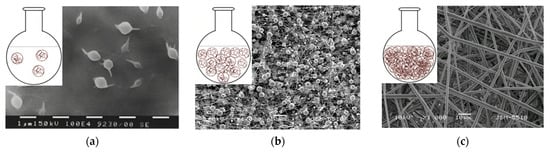
Figure 2. Schematic representation of polymer chains at different concentration regimes and SEM micrographs obtained from solutions with different concentrations: (a) dilute solution, (b) semi-dilute without entanglement between polymer chains and (c) semi-dilute with entanglement between polymer chains.
It is well known that the solution concentration and viscosity are strongly correlated. As the viscosity or concentration of the solution increases, the distribution of mean fiber diameters becomes more uniform [19]. The only objects produced are spheres or fibers with spherical defects by applying a high voltage to polymer solutions with low viscosity. However, above a certain critical concentration, a continuous fibrous structure is formed, and the concentration of the solution has a crucial impact on the fibrous morphology [20].
The electrical conductivity of the solution is another factor that influences the diameter of the obtained fibers. It depends on the polymer type, the solvent used and the presence of ionized salts. Salts added to polymer solutions increase the charge density of the jet, leading to repulsion between individual charges, thereby increasing the extent of jet elongation and forming fibers with smaller diameters [21].
The surface tension of the spinning solution also has an effect on the morphology of the resulting fibers. For the electrospinning process to take place, the solution must overcome the surface tension. It may depend on the type of solvent used. Generally, higher solution surface tension values hinder the electrospinning process and lead to the formation of particles or fibers with defects [22].
The applied voltage is a critical process factor. Typically, a positive or negative voltage of over 6 kV is required for the jet to form a Taylor cone. When the voltage is low, the electrostatic forces cannot overcome the solution drop’s surface tension, and as a result, a jet is not drawn, but a drip occurs [23]. As the applied voltage increases, the electrostatic forces also increase, which leads to the formation of a jet and the process of electrospinning begins [24]. Some authors believe that increasing the applied voltage leads to the creation of a stronger electric field. This, consecutively, contributes to the production of thinner fibers [25]. Other researchers contend that the strong electric field increases the jet’s acceleration and shortens its flight time. A longer jet flight time means more time for the fibers to stretch and elongate before being deposited on the collector. Thus, the lower voltage, reduced acceleration and weaker electric field can increase the flight time of the jet, resulting in the formation of finer fibers.
The distance between the capillary tip and the collector, the flow rate of the spinning solution, the rotation speed of the collector and the type of collector, are all electrospinning process parameters that influence the morphology of the fibers as well. Above a certain critical value of solution flow rates, uniform fibers with no defects are produced. An increase in the rate above the critical value leads to particle formation [26].
The time of flight for the jet is found to be shortened when the distance between the tip of the capillary and the collector surface is decreased. If the distance is too short, there is insufficient time for the solvent to evaporate, and the fibers do not dry before they reach the collector. Subsequently, the solvent-containing fibers are deposited [27]. Some authors reported that a larger distance favors the formation of thinner fibers [28]. It is also known that solutions of water-soluble polymers require the use of a greater distance for the formation of dry fibers compared to systems using volatile organic solvents [9].
Environmental factors such as temperature and humidity have a significant impact on morphology, diameter and diameter distribution of the electrospun fibers. It was observed that when the temperature rises, thinner fibers are produced. This is because high temperatures cause the polymer solutions’ viscosity to decrease [29]. The predominant number of studies on the electrospinning of polymer solutions were performed in air environment conditions. The formation of thicker fibers or fibers with defects at higher relative humidity is detected which is most probably due to the fact that a greater discharge of electrostatic charges from the surface of the polymer solution occurs, causing a decrease in the forces of the electrical charges [30].
The use of various collectors and focusing devices can alter the trajectory of the jet and change the morphology and orientation of the prepared fibers. A stationary metal plate or foil, positioned at a certain distance from the needle, serves as the most basic collector in electrospinning. Fibers are typically deposited randomly on the metal plate when using this type of collector [31]. The use of a drum or disk collector has the advantage of producing oriented fibers and long oriented bundles [32]. However, one disadvantage of their use is that the fiber orientation decreases with layer thickness. Collectors that are wire-wrapped, wire-built or bladed can also be used to create highly oriented fibers [33].
The electrospinning method provides the possibility for creation of novel nanofibrous architectures possessing a complex of desired properties. As it was presented, many diverse parameters influence this electrohydrodynamic process. Therefore, the fabrication of these nanostructures requires knowing and being able to control the solution and process parameters. The addition of bioactive compounds such as medicinal and aromatic plant extracts impacts the spinnability of the solution and therefore is a crucial factor in determining the morphology, structure and properties of the obtained fibrous materials as well.
2. Plant Extracts Incorporated by Electrospinning
In the last several years there has been a great interest in the fabrication of nanofibrous materials containing bioactive plant extracts by electrospinning. Up to now, the numbers of publications concerning the topic of fabrication of nanofibrous materials containing plant extract by electrospinning are much fewer compared to publications discussing the preparation of drug-containing fibers by electrospinning. Nevertheless, the number of studies increased due to the significant advantages of the natural compounds such as inherent medicinal activity, non-toxicity, lack of side effects, environmental sustainability, cost-effectiveness and easy availability. Moreover, the created hybrid structures possess antibacterial, antifungal, anti-inflammatory, antioxidant and anticancer activities which make these fibrous materials attractive for biomedical, pharmaceutical, agricultural and industrial applications. For the most part, articles on prepared nanofibers containing plant extracts were addressed to their prospective use in medicine.
Many researchers have reported the incorporation of C. longa in different polymer fibrous materials by electrospinning [34]. Moreover, this medicinal plant is the first to be loaded into electrospun fibers. The first report concerning the loading of curcumin in cellulose acetate (CA) was published in 2007 [35]. For the fabrication of the hybrid mats, CA with Mw = 30,000 Da and a degree of acetyl substitution of ~2.4 was used. The CA solution concentration was 17 wt% in mixed solvent acetone/dimethylacetamide 2:1 v/v. The curcumin was in the amount of 5 to 20 wt%. The obtained fibers were smooth with the average diameters of the curcumin-loaded CA fibers measuring up to 340 nm. The authors have proved that electrospun curcumin-loaded CA materials are non-toxic to normal human dermal fibroblasts.
Curcumin was incorporated in CA and polyvinylpyrrolidone (PVP) fibrous materials by one-pot electrospinning or dual spinneret electrospinning enabling modulating drug release [36]. PVP assisted faster curcumin release while curcumin imparted antimicrobial properties to the novel mats rendering the created materials suitable for wound dressing applications.
Innovative materials that allow the enhanced release of curcumin in aqueous medium were obtained from CA with electrosprayed curcumin/polyvinylpyrrolidone (Curc/PVP) particles [34][36]. The use of PVP led to hydrophilization of the mats and facilitated Curc release. Different solvent systems for the dissolution of Curc/PVP were studied: acetone/water (70/30) and ethanol/acetone (50/50). The representative SEM micrographs of the prepared Curc/PVP-on-CA mats using different solvents were presented in Figure 3. As it can be seen, the used solvent system for the dissolution of Curc/PVP strongly influenced the particles’ morphology. The electrosprayed particles possessed a polygonal shape which is attributed to the rapid solvent evaporation. Particles with larger sizes were obtained by dissolving Curc/PVP in a more rapidly evaporated ethanol/acetone solvent system. Moreover, the authors proved that the created curcumin-containing fibrous mats possessed antibacterial activity against E. coli and S. aureus along with high in vitro cytotoxicity towards HeLa tumor cells. These features make the prepared materials promising candidates for wound dressing applications and local cancer treatment.
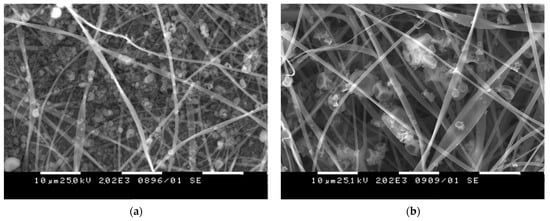
Figure 3. SEM micrographs of Curc/PVP-on-CA mats using (a) acetone/water and (b) ethanol/acetone solvent system.
Curcumin was successfully loaded along with cyclodextrin (CD) in electrospun polyvinyl alcohol (PVA) nanofibers. The influence of the plant addition to the fiber morphology and structure was studied. It was proven that curcumin was presented as crystalline aggregates into the fibrous mat while preserving its chemical structure. The incorporation of curcumin in the fibers increased its thermal stability [37]. PVA was chosen as a polymer for incorporation of curcumin by electrospinning by Mahmud et. al. as well [38]. The prepared fibrous PVA/curcumin crosslinked through heat and UV treatment materials showed antibacterial efficacy against S. aureus and E. coli bacteria for biomedical applications.
A blended solution of PVA, honey and C. longa extract was used for the preparation of antibacterial wound dressing electrospun nanofibrous material [39]. The average diameter of the obtained nanofibers was about 340 nm and the fibers had better moisture management properties compared to nanofibrous PVA material alone. In addition, the created novel material reveals antibacterial activity. Curcumin–honey-loaded multilayered PVA/CA electrospun nanofibrous mats were obtained as well [40]. They were fabricated to serve as bioactive wound dressings. The prepared hybrid novel materials show potential resistance towards E. coli and ca. 90% antioxidant activity when used against diphenyl-picrylhydrazyl (DPPH) radical scavenging. In addition, the obtained material possessed excellent absorption with controlled transmission of wound exudate.
There are reports in the literature revealing the creation of nanofibers based on curcumin and PLA by electrospinning for wound healing and cancer treatment. Pankongadisak et al. have varied the amount of loaded curcumin at 0.2, 0.5 and 1.0% w/w (based on the weight of PLLA) in the PLLA solution. The developed fibers possessed a smooth surface revealing a complete curcumin incorporation. The mean diameter of the prepared fibers was between 333 and 386 nm. The higher loading of curcumin resulted in an increased release amount. It was demonstrated that the obtained fibrous materials were non-toxic to human adult dermal fibroblast cells and supported cell attachment and proliferation [41].
Bharathi et al. loaded curcumin in chitosan/PLA nanofibers by using the electrospinning method. The suitability of created nanofibers was studied by antioxidant, drug release and in vitro cytotoxicity studies. In vivo wound healing tests on wounds using a rat model revealed a significant reduction in wound area. The authors concluded that the better healing efficiency could be attributed to the presence of curcumin and chitosan in the fibrous materials [42].
One-pot electrospinning was used to obtain fibrous mats based on PLA and PVP or polyethylene glycol (PEG) loaded with curcumin. The loading of curcumin into the fibers resulted in curcumin shielding from photodestruction and an increase in the mechanical properties of the fibers. In addition, the drug release was facilitated by the formation of hydrogen bonds between curcumin and PVP or PEG. The release of the natural extract provides the antibacterial and anticoagulant activity of the curcumin-loaded mats and prevents adhesion and aggregation of platelets onto the surface of the prepared mats [43].
Electrospun membranes containing curcumin were obtained from poly(L-co-D,L-lactic) acid and PVP [44]. It was determined that the dynamic viscosities of the spinning solutions depended on the amount of curcumin, which influenced the average fiber diameter and fiber shape. The authors proved that upon UV-Vis irradiation the amount, physico-chemical and therapeutic properties of curcumin were preserved, revealing the possibility of sterilizing the fibrous biomaterials by UV light.
Pegylated curcumin derivatives were obtained by a direct esterification reaction between poly(ethylene glycol)diacid and curcumin in the presence of N,N′-dicyclohexylcarbodiimide (PEG600-Curc). The prepared pegylated curcumin derivative is water-soluble. The synthesized product possessed cytotoxic activity against Graffi cell lines along with antibacterial activity [45].
A PHB polymer was used for the design of curcumin-loaded electrospun mats as a wound healing material [46]. The microscopic images of the fibers revealed that the fiber diameter increased with the increase in curcumin concentration. In addition, an increase in the curcumin amount resulted in an increase in the elongation of the fibrous material. The results from the cytotoxicity study show that the samples with a lower curcumin amount showed better biocompatibility. The authors concluded that the electrospun curcumin-loaded PHB mats could be potential candidates for wound healing applications.
In general, the research studies showed the successful incorporation of curcumin in different biopolymers by electrospinning and revealed the perspective for applications of the obtained novel materials as wound dressings as well as for anticancer treatment.
M. officinalis L. is a medicinal plant of the Lamiaceae family that is known as lemon balm [47]. Many pharmacological studies reveal the diverse inherent properties of M. officinalis. Up to now, there have been few studies reporting the incorporation of an M. officinalis plant extract into polymer fibers by electrospinning and investigating their characteristic properties. Bioactive nanofibers based on collagen hydrolysate-chitosan and the essential oil of lemon balm (M. officinalis L.) and dill (Anethum graveolens L.) were obtained by coaxial electrospinning for potential wound dressing applications [48]. The authors report that the synergetic effect of the used essential oils improves the antimicrobial activity of collagen hydrolysate-chitosan nanofibers against the following bacterial, yeast and fungal strains: S. aureus, E. coli, Enterococcus faecalis, Salmonella typhimurium, C. albicans, C. glabrata and Aspergillus brasiliensis. The results from the in vivo test showed good biocompatibility of electrospun materials based on collagen hydrolysate/chitosan and containing bioactive compounds (dill and/or lemon balm essential oils). This makes the obtained materials suitable for wound healing applications.
Some have reported for the first time a study revealing the successful incorporation of M. officinalis plant extract into PLA and PLA/PEG fibers by electrospinning [49]. The optimal parameters of the process needed for the fabrication of defect-free hybrid fibers are the following: applied voltage—25 kV, tip to collector distance—15 cm, collector rotation speed—1000 rpm, constant feed rate—3 mL/h, room temperature—21 °C and a relative humidity of 53%. The concentration of the plant extract was varied (0, 5 or 10 wt% in respect to the polymer weight). The measured average fiber diameters of the PLA, PLA/M. officinalis (5 wt%) and PLA/M. officinalis (10 wt%) were 1370 ± 220 nm, 1398 ± 233 nm and 1506 ± 242 nm, respectively. The slight increase in mean fiber diameter is due to the increase in M. officinalis concentration. The presence of the polyether PEG into the hybrid materials resulted in hydrophilization of the resulted electrospun mats. M. officinalis-containing fibrous mats possessed high antioxidant activity as determined by the DPPH free radical method. After being in contact with PLA/M. officinalis and PLA/PEG/M. officinalis fibrous materials, the color of the DPPH solution changed to yellowish and the absorbance of the radical has dropped by 88.7% and 91%, respectively. The reported results revealed the optimal conditions for fabrication of PLA or PLA/PEG fibrous materials incorporated with M. officinalis plant extract that were promising candidates for pharmaceutical, cosmetic and biomedical applications.
Rosmarinus officinalis (R. officinalis) is a plant belonging to the family Lamiaceae and native to the Mediterranean region. R. officinalis is used as a spice in cooking, as a natural preservative in the food industry and as medicinal plant [50]. This medicinal plant contains diverse bioactive molecules that were responsible for its anti-inflammatory, antioxidant, antimicrobial, antiproliferative, anticancer and protective properties. Several phytocompounds could be isolated from the R. officinalis L.: caffeic acid, rosmarinic acid (RA), carnosic acid, chlorogenic acid, monomeric acid, oleanolic acid, ursolic acid, alpha-pinene, camphor, carnosol, eucalyptol, rosmadial, rosmanol, rosmaquinones A and B, secohinokio, and derivatives of eugenol and luteolin [51]. For instance, there is data that, as an excellent antioxidant, RA prevents cell damage and thus lowers the risk of cancer and atherosclerosis. There is no evidence of harm from the use of rosmarinic acid in the literature. However, despite the high therapeutic potential, limited solubility in water and body fluids, chemical instability, poor absorption, rapid metabolism and elimination from the human body determine its low bioavailability, which significantly limits its use in clinical practice as a therapeutic agent [52]. This necessitates the development of new biomaterials as suitable and effective carriers of rosmarinic acid that increase its bioavailability or the finding of new rational solutions and approaches to improve the existing ones. Recently, fibrous polymer materials obtained by electrospinning have been of great interest as carriers of RA [53]. So far, fibrous materials obtained by electrospinning of poly(ε-caprolactone) solutions containing RA and magnetite with the potential application as a drug delivery system have been reported [54]. The electrospinning of CA containing RA in concentrations of 5 and 10% is also reported [55]. A disadvantage of electrospinning in this case is the use of an extremely low solution feeding rate (250 μL/h), which significantly extends the time to obtain the non-woven textile.
Portulaca oleracea L. (P. oleracea) is an annual weed from the family Portulacaceae that is native to the Mediterranean region and has spread worldwide. The World Health Organization classified it as one of the most used therapeutic plants [56]. Flavonoids, alkaloids, polysaccharides, fatty acids, terpenoids, sterols, proteins, vitamins and minerals were isolated from this herb [57]. Because of these beneficial and diverse compounds, P. oleracea possesses antioxidant, antimicrobial, anti-inflammatory, cardioprotective, neuroprotective, antidiabetic and immunomodulatory activities [58]. Nevertheless, up to now there are only two reports reporting the fabrication of fibrous electrospun materials loaded with P. oleracea extract [59][60]. PLA was used to create electrospun materials loaded with P. oleracea plant extract obtained by supercritical carbon dioxide. The obtained novel materials were morphologically, physico-chemically, mechanically and biologically characterized. The observation of the obtained fibrous mats by SEM revealed that smooth and defect-free fibers with diameters in the micron scale were obtained. The mechanical properties of the prepared samples were examined, and the results revealed that the tensile strength values of PLA fibrous materials and the hybrid material loaded with P. oleracea were very similar. The tensile strength of the PLA/plant extract mat reached ~3.78 MPa while the tensile strength of the PLA fibrous material was ca. 3.9 MPa.
Hypericum perforatum (H. perforatum), known as St. John’s wort, is a perennial plant with worldwide distribution grown for its medicinal use. There are reports showing the incorporation of H. perforatum L. in electrospun dressing materials which are helpful for preventing infections in wounds. A novel double-layer dressing material based on nanofibers was fabricated using poly (L-lactic acid) in the outer layer and a mixture of poly(ethylene) oxide and chitosan loaded with H. perforatum [61]. The extract-loaded, fibrous material possessed no cytotoxicity to normal human dermal fibroblast cells along with ability to inhibit S. aureus and P. aeruginosa. These results reveal the possibility of applying it as an antibacterial wound dressing to treat skin lesions. Furthermore, fibrous mats based on PLA, PVA and chitosan incorporated with the extract were fabricated by emulsion electrospinning of homogeneous gel-like suspensions of different and incompatible polymer solutions [61]. The results revealed the possibility of applying the created material as an antibacterial nanofibrous wound dressing preventing infections and enhancing the wound healing [62].
References
- Wang, W.-C.; Cheng, Y.-T.; Estroff, B. Electrostatic Self-Assembly of Composite Nanofiber Yarn. Polymers 2021, 13, 12.
- Xie, Y.; Kocaefe, D.; Chen, C.; Kocaefe, Y. Review of Research on Template Methods in Preparation of Nanomaterials. J. Nanomater. 2016, 2016, 2302595.
- Wei, J.; Liang, W.; Zhang, J. Preparation of Mechanically Stable Superamphiphobic Coatings via Combining Phase Separation of Adhesive and Fluorinated SiO2 for Anti-Icing. Nanomaterials 2023, 13, 1872.
- Yadavalli, N.; Asheghali, D.; Tokarev, A.; Zhang, W.; Xie, J.; Minko, S. Gravity Drawing of Micro- and Nanofibers for Additive Manufacturing of Well-Organized 3D-Nanostructured Scaffolds. Small 2020, 16, 1907422.
- Botta, L.; Teresi, R.; Titone, V.; Salvaggio, G.; La Mantia, F.; Lopresti, F. Use of Biochar as Filler for Biocomposite Blown Films: Structure-Processing-Properties Relationships. Polymers 2021, 13, 3953.
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A Review on Polymer Nanofibers by Electrospinning and Their Applications in Nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253.
- Islam, S.; Ang, B.; Andriyana, A.; Afifi, A. A Review on Fabrication of Nanofibers via Electrospinning and Their Applications. SN Appl. Sci. 2019, 1, 1248–1263.
- Reneker, D.; Yarin, A. Electrospinning Jets and Polymer Nanofibers. Polymer 2008, 49, 2387–2425.
- Al-Abduljabbar, A.; Farooq, I. Electrospun Polymer Nanofibers: Processing, Properties, and Applications. Polymers 2023, 15, 65.
- Bhushani, J.; Anandharamakrishnan, C. Electrospinning and Electrospraying Techniques: Potential Food Based Applications. Trends Food Sci. Technol. 2014, 38, 21–33.
- Spasova, M.; Stoyanova, N.; Nachev, N.; Ignatova, M.; Manolova, N.; Rashkov, I.; Georgieva, A.; Toshkova, R.; Markova, N. Innovative Fibrous Materials Loaded with 5-Nitro-8-hydroxyquinoline via Electrospinning/Electrospraying Demonstrate Antioxidant, Antimicrobial and Anticancer Activities. Antioxidants 2023, 12, 1243.
- Fokin, N.; Grothe, T.; Mamun, A.; Trabelsi, M.; Klöcker, M.; Sabantina, L.; Döpke, C.; Blachowicz, T.; Hütten, A.; Ehrmann, A. Magnetic Properties of Electrospun Magnetic Nanofiber Mats after Stabilization and Carbonization. Materials 2020, 13, 1552.
- Ignatova, M.; Rashkov, I.; Manolova, N. Drug-Loaded Electrospun Materials in Wound-Dressing Applications and in Local Cancer Treatment. Expert. Opin. Drug. Deliv. 2013, 10, 469–483.
- Ignatova, M.; Nachev, N.; Spasova, M.; Manolova, N.; Rashkov, I.; Naydenov, M. Electrospun 5-Chloro-7-iodo-8-hydroxyquinoline (Clioquinol)-Containing Poly(3-hydroxybutyrate)/Polyvinylpyrrolidone Antifungal Materials Prospective as Active Dressings against Esca. Polymers 2022, 14, 367.
- Korina, E.; Stoilova, O.; Manolova, N.; Rashkov, I. Polymer Fibers with Magnetic Core Decorated with Titanium Dioxide Prospective for Photocatalytic Water Treatment. J. Environ. Chem. Eng. 2018, 6, 2075–2084.
- Shenoy, S.; Bates, W.; Frisch, H.; Wnek, G. Role of Chain Entanglements on Fiber Formation During Electrospinning of Polymer Solutions: Good Solvent, Non-specific Polymer–Polymer Interaction Limit. Polymer 2005, 46, 3372–3384.
- Spasova, M.; Manolova, N.; Paneva, D.; Rashkov, I. Preparation of Chitosan Containing Nanofibers by Electrospinning Chitosan/Poly(Ethylene Oxide) Mixed Solutions. e-Polymers 2004, 056, 1–12.
- Stoyanova, N.; Paneva, D.; Mincheva, R.; Toncheva, A.; Manolova, N.; Dubois, P.; Rashkov, I. Poly(L-Lactide) And Poly(Butylene Succinate) Immiscible Blends: From Electrospinning to Biologically Active Materials. Mater. Sci. Eng. C 2014, 41, 119–126.
- Tarus, B.; Fadel, N.; Al-Oufy, A.; El-Messiry, M. Effect of Polymer Concentration on the Morphology and Mechanical Characteristics of Electrospun Cellulose Acetate and Poly (Vinyl Chloride) Nanofiber Mats. Alex. Eng. J. 2016, 55, 2975–2984.
- Bhardwaj, N.; Kundu, S. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347.
- Topuz, F.; Abdulhamid, M.; Holtzl, T.; Szekely, G. Nanofiber Engineering of Microporous Polyimides Through Electrospinning: Influence of Electrospinning Parameters and Salt Addition. Mater. Des. 2021, 198, 109280.
- Haider, A.; Haider, S.; Kang, I.-K. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188.
- Laudenslager, M.; Sigmund, W. Electrospinning. In Encyclopedia of Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 769–777.
- Angammana, C.; Jayaram, S. Fundamentals of Electrospinning and Processing Technologies. Part. Sci. Technol. 2015, 34, 72–82.
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415.
- Megelski, S.; Stephens, J.; Chase, D.; Rabolt, J. Micro- And Nanostructured Surface Morphology on Electrospun Polymer Fibers. Macromolecules 2002, 35, 8456–8466.
- Lee, J.; Choi, K.; Ghim, H.; Kim, S.; Chun, D.; Kim, H.; Lyoo, W. Role of Molecular Weight of Atactic Poly(Vinyl Alcohol) (PVA) In the Structure and Properties of PVA Nanofabric Prepared by Electrospinning. J. Appl. Polym. Sci. 2004, 93, 1638–1646.
- Jabur, A.; Abbas, L.; Aldain, S. Effects of Ambient Temperature and Needle to Collector Distance on PVA Nanofibers Diameter Obtained From Electrospinning Technique. Eng. Technol. J. 2017, 35, 340–347.
- Yang, G.-Z.; Li, H.-P.; Yang, J.-H.; Wan, J.; Yu, D.-G. Influence of Working Temperature on The Formation of Electrospun Polymer Nanofibers. Nanoscale Res. Lett. 2017, 12, 55.
- Park, B.K.; Um, I.C. Effect of Relative Humidity on the Electrospinning Performance of Regenerated Silk Solution. Polymers 2021, 13, 2479.
- Zander, N. Hierarchically Structured Electrospun Fibers. Polymers 2013, 5, 19–44.
- Nayak, R.; Padhye, R. Nano Fibres by Electro Spinning, Properties and Applications. J. Text. Eng. Fash. Technol. 2017, 2, 486–497.
- Subrahmanya, T.; Arshad, A.; Lin, P.; Widakdo, J.; Makari, H.; Austria, H.; Hu, C.-C.; Lai, J.-Y.; Hung, W.-S. A Review of Recent Progress in Polymeric Electrospun Nanofiber Membranes in Addressing Safe Water Global Issues. RSC Adv. 2021, 11, 9638–9663.
- Tsekova, P.; Spasova, M.; Manolova, N.; Markova, N.; Rashkov, I. Electrospun Curcumin-Loaded Cellulose Acetate/Polyvinylpyrrolidone Fibrous Materials with Complex Architecture and Antibacterial Activity. Mater. Sci. Eng. C 2017, 73, 206–214.
- Suwantong, O.; Opanasopit, P.; Ruktanonchai, U.; Supaphol, P. Electrospun Cellulose Acetate Fiber Mats Containing Curcumin and Release Characteristic of the Herbal Substance. Polymer 2007, 48, 7546–7557.
- Tsekova, P.; Spasova, M.; Manolova, N.; Rashkov, I.; Markova, N.; Georgieva, A.; Toshkova, R. Electrospun Cellulose Acetate Membranes Decorated with Curcumin-PVP Particles: Preparation, Antibacterial and Antitumor Activities. J. Mater. Sci. Mater. Med. 2018, 29, 9.
- Sun, X.-Z.; Williams, G.; Hou, X.-X.; Zhu, L.-M. Electrospun Curcumin-Loaded Fibers with Potential Biomedical Applications. Carbohydr. Polym. 2013, 94, 147–153.
- Mahmud, M.; Zaman, S.; Perveen, A.; Jahan, R.; Islam, F.; Arafat, M. Controlled Release of Curcumin from Electrospun Fiber Mats with Antibacterial Activity. J. Drug Deliv. Sci. Technol. 2020, 55, 101386.
- Shahid, A.; Ali, A.; Uddin, N.; Miah, S.; Islam, S.; Mohebbullah, M.; Jamal, M. Antibacterial Wound Dressing Electrospun Nanofibrous Material from Polyvinyl Alcohol, Honey and Curcumin longa Extract. J. Ind. Text. 2021, 51, 455–469.
- Gaydhane, M.; Kanuganti, J.; Sharma, C. Honey and Curcumin Loaded Multilayered Polyvinylalcohol/Cellulose Acetate Electrospun Nanofibrous Mat for Wound Healing. J. Mater. Res. 2020, 35, 600–609.
- Pankongadisak, P.; Sangklin, S.; Chuysinuan, P.; Suwantong, O.; Supaphol, P. The Use of Electrospun Curcumin-Loaded Poly(L-Lactic Acid) Fiber Mats as Wound Dressing Materials. Drug Deliv. Sci. Technol. 2019, 53, 101121.
- Dhurai, B.; Saraswathy, N.; Maheswaran, R.; Sethupathi, P.; Vanitha, P.; Vigneshwaran, S.; Rameshbabu, V. Electrospinning of Curcumin Loaded Chitosan/Poly (Lactic Acid) Nanofilm and Evaluation of Its Medicinal Characteristics. Front. Mater. Sci. 2013, 7, 350–361.
- Yakub, G.; Toncheva, A.; Manolova, N.; Rashkov, I.; Danchev, D.; Kussovski, V. Electrospun Polylactide-Based Materials for Curcumin Release: Photostability, Antimicrobial Activity, and Anticoagulant Effect. J. Appl. Polym. Sci. 2016, 133, 5738.
- Yakub, G.; Toncheva, A.; Kussovski, V.; Toshkova, R.; Georgieva, A.; Nikolova, E.; Manolova, N.; Rashkov, I. Curcumin-PVP Loaded Electrospun Membranes with Conferred Antibacterial and Antitumoral Activities. Fibers Polym. 2020, 21, 55–65.
- Yakub, G.; Manolova, N.; Rashkov, I.; Markova, N.; Toshkova, R.; Georgieva, A.; Mincheva, R.; Toncheva, A.; Raquez, J.-M.; Dubois, P. Pegylated Curcumin Derivative: Water-Soluble Conjugates with Antitumor and Antibacterial Activity. ACS Omega 2022, 41, 36403–36414.
- Ghavami, R.; Biazar, E.; Taleghani, A.; Keshel, S. Design of Curcumin-Loaded Electrospun Polyhydroxybutyrate Mat as a Wound Healing Material. Nano Biomed. Eng. 2020, 12, 14–20.
- Papoti, V.T.; Totomis, N.; Atmatzidou, A.; Zinoviadou, K.; Androulaki, A.; Petridis, D.; Ritzoulis, C. Phytochemical Content of Melissa officinalis L. Herbal Preparations Appropriate for Consumption. Processes 2019, 7, 88.
- Râpa, M.; Gaidau, C.; Mititelu-Tartau, L.; Berechet, M.-D.; Berbecaru, A.C.; Rosca, I.; Chiriac, A.P.; Matei, E.; Predescu, A.-M.; Predescu, C. Bioactive Collagen Hydrolysate-Chitosan/Essential Oil Electrospun Nanofibers Designed for Medical Wound Dressings. Pharmaceutics 2021, 13, 1939.
- Stoyanova, N.; Spasova, M.; Manolova, N.; Rashkov, I.; Kamenova-Nacheva, M.; Staleva, P.; Tavlinova-Kirilova, M. Electrospun PLA-Based Biomaterials Loaded with Melissa officinalis Extract with Strong Antioxidant Activity. Polymers 2023, 15, 1070.
- de Oliveira, J.; Camargo, S.E.; de Oliveira, L. Rosmarinus officinalis L. (Rosemary) As Therapeutic and Prophylactic Agent. J. Biomed. Sci. 2019, 26, 5.
- Borges, R.; Ortiz, B.L.; Pereira, A.C.; Keita, H.; Carvalho, J.C. Rosmarinus officinalis Essential Oil: A Review of Its Phytochemistry, Anti-inflammatory Activity, and Mechanisms of Action Involved. J. Ethnopharmacol. 2019, 229, 29–45.
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273.
- Wen, P.; Zong, M.-H.; Linhardt, R.J.; Feng, K.; Wu, H. Electrospinning: A Novel Nano-Encapsulation Approach for Bioactive Compounds. Trends Food Sci. Technol. 2017, 70, 56–68.
- Saad, E.; El Gohary, N.; El-Shenawy, B.; Handoussa, H.; Klingner, A.; Elwi, M.; Hamed, Y.; Khalil, I.; El Nashar, R.; Mizaikoff, B. Fabrication of Magnetic Molecularly Imprinted Beaded Fibers for Rosmarinic Acid. Nanomaterials 2020, 10, 1478.
- Vatankhah, E. Rosmarinic Acid-Loaded Electrospun Nanofibers: In Vitro Release Kinetic Study and Bioactivity Assessment. Eng. Life Sci. 2018, 18, 732–742.
- Azuka, O.; Mary, A.; Abu, O. A Review on Portulaca oleracea (Purslane) Plant- Its Nature and Biomedical Benefits. Int. J. Biomed. Res. 2014, 5, 75–80.
- Zhou, Y.-X.; Xin, H.-L.; Rahman, K.; Wang, S.-J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A Review of Phytochemistry and Pharmacological Effects. BioMed Res. Int. 2015, 2015, 925631.
- Iranshahy, M.; Javadi, B.; Iranshahi, M.; Jahanbakhsh, S.; Mahyari, S.; Hassani, F.; Karimi, G. Review of Traditional Uses, Phytochemistry and Pharmacology of Portulaca oleracea L. J. Ethnopharmacol. 2017, 205, 158–172.
- Spasova, M.; Stoyanova, N.; Manolova, N.; Rashkov, I.; Taneva, S.; Momchilova, S.; Georgieva, A. Facile Preparation of Novel Antioxidant Fibrous Material Based on Natural Plant Extract from Portulaca oleracea and PLA by Electrospinning for Biomedical Applications. Polym. Int. 2022, 71, 689–696.
- Stoyanova, N.; Spasova, M.; Manolova, N.; Rashkov, I.; Taneva, S.; Momchilova, S.; Georgieva, A. Physico-Chemical, Mechanical, and Biological Properties of Polylactide/Portulaca oleracea Extract Electrospun Fibers. Membranes 2023, 13, 298.
- Mouro, C.; Gomes, A.P.; Gouveia, I.C. Double-Layer PLLA/PEO_chitosan Nanofibrous Mats Containing Hypericum perforatum L. As an Effective Approach for Wound Treatment. Polym. Adv. Technol. 2020, 32, 1493–1506.
- Mouro, C.; Gomes, A.P.; Gouveia, I.C. Emulsion Electrospinning of PLLA/PVA/Chitosan with Hypericum perforatum L. as an Antibacterial Nanofibrous Wound Dressing. Gels 2023, 9, 353.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
775
Revisions:
2 times
(View History)
Update Date:
25 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





