1. Introduction
Hydrogen stands out as an exceptionally versatile fuel that can be generated using a wide spectrum of energy sources, encompassing coal, oil, natural gas, biomass, renewables, and nuclear power. The production methods are equally diverse, spanning processes like reforming, gasification, electrolysis, pyrolysis, water splitting, and numerous others. In recent times, distinct colors have been assigned to signify different pathways of hydrogen production: “green” for hydrogen extracted through electrolysis with electricity from renewable sources; “grey” for H2 produced from fossil raw materials such as natural gas (this generates around 10 tons of CO2 per ton of H2); “blue” for production derived from fossil fuels with carbon capture, utilization, and storage (CCUS); “turquoise” for H2 produced by splitting natural gas at high temperatures (only solid carbon is produced in the process, i.e., no CO2 is emitted if the process runs on heat from renewable energy sources). Due to the multitude of energy sources that can be employed, the environmental consequences associated with each method of hydrogen production can differ significantly. Moreover, the geographic location and the specific configuration of the process also play a crucial role in shaping these environmental impacts.
When hydrogen is burned, the primary resultant is water (H2O), rendering it a genuinely emission-free fuel in terms of CO2. The ultimate objective is to utilize 100% green hydrogen for combustion, entirely supplanting natural gas. In the interim, a more immediate goal involves blending hydrogen with natural gas to be used in gas turbines and other industrial combustion applications, thereby achieving a partial reduction in CO2 emissions. Nowadays, the term fuel-flexibility refers to combustion-based power generation system (especially gas turbines) capabilities to operate with hydrogen blends as fuel in a stable, safe, and reliable way when the H2 content unpredictably varies in time due to intermittent production from renewables. Such blends range from hydrogen-enriched natural gas (HENG) to ammonia (a promising H2-carrier).
2. Features of Hydrogen Combustion
When employing hydrogen either alongside natural gas or as a complete substitute, it is crucial to grasp the distinctions between these two fuels.
Hydrogen possesses a density that is one-ninth that of natural gas, and it holds the distinction of being the smallest known molecule. This characteristic introduces challenges regarding transportation and sealing. Furthermore, hydrogen’s heating value is merely one-third that of natural gas (on a volumetric basis), signifying that three times the amount of hydrogen fuel is necessary to generate an equivalent power output compared to natural gas. In hydrogen combustion, although a higher volume flow of fuel is needed for equivalent energy production, about 20% less air by volume is necessary to generate a flame comparable to natural gas. This reduction in the air volume requirement results in a decreased mass flow through the combustor, subsequently reducing convective heat transfer. From this standpoint, flue gas recirculation can be employed to increase the mass flow of air into the combustor, thereby increasing convective heat transfer and lowering the flame temperature, as discussed in
[1][2][3].
Moreover, hydrogen has a considerably broader flammability range than natural gas, leading to heightened concerns regarding environmental, health, and safety aspects during both hydrogen transportation and combustion. Although it has recently been observed
[4] that mixtures containing higher
H2 concentrations are slower to ignite compared to those with higher
CH4 concentrations at low temperatures (below
930K, for hydrogen combustion, the self-recombination of
HO2 radicals leads to chain propagation which inhibits reactivity, whereas for methane combustion, the reaction between radicals and
HO2 leads to chain branching, increasing reactivity), for higher temperatures hydrogen largely enhances the reactivity of fuel blends: the ignition delay time decreases
[5][6][7][8]; at room temperature and pressure, the flammability limits (0.1–7.1) are well wider than those of natural gas (0.5–1.67); the flame speed is higher
[9] and the critical strain rate increases, thus reducing potential flame quenching
[10][11].
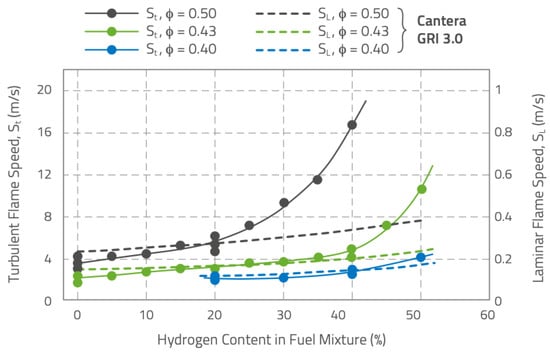
Figure 1 reports data on laminar and turbulent flame speed from
[12][13] for
CH4/H2 fuel mixtures with hydrogen content up to 50% vol. The data are specific to lean premixed fuel/air mixtures (
0.4<𝜙<0.5) at 5 bar, preheated at
673K, flowing at
40m/s, and with a 15% inlet turbulence intensity. Laminar flame speed values were calculated based on the GRI3.0 chemical mechanism. The data on turbulent flame speed show the unexpected effects of increasing the hydrogen content. Initially, there is a moderate, nearly linear increase in turbulent flame speed, following the trend of calculated laminar flame speed, but the linearity varies with the equivalence ratio, is longer for leaner mixtures. This suggests complex interactions between hydrogen addition and combustion properties. The differences (evident in the richer mixtures in
Figure 1) highlight the presence of both the chemical kinetics and physical (diffusivity, thermal conductivity, turbulence) effects of the hydrogen addition: the linear trends depict the chemical kinetics reaction properties of the fuel gas mixtures; beyond the limits of the linear region, the turbulent flame speed rapidly diverges (similar trends are observed for the leaner conditions but shifted towards higher hydrogen concentrations). Hence, besides the chemical kinetics effects influenced by hydrogen, which are reflected in changes in the laminar flame speed, the physical properties of hydrogen also accelerate the consumption rate of the fuel species.
Figure 1. Laminar and turbulent flame speed for
CH4/H2 fuel mixtures with different hydrogen from
[12]. Laminar flame speed data come from chemical kinetics calculations based on the GRI3.0 chemical mechanism. Turbulent flame speeds come from experimental measurements.
The higher hydrogen combustion temperatures produce an increase in nitrogen oxides,
NOx, most of which is thermal, i.e., coming from high-temperature regions with sufficiently long residence times
[14]. This is one of the main concerns in operating burners with fuel blends having a high
H2 content. Also, turbulence plays its role in the formation of
NOx, e.g., they are reduced as the Karlovitz number and the
H2 content are increased, and the turbulent flame brush thickened
[15].
Hydrogen reactions play a central role in the combustion kinetics of any fuel. Specifically in the context of hydrogen combustion, the Konnov mechanism
[16][17] has been recently developed, also in conjunction with carbon chemistry. These mechanisms are highly detailed and exhibit good agreement with experimental data, as well as with other significant kinetic models. In
[16], a comparison was made between experimental and computational results employing two modern and comprehensive chemical kinetic mechanisms, Glarborg
[18] and POLIMI
[19]. Discrepancies were observed, particularly at rich equivalence ratios, but the Konnov model
[16] demonstrated a strong overall performance in replicating laminar burning velocities and
NO concentrations. At lean equivalence ratios, the adiabatic burning velocities from both numerical and experimental studies matched quite closely, with minor distinctions emerging under rich conditions, where the Konnov model aligned more closely with experimental values despite inherent uncertainties.
2.1. Effects on Flame Stability
Combustion in excess of air can mitigate
NOx production
[20], but at these conditions of extremely lean combustion, the gaseous mixtures rich in hydrogen are characterized by a disparity between the diffusion by mass and the thermal one, inducing phenomena of intrinsic instability with a strong impact on the laminar and turbulent combustion speed, and thus altering the flame topology.
These effects have been theoretically predicted
[21][22][23], as well as experimentally
[24][25] and numerically visualized (numerical simulations)
[26][27][28]. In particular, these reactive mixtures are extremely sensitive to some external perturbations (usually present in every burner) producing exponential growths in the extension of the flame front, in pressure fluctuations, as well as in temperature, with a negative impact on the performance of thermal machines, their polluting emissions, and even their life time. However, with an accurate design of the combustion chamber, it is possible to maximize the advantages deriving from these instabilities, i.e., the increase in the average combustion speed (and therefore, in the thermal power) and compactness of flames (smaller size of the devices), while eliminating or mitigating the negative aspects, such as high pressure and temperature fluctuations, localized quenching and flashbacks.
Hydrogen, and light species in general, diffuse preferentially with respect to other species in a mixture. In recent decades, so-called asymptotic theories
[21][22][23] have been developed which predict the existence of perturbations (in specific wavelength ranges) that are particularly effective in destabilizing combustion processes, which strongly depend on the Lewis number of the mixture. The Lewis number (Le) represents a measure of the disparity between thermal and mass diffusivity, through their ratio: therefore, a hydrogen-rich mixture, which has a mass diffusivity that is extremely greater than the methane molecule, will be characterized by a Lewis number less than unity. In a premixed combustion, this makes the local equivalence ratio lower or higher than the inlet nominal equivalence ratio of the considered fuel blend depending on the local flame curvature.
Mixtures with Lewis numbers smaller than a critical value (
Lec, generally less than one) will be characterized by small-scale (thermo-diffusive) and large-scale (hydro-dynamic) instabilities. With a Lewis number close to the
Lec or greater, mainly hydro-dynamic instabilities will survive. This type of instability is based on different formation mechanisms. In the case of thermo-diffusive instabilities, the high diffusivity of the mixture (in particular of
H2) will give rise, when the flame front is perturbed/corrugated, to inhomogeneity of the equivalence ratio of the mixture and of the local temperatures with a consequent variation of the local laminar combustion speeds, which in turn will amplify the initial perturbations of the front, making it even more corrugated. Such corrugations increase the turbulent flame speed, that reports the ratio between the turbulent and laminar flame speeds of a premixed
CH4/H2/Air Bunsen flame: the lower the Lewis number and the higher the turbulence intensity, the higher the turbulent flame speed will be
[29][30].
This phenomenon is more effective with more perturbations, and the corrugations of the front are small in scale. In the case of Le>Lec, this phenomenon is strongly limited, and what arises is a hydro-dynamic type mechanism, in which the large-scale corrugations of the front compress or expand the stream lines of the flow field, thus channeling areas with low or high velocity upstream of the flame front.
The presence of thermo-diffusive instabilities alone is not possible. In fact, since the a phenomenon of hydro-dynamic instability is always present in a flame, due to the difference in temperature between reactants and combustion products, that of the thermo-diffusive type is driven by the disparity between the mass and thermal diffusivity, which can be eliminated by acting on the composition of the reactant mixture.
Due to its elevated combustion temperature and faster flame speed, hydrogen combustion presents an increased susceptibility to instability, potentially leading to flameouts and flashback incidents. Specifically, the heightened reactivity of hydrogen inherently raises the risk of self-ignition and flashbacks, primarily within the premix section. This concern becomes even more critical in systems characterized by exceedingly high inlet air temperatures, such as those found in highly recuperated gas turbines, as referenced in
[31][32].
2.2. Effects on Pollutant Emissions
The main emission indicators on which the performance of burners are based are mainly the nitrogen oxides NOx, composed of NO and NO2, CO and carbon dioxide CO2. There is no fundamental chemical kinetic reason why H2 flames should produce more NOx than natural gas flames. Since most NOx is thermal, i.e., produced where reactants are at a high temperature for a sufficiently long residence time (above a certain threshold temperature, around 1800 K, the production of NOx increases dramatically), more lean mixtures have to be adopted to reduce the peak temperatures when burning fuel blends with higher a H2 content.
Apart from
NOx predictions and expectations from chemical kinetics calculations, some direct measurements of
NOx emissions in a specific premixed Bunsen burner at 1 atmosphere are here reported due to the interesting operational strategy implemented to avoid flashback occurrence
[30]: as the hydrogen content increases in the
CH4/H2 fuel blend, driving the increase in flame propagation speed, the value of the equivalence ratio is decreased by increasing the excess air. This strategy allowed to maintain the flame propagation speed constant (
0.33m/s), and at the same time, lowered the adiabatic flame temperature; additionally, the power was kept constant (
7.1kW) by raising the flow rate of the reactant mixture. Such a strategy avoided a flashback up to the maximum jet Reynolds number (explored range, 10.000–13.900) in
[30]: it would be interesting to see what happens at higher turbulent jet Reynolds numbers to check the effects on the nonlinear increase in the turbulent flame speed. The measurements of emissions were carried out at the chimney, taking a small quantity of combustion products and analyzing them by means of the FTIR (Fourier transform infrared spectroscopy) technique
[33][34].
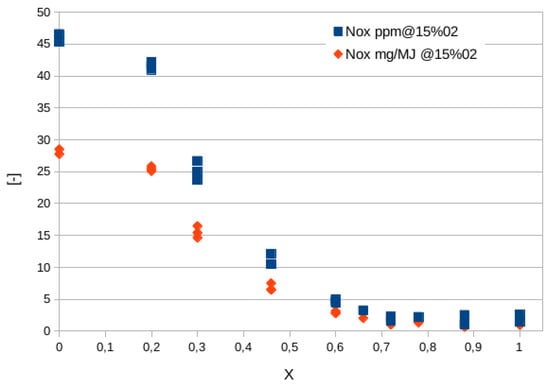
Figure 2 shows the quantity of
NOx expressed in parts per million (ppm), scaled to a value of oxygen in the combustion equal to 15%, as well as in mass normalized by the fuel energy actually converted into heat, to take into account the different composition of exhausts while increasing the hydrogen content
[30]. Additionally, in this way, the emission values of machines of significantly different sizes (power) can be compared. It is observed that, above a hydrogen mole fraction of 45–50%, emissions decrease dramatically. This is due to the regulation strategy adopted in
[30]: while the hydrogen content is increased, the equivalence ratio is decreased, thus lowering the flame temperature and thermal
NOx contribution (the most relevant). In fact, the temperature changes from about 2100 K for the methane flame to nearly 1600 K for the hydrogen one.
Figure 2. NOx concentration in ppm (blue squared symbols) and mg/MJ (red diamonds) as the hydrogen content (expressed in molar fraction,
X) varies in the premixed
CH4/H2/Air Bunsen flames studied in
[30]. Values are scaled at an oxygen content of 15%.
2.3. Effects on Radiant Energy Transfer
Radiative transfer of energy (RTE) is a highly significant mechanism in a multitude of applications, including high-pressure and high-temperature engine combustion chambers, rocket propulsion, hypersonic vehicles, spacecraft atmospheric reentry, ablating thermal protection systems, glass manufacturing, plasma generators, and nuclear fusion.
It is important to note that hydrogen flames are notably less visible compared to flames produced by natural gas. This reduced visibility is a result of the lower concentration of radiant species, including soot,
CO2, and radicals in hydrogen flames. Consequently, detecting these flames presents a more intricate challenge. Infrared flame detection methods are ineffective for hydrogen flames, necessitating the use of ultraviolet systems, as discussed in
[35][36][37][38].
At the same time, this also affects the radiative heat transfer from the flame, with important implications in some applications, such as glass furnaces. The lower emissivity and lower mass flow rate (less air is required while increasing the H2 content to generate a flame comparable to natural gas, i.e., with the same heat release) of the combustor, therefore changing the heat transfer balance.
The radiative absorption properties hinge on the absorption coefficient, denoted by
𝜅𝜆. In gases, this coefficient often exhibits significant variations with respect to wavelength, temperature, and pressure. It also increases in direct proportion to the concentration of participating species. While solid surfaces can generally be assumed as opaque, the absorption or emission properties of gases exhibit irregularities within the wavelength domain, becoming particularly noteworthy at temperatures below a few thousand Kelvin. As the temperature increases, the absorption coefficient for cold lines diminishes, but hot lines emerge at higher wavelengths, slightly increasing the absorption coefficient. Elevating the pressure results in spectral line broadening, primarily due to molecular collisions
[39]. This broadening leads to wider and more overlapping lines, especially at higher pressures, rendering the gas more “gray” or opaque.
At the temperatures typically encountered in industrial furnaces and combustion chambers, the gaseous species that play a significant role in absorbing and emitting radiation are carbon dioxide (CO2), water vapor (H2O), carbon monoxide (CO), sulfur dioxide (SO2), nitrogen oxide (NO), and methane (CH4). Other gases, such as nitrogen (N2), oxygen (O2), and hydrogen (H2), are essentially transparent to infrared radiation and do not significantly contribute to emission or absorption. However, their importance as absorbing and emitting contributors becomes pronounced at extremely high temperatures.
Regarding flames, it is well established that disregarding radiation under atmospheric pressure conditions can result in an overestimation of temperature by as much as
200K. However, it is important to note that numerical predictions are highly dependent on the radiative transfer of energy (RTE) model employed. The commonly used optically thin or gray radiation models tend to underestimate the temperature by up to
100K or more, as discussed in
[40][41].
In systems where RTE is a significant factor, it has been demonstrated that radiation can be considered equivalent to a substantial nonlinear diffusion term. In fact, the transfer of heat through radiation can function as a preheating mechanism, similarly to heat conduction. As a result, this preheating effect contributes to higher flame speeds, as discussed in the work by Mercer in
[42].
When it comes to the peak flame temperature, radiation typically reduces it in low-pressure flames. Although radiation effects become more pronounced with increasing pressure, primarily due to the heightened emission and absorption of energy, the peak flame temperature is less affected by radiation. This is attributed to the faster chemical reactions that occur at higher pressures, a phenomenon also observed in
[43] (p. 139).
It is known that hydrogen flames have a lower emissivity than natural gas flames. Such radiative emission changes may have important implications in some applications
[44].
As the hydrogen content in the mixture increases, the contribution of carbon to the emission of radiative energy progressively decreases until, in the mixture of pure hydrogen, only the emission peak of water and H2 survives, strongly reducing the ratio between radiant/convective energy emitted. In all mixtures, the emission peak of the OH radical, located in the ultraviolet around a wavelength between about 260 and 310 nm, is substantially absent, despite being a chemical species strongly present inside the flame front.
A lower radiative emission translates into a greater quantity of energy which is transmitted by convection, which mainly follows an axial direction, whereas the radiative one is emitted in all directions, and, given the cylindrical symmetry of the flame, preferentially in a radial direction. While this is beneficial for gas turbine applications (lower heat losses to walls and less cooling issues), it could be a serious issue in other industrial applications such as raw-material melting kilns or product cooking kilns.
2.4. Effects on Materials
Besides affecting combustion characteristics, hydrogen enrichment to natural gas also affects materials. Hydrogen can be absorbed by certain containment materials and piping, potentially leading to the loss of ductility or embrittlement in them. Hydrogen embrittlement becomes evident shortly after its introduction into a system and is an irreversible process. This phenomenon diminishes the material’s yield stress, consequently compromising its fatigue resilience, particularly under low-cycle fatigue conditions
[45]. The extent and rapidity of embrittlement is accelerated by factors such as high temperature, pressure, and stress levels. It is important to note that not all materials exhibit the same susceptibility to hydrogen embrittlement. Stainless steels and nickel alloys, which are frequently employed in gas turbine combustion systems, are particularly prone to heightened embrittlement at elevated temperatures.
A 100% hydrogen flame is typically shorter and positioned much closer to the burner when compared to a methane flame operating under the same conditions. This difference is primarily due to the higher flame speed and shorter ignition times associated with hydrogen, as highlighted in
[46]. Conversely, industrial experience has shown that, when high percentages of water or steam are added to the fuel, the flame length can significantly increase. This, in combination with the elevation of flame temperature, carries important implications for the potential impact on combustor materials, as discussed in
[47]. The most significant worry concerning hot gas path components arises from the fact that hydrogen’s elevated combustion temperature leads to a rise in turbine firing temperature. Furthermore, the gas temperature profile upon leaving the combustor will be hotter and exhibit distinct characteristics when burning hydrogen in comparison to natural gas.
It is also crucial to take into account that the combustion of hydrogen results in a change in the composition of the exhaust gases compared to the use of natural gas. This alteration in the composition of combustion products can have a notable impact in direct combustion equipment, particularly in situations where the combustion gases directly interact with the products being produced, as mentioned in
[48][49].
3. Gas Turbine and Piston Engines Power Generation
3.1. Hydrogen Piston Engines
Hydrogen can be effectively employed in piston engines when blended with hydrocarbons, utilizing fuel and injection systems similar to conventional gasoline engines. Consequently, hydrogen-powered vehicles represent a promising way to diminish the reliance on fossil fuels within the automotive sector; a history of hydrogen combustion vehicles can be found in
[50].
Some of the already mentioned properties of hydrogen can have a positive impact on the use of
H2 as fuel in piston engines: its wider flammability range; its very low ignition energy (an order of magnitude less than gasoline); its high octane number (due to the self-ignition temperature being higher than gasoline), above 130, offering a high resistance to knocking combustion
[51]; its high diffusivity, facilitating the formation of uniform fuel–air mixtures; its high flame speed (an order of magnitude higher than gasoline); and injected in a gaseous state, it provides optimal cold start performance.
Using hydrogen as a fuel in piston engines requires significant engine modifications, particularly in the areas of fuel–air mixture injection, compression, and ignition.
The mode of injection plays a crucial role in influencing airflow distribution, impacting the mixing and combustion of hydrogen and air. For hydrogen, direct high-pressure or low-pressure injection (depending on the phase of the cycle) is considered the optimal choice
[51][52][53]. This method involves injecting fuel with the intake valve closed, reducing the risk of premature mixture ignition and preventing backfires in the delivery channel. However, injecting hydrogen into the intake port can lead to airflow blockage, potentially decreasing engine power and even causing engine shutdown, especially when higher hydrogen levels are required for increased power. Attempts to enhance power through supercharging introduce challenges such as increased risk of hot spot formation, backfires, and pre-ignition, which require leaner fuel ratios, limiting potential power gains.
Achieving the highest efficiency with hydrogen in piston engines might necessitate a higher compression ratio compared to gasoline engines
[54]. This is because the self-ignition temperature of hydrogen is higher. The temperature increase during compression is directly linked to the compression ratio, so a higher self-ignition temperature permits a higher compression ratio, and this, in turns, improves efficiency.
Regarding spark plugs, they must prevent the electrode temperature from exceeding the ignition threshold and causing backfires. It is worth noting that platinum electrode spark plugs are not suitable for hydrogen use
[54], as their catalytic properties could encourage hydrogen oxidation.
Direct injection
[53] prevents flame propagation within the intake circuit but may not necessarily prevent pre-ignition inside the combustion chamber. To address pre-ignition issues, techniques like exhaust gas recirculation (EGR) are employed to introduce thermal dilution
[55]. EGR helps lower the temperature of hot spots, reducing the chances of pre-ignition.
Although the most advanced gas-reciprocating (or piston) internal combustion engines are capable of accommodating gases containing hydrogen content of up to 70% on a volumetric basis
[56] and several manufacturers have showcased the feasibility of engines that operate using 100% hydrogen (anticipated to become commercially accessible in the near future), they can be generally fed with a fraction of about 30%, going beyond 50% by adjusting the compression and power in some cases.
3.2. Hydrogen Gas Turbines
Thermo-electric power generation with gas turbines is one of the most immediately applicable solutions for reducing
CO2 emissions. However, a significant share of
CO2 emissions in the power generation sector can be attributed to gas turbines: utilizing hydrogen combined with natural gas will progressively become more significant in the effort to decrease
CO2 emissions. While renewable energy sources are making progress on the global energy stage, gas turbines will maintain their prominence in power generation both during the energy transition period and in the future energy scenario with an increasing percentage of non-programmable renewable sources, thanks to their capability to provide flexibility to the electric grid system
[57][58]. In this vision, it has to be accepted that, over the years, the very concept of the gas turbine will undergo a natural evolution, adapting to the technological advances offered by the market. The flexibility of gas turbines gathers both their “load-flexibility” or “operational flexibility”, and their “fuel-flexibility”
[59]. Load flexibility, in the context of gas turbines, pertains to the turbine’s ability to swiftly adjust its power output, contributing to the rapid stabilization of the electric grid. Various solutions have been suggested and put into practice in recent years, as noted in
[60][61][62]. These efforts have resulted in the achievement of a power ramp rate of 10% of the nominal power per minute, which appears to be satisfactory for gas turbine users at present. This capability should be maintained as we transition to hydrogen gas turbines in the future. Fuel flexibility, on the other hand, relates to a gas turbine’s capacity to operate effectively with various types of fuels, and more specifically for the present time, with fuel blends with a varying hydrogen content
[63]. However, despite the considerable efforts and investments made by various manufacturers in recent years, the actual fuel-flexibility of the machines, i.e., their stable, efficient, clean, reliable, and safe operation from 100% natural gas to 100% hydrogen with varying hydrogen content, is a challenge yet to be overcome due to technological issues in the combustion system.
Hydrogen significantly modifies the combustion characteristics of a reacting mixture in comparison to natural gas. Specifically, the following changes are observed: a decrease in the ignition delay time; the enlargement of flammability limits; an increase in laminar flame speed; an increase in turbulent flame speed, which also becomes more pressure-dependent
[64][65]; an increase in the adiabatic flame temperature. Hence, apart from issues related to its production, storage (lower vapor density), and transportation, safety issues also arise from such changes. In particular, the broader flammability range amplifies the potential for fuel ignition within the mixing passages; combustion dynamics can be dramatically affected by dangerous flashbacks due to the increased flame speed
[66] and/or thermo-acoustic instabilities
[67][68] that can lead to unexpected maintenance interventions, consequently diminishing the overall system’s reliability. Despite extensive research and industrial efforts over the years, the issue of combustion instability remains partially unresolved. The strategies typically employed to mitigate the potentially hazardous consequences of these instabilities in modern gas turbines primarily rely on empirical methods.
Controlling temperature peaks to restrict
NOx emissions requires special consideration. Keeping these peaks below the existing natural gas limits of 25 parts per million by volume (ppmv) seems to be a challenging endeavor for gas turbines operating within the 0–100% hydrogen range until 2030. Achieving this goal is difficult because higher temperatures can be easily reached in the process. It has been observed that hydrogen changes the exhaust gas composition (more water) and that the gas turbine power should also be considered. For these reasons, there is an open discussion in gas turbine associations and OEMs about how to take into account such operating conditions in the law limits: a very promising suggestion to discuss with regulatory entities is to quantify such emissions as the ratio between the mass of
NOx at the exit and the input fuel energy (mg/MJ)
[69].
Burning fuel mixtures with a high hydrogen content also has another significant impact—an increase in the steam content within the exhaust gases. This, in turn, leads to heightened heat exchange in the hot section of the gas turbine, necessitating additional cooling, as mentioned in
[70]. However, this increased exposure to steam also raises concerns about hot corrosion and shortens component lifecycles. To address these challenges, a commonly adopted solution is to reduce the turbine inlet temperature, which results in a reduction in the machine’s power output. While this approach can help mitigate the risk of hot corrosion, it comes at the cost of a 2% point reduction in efficiency, making it another challenging aspect to maintain until 2030, as discussed in
[71].
In the end, necessary modifications in design are highly contingent on the proportion of hydrogen intended for combustion. The utilization of hydrogen can be categorized into groups based on the volume percentage: low (5–10%), medium (10–50%), and high (more than 50%) blends. Turbines operating with a low hydrogen blend may not necessitate design alterations, as the fuel-burning characteristics closely resemble those of a pure natural gas stream. In the case of medium blends, the overall architecture and combustor of the turbine might remain largely unchanged, but adjustments to combustor materials, fuel nozzles, and control systems will be essential. The development of retrofit solutions for existing gas turbines is certainly a key factor. When dealing with higher hydrogen blends exceeding 50%, extensive modifications to the turbines become imperative, and a comprehensive retrofit of the combustion system is likely required. Numerous original equipment manufacturers (OEMs) are presently engaged in developing new combustion systems capable of accommodating elevated hydrogen blend levels.
4. Hard-to-Abate Industry
Constituting 38% of the total final energy demand, the industrial sector stands as the most substantial end-use segment. Notably, this sector is responsible for 26% of the global energy system’s
CO2 emissions
[72]. Involving processes heavily relying on fossil fuels, the hard-to-abate sector is the most challenging in reducing pollutant emissions
[44][73]: electrification is not a straightforward solution in most related industrial processes and the potential implementation of hydrogen opens some critical issues regarding the process itself and the quality of the product. Within the industrial landscape, hydrogen plays a role in utilizing 6% of the overall energy demand. This hydrogen is predominantly employed as feedstock in chemical production and as a reducing agent in the manufacturing of iron and steel. The annual industrial demand for hydrogen amounts to 51 million metric tons
[72].
Producers sell burners (generally working at atmospheric pressure) able to operate up to 100% H2 usually in the chemical or refining sectors, where a subproduct of hydrogen is produced. Due to the high flame temperature, combustors’ material have to be chosen suitably. In fact, the flame can easily attach close to the nozzles, making the choice of materials a serious concern. High-frequency noise can be avoided by means of silencers or damping devices. Some burners operating at 100% H2 are non-premixed to reduce NOx (for example, in the MILD or flameless combustion regime); others are DLE/DLN premixed. There exist burners without flashback up to 100% H2.
Almost all glass is produced in furnaces where a mixture of raw materials is combined and melted into a homogeneous mixture. Like many industrial production processes, glass melting can be classified as a continuous or discontinuous (batch) process. The melting point of most glass is around 1400–1600 °C, depending on its composition. Consequently, glass production requires a large amount of thermal energy. Because of this high energy demand, glass furnaces are built to minimise heat loss and often feature some form of waste heat recovery system: regenerative, recuperative, electrical. The most commonly used fuels for glass melting furnaces are natural gas, light and heavy fuel oil, and liquefied petroleum gas.
For the lime industry, in the most advanced dry process, raw materials are calcined at around 900–1250 °C in a pre-calciner to transform the limestone into lime, which releases
CO2 as a by-product. The materials are then fed into a rotary kiln, where they aggregate to form clinker at 1450 °C with flame temperatures reaching 2000 °C. The fuel can be coal gas or hydrogen-rich syngas. In the lime industry, the water content of the flue gas would pose a significant challenge
[44]. Calcium oxide in fact reacts with steam, causing problems with the quality, efficiency, durability, and safety of the product.
Besides being used as a reducing agent, hydrogen has great potential as a fuel in the steel production process. The possible applications of hydrogen mainly concern the production of pellets, the sintering process, reheating, and heat treatment furnaces. Several manufacturers of combustion systems for the steel industry already market some MILD or flameless type furnaces, which have been successfully tested with a variety of mixtures of natural gas and hydrogen, up to 100% hydrogen. In the MILD combustion mode, the heat release is distributed over a large volume, ensuring an even heat distribution, which is very effective for the material being processed.
Roller kilns are commonly utilized in the ceramics industry because of their efficiency in achieving temperature variations. They achieve this by moving the product through zones of different temperatures along the length of the kiln, rather than periodically heating and cooling the entire kiln. Additionally, they enhance productivity by allowing for continuous production. In the ceramics industry, industrial kilns used for ceramic tile production are predominantly of the roller type, typically fueled by gas. These kilns can be quite extensive, reaching lengths of up to 300 m, and they may have several hundreds of burners positioned along their length. Depending on the specific type of production, these kilns must be capable of maintaining temperatures of up to 1250 °C, while it is preferable to keep the silicon carbide (SiC) burner tubes, which contain the flame, at temperatures below 1300 °C. Fuels can be natural gas, LPG, diesel, paraffin, and other liquid fuels up to temperatures of 1800 °C. In the case of hydrogen, the great challenge concerns the moisture content of the combustion gases, since the ultimate goal of ceramic production is to remove moisture .
5. Conclusions
Hydrogen introduces a remarkable potential for a substantial reduction in CO2 emissions. Numerous technologies are currently in existence or in development, poised to facilitate the integration of hydrogen into the future energy landscape. While these technologies hold great promise, the primary hurdles lie in achieving the necessary scale and ensuring the economic viability and genuine environmental friendliness of hydrogen generation, distribution, and utilization.
In the power sector, gas turbines will persist as a crucial component within the global energy framework, as they complement renewable energy sources and possess a significant existing installed base. Gas turbines are called upon to serve as a backup (seasonal and peak), supporting variable renewable energy sources and maintaining the stability of the electric grid, including voltage and frequency control. This contribution to electric system flexibility is expected to be significant, even in a projection for the year 2040, which estimates that the annual operating hours will be less than 3000. In this context, post-combustion carbon capture technologies are deemed impractical, both from a technical and economic perspective, as indicated in a report by AECOM in 2020
[75]. It is worth noting that the potential annual reduction in carbon dioxide emissions is achievable with 100% hydrogen gas turbines exceeding 450 million tons in this scenario.
Despite the long history of gas turbines and their diverse applications across various sectors, ongoing research and development efforts are necessary to ensure their reliable and safe operation with a higher hydrogen content. The aim is to reach a hydrogen content of at least 80% by volume, which can lead to a substantial reduction in CO2 emissions. Original equipment manufacturers (OEMs) are dedicating substantial resources to incorporating hydrogen-burning technologies into their new engines and crafting modification packages for existing engines.
In the hard-to-abate sector, the reduction in pollutant emission is even more challenging. Electrification cannot be implemented in most industrial processes and hydrogen combustion cannot also be adopted in a straightforward way. In fact, substituting fuel blends with a high content for natural gas produces changes in the radiative energy emission and in exhaust gases composition that can have important effects on the industrial process and the quality of the products. Hence, some research is still needed and more importantly, communication between industries and research should be enhanced, especially in the glass and ceramic sectors. The application of hydrogen in furnaces for the steel industry is instead at an advanced stage.