You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yongjun Wei | -- | 2639 | 2023-10-23 09:55:49 | | | |
| 2 | Jessie Wu | Meta information modification | 2639 | 2023-10-23 10:09:23 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang, X.; Tang, B.; Wen, S.; Wang, Y.; Pan, C.; Qu, L.; Yin, Y.; Wei, Y. Epimedium Flavonoids. Encyclopedia. Available online: https://encyclopedia.pub/entry/50664 (accessed on 29 December 2025).
Zhang X, Tang B, Wen S, Wang Y, Pan C, Qu L, et al. Epimedium Flavonoids. Encyclopedia. Available at: https://encyclopedia.pub/entry/50664. Accessed December 29, 2025.
Zhang, Xiaoling, Bingling Tang, Sijie Wen, Yitong Wang, Chengxue Pan, Lingbo Qu, Yulong Yin, Yongjun Wei. "Epimedium Flavonoids" Encyclopedia, https://encyclopedia.pub/entry/50664 (accessed December 29, 2025).
Zhang, X., Tang, B., Wen, S., Wang, Y., Pan, C., Qu, L., Yin, Y., & Wei, Y. (2023, October 23). Epimedium Flavonoids. In Encyclopedia. https://encyclopedia.pub/entry/50664
Zhang, Xiaoling, et al. "Epimedium Flavonoids." Encyclopedia. Web. 23 October, 2023.
Copy Citation
Epimedium is a classical Chinese herbal medicine, which has been used extensively to treat various diseases, such as sexual dysfunction, osteoporosis, cancer, rheumatoid arthritis, and brain diseases. Flavonoids, such as icariin, baohuoside I, icaritin, and epimedin C, are the main active ingredients with diverse pharmacological activities.
flavonoids
pharmacological activities
extraction methods
biotransformation
biosynthesis
1. Introduction
The Epimedium genus, belonging to the Berberidaceae family, contains 68 species worldwide, with 58 of them (85.3%) distributed in China [1]. China is the center of geographical distribution and varieties of Epimedium. Over 15 Epimedium species have a long history of use in Traditional Chinese Medicine (TCM) and are believed to have kidney-nourishing and Yang-reinforcing properties [2]. Tao Hongjing, a renowned medical scientist, learned from shepherds that male sheep consuming a certain plant experienced significantly increased penile erections and mating frequency. Tao believed that this plant could enhance “Yang” energy, and named it “Yin-Yang-Huo” in Chinese [3]. It was later discovered that this was an Epimedium plant.
Epimedium was first mentioned over 2000 years ago in the “Shen Nong Ben Cao Jing”. It was later listed as a medium-grade herb in the “Ben Cao Gang Mu” by Li Shizhen during the Ming Dynasty [4]. In the Chinese Pharmacopoeia (2020 edition), Epimedii Folium (EF) refers to the dried leaves of four Epimedium plants, namely E. brevicornum Maxim, E. sagittatum (Sieb. et Zucc.) Maxim, E. pubescens Maxim, and E. koreanum Nakai [5]. EF is a classical herbal medicine. Alone, or combined within diverse prescriptions, it has been used to treat various diseases, including sexual dysfunction [6][7], osteoporosis [8], cancer [9], rheumatoid arthritis [10], and brain diseases [11]. Additionally, EF has been used in functional food production, and is available in alcoholic health beverages, health tea, and medicated gruel and noodle diets [2][12].
2. The Pharmacological Activities of Major Epimedium Flavonoids
More than 379 compounds have been detected in EF, including flavonoids, lignans, organic acids, terpenoids, dihydrophenanthrene derivatives, alkaloids, and other constituents [13]. Flavonoids, such as epimedin A, epimedin B, epimedin C, icariin, baohuoside I (also known as icariside II), icariside I, and icaritin have been recognized as major phytochemical and pharmacological active ingredients (Figure 1) [13][14]. These compounds differ in varying degrees of glycosylation at the C-3 and C-7 of icaritin [2][14]. There were great variations among the flavonoid contents in Epimedium from different species, collection and/or storage times and/or locations [15][16]. Icariin was the most abundant component in E. brevicornum Maxim and E. koreanum Nakai, followed by epimedin B, epimedin C, and epimedin A. However, epimedin C was the most abundant component in E. sagittatum (Sieb. et Zucc.) Maxim, E. pubescens Maxim, and E. wushanense T.S. Ying, followed by icariin, epimedin B, and epimedin A [15][16]. The average proportions of the total contents of epimedin A, B, C, and icariin to the 15 investigated flavonoid contents were 85.6%, 82.3%, 68.8%, 74.9%, and 69.8% in E. brevicornum Maxim, E. koreanum Nakai, E. sagittatum (Sieb. et Zucc.) Maxim, E. pubescens Maxim, and E. wushanense T.S. Ying, respectively [15][16]. In another study, epimedin A, B, C, and icariin accounted for over 52% of the total flavonoid contents in E. brevicornum Maxim [17]. In the Chinese Pharmacopoeia (2020 edition), the total amount of epimedin A, B, C and icariin was identified as the quality control indicator for the EF herb [5]. The major flavonoids in EF exhibit significant and diverse pharmacological activities (Figure 1).
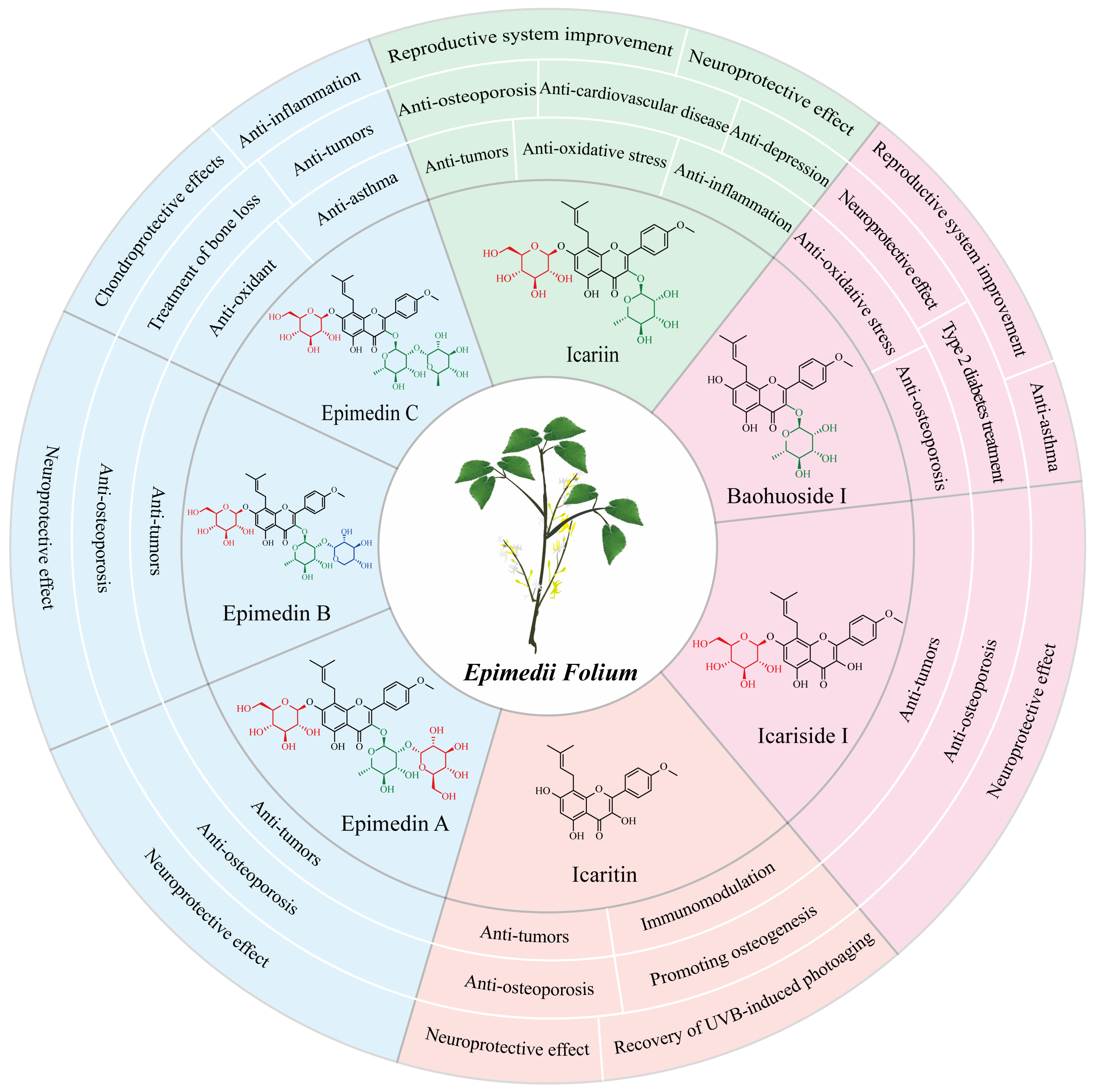
Figure 1. Chemical structures and pharmaceutical properties of the major flavonoids from Epimedii Folium.
2.1. Icariin and Its Pharmaceutical Effects
Icariin, the major bioactive component in EF (about 1%) [17], has been found to possess various pharmacological effects. These include improved reproductive system function, a neuroprotective effect, an anti-osteoporosis effect, protective effects from cardiovascular disease, an anti-inflammation effect, an anti-oxidative stress effect, an anti-depressive effect, and an anti-tumor effect [18][19][20][21]. In ancient China, EF was commonly used to treat sexual dysfunction [3]. Icariin can enhance erectile function in spontaneously hypertensive rats by reducing endothelial microparticle levels in the blood and inhibiting platelet activation [22]. In male mice, icariin can improve sexual function through the PI3K/AKT/eNOS/NO signaling pathway [23]. In the female reproductive system, icariin promotes estrogen biosynthesis in human ovarian granulosa-like KGN cells, and upregulates the expression of aromatase, which is responsible for the conversion of androgens to estrogens in vertebrates [24]. Additionally, icariin has exhibited protective effects in various nervous system disorders, including Alzheimer’s disease, Parkinson’s disease, and depressive disorder [20][25][26][27]. Moreover, icariin is regarded as a potential drug for osteoporosis treatment. Recent studies have demonstrated that icariin could prevent bone loss in ovariectomized rat models by modulating gut microbiota and regulating metabolite alterations [28] or by activating autophagy [29], as well as protect against iron overload-induced bone loss via suppressing oxidative stress [30].
2.2. Baohuoside I and Its Pharmaceutical Effects
Baohuoside I, although presents in low contents (<0.15%) in the raw material of EF compared to icariin, exhibits a wider range of pharmacological activities [31][32]. Baohuoside I has better bioavailability in vivo than icariin, as it is more easily absorbed by the capillaries of intestinal epithelial cells because of its lower polarity [33]. Cheng et al. found that 91.2% of icariin was converted to baohuoside I after oral administration in rats [34]. Similarly, human intestinal microflora metabolized most icariin to baohuoside I in a short time before absorption in the human intestine [35]. Baohuoside I has been proved to have a significant therapeutic effect on various diseases, such as sexual dysfunction, osteoporosis, and cancers [9][36][37][38]. For improving erectile dysfunction, baohuoside I could facilitate the differentiation of adipose-derived stem cells into Schwann cells and preserve the erectile function of bilateral cavernous nerve injury (BCNI) in rats [36][39]. The anti-osteoporotic activity of baohuoside I was suggested to be associated with its ability to induce bone marrow stromal cell differentiation into osteoblasts while inhibiting adipocyte formation, regulating immune functions, and providing antioxidant activity [40]. Baohuoside I could inhibit osteoclastogenesis and protect against ovariectomy-induced bone loss in mice, surpassing the effects of icariin [38]. Current studies have shown that baohuoside I exhibits promising anti-tumor effects on lung cancer cells [41], melanoma cells [42], breast cancer cells [43], prostate cancer cells [44], and osteosarcoma cells [45]. Furthermore, baohuoside I has shown its potential application in type 2 diabetes treatment [46], neuroprotection [47], and asthma inhibition [48].
2.3. Icaritin and Its Pharmaceutical Effects
Icaritin is a flavonoid aglycone in EF [14], which can be generated by hydrolytic reactions that remove the glycone parts of icariin, baohuoside I, and icariside I [49][50]. Icaritin possesses diverse pharmacological activities [51], including protection of neurons against amyloid-induced neurotoxicity [52], promotion of differentiation from embryonic stem cells into cardiomyocytes [53], anti-osteoporosis effects and osteogenesis promotion [54][55], immunomodulation [56][57], and recovery of UVB-induced photoaging of human keratinocytes [58]. Moreover, icaritin is considered as a promising candidate for the treatment of various cancers [59][60], including hepatocellular carcinoma [61][62][63], breast cancer [64], lung cancer [65], ovarian cancer [66], endometrial cancer [67], human oral squamous cell carcinoma [68], and multiple hematological malignancies [59][69][70][71]. In the treatment of hepatocellular carcinoma, icaritin can suppress cell growth and promote cell apoptosis via down-regulating alpha-fetoprotein gene expression in hepatocellular carcinoma [62] and inducing anti-tumor immune responses [61][63]. In 2022, an icaritin soft capsule was marketed as a small molecule immunomodulatory drug, providing a solution for patients with advanced hepatocellular carcinoma with poor prognosis, and significantly improving the life quality of patients with hepatocellular carcinoma [72][73].
2.4. Epimedin C and Its Pharmaceutical Effects
Epimedin C is a trioglycoside ingredient in EF, with the highest content among all flavonol glycosides in certain Epimedium species, such as E. brevicornu Maxim [74], E. wushanense T.S. Ying, and E. sagittatum Maxim [75]. Epimedin C is considered as the quality control standard for evaluating the quality of E. wushanense T.S. Ying in the Chinese Pharmacopoeia (2020 edition) [5]. The pharmacological activities of epimedin C mainly include treatment of bone loss, anti-oxidant effects, and anti-inflammation. Epimedin C has shown significant anti-inflammatory and chondroprotective effects by increasing the expression of extracellular matrix components in osteoarthritis chondrocytes [76]. Epimedin C could alleviate the suppressive impact of dexamethasone on the osteogenesis of larval zebrafish and MC3T3-E1 cells via triggering the PI3K/AKT/RUNX2 signaling pathway [77]. Notably, epimedin C has a stronger anti-osteoporosis effect than icariin at the same dose on dexamethasone-induced osteoporosis in a mouse model [78]. Furthermore, epimedin C has been found to protect against H2O2-induced peroxidation injury by enhancing the function of endothelial progenitor human umbilical vein endothelial cells, which plays an important role in repairing endothelial cell vascular injury [79]. In an ovalbumin-induced murine asthma model, epimedin C was demonstrated to dose-dependently decrease the protein levels of p52 and RelB, and the phosphorylation of ERK1/2, and p38 MAPK, which are pivotal in the development of Th9 cells and Treg cells, thereby inhibiting airway inflammation [80].
2.5. Other Flavonoids and Their Pharmaceutical Effects
Other flavonoids presented in EF include epimedin A and B, icariside I, and sagittatoside A, B, and C [2][13]. Their contents in EF are very low, and limited pharmacological research is available on them. However, similar to the major flavonoids described above, icariside I, epimedin A, and epimedin B also exhibit anti-osteoporosis, neuroprotective, and anti-cancer effects. Epimedin A has shown excellent efficacy against senile osteoporosis [8], and in vitro and in vivo experiments demonstrated that a complex epimedin A drug significantly enhances bone regeneration [81]. In addition, epimedin A could ameliorate 2,4-dinitrofluorobenzene (DNFB)-induced allergic contact dermatitis in mice, due to its ability to suppress the NF-κB/NLRP3 pathway, enhance the Nrf2 pathway, and modulate local inflammation [82]. Diao et al. provided evidence that epimedin B ameliorates osteoporosis in male mice via regulating PI3K-Akt, MAPK, and PPAR signaling pathways [83]. Additionally, epimedin B can exert a neuroprotective effect against Parkinson’s disease in an MPTP-induced mouse model [84]. Chen et al. suggested that icariside I performed tumor immunotherapy activity by blocking the kynurenine-AhR pathway and tumor immune escape [85]. Icariside I could significantly inhibit B16F10 melanoma growth in vivo through regulation of gut microbiota and host immunity [85]. Moreover, icariside I also effectively ameliorated estrogen deficiency-induced osteoporosis in an ovariectomy mouse model [86].
In addition to the various beneficial effects of EF flavonoids, it is important to note that EF can potentially cause drug-induced liver injury (Table 1). In clinical applications, there are increasing evidences indicate that Zhuangguguanjie pills and Xianlinggubao capsules have toxic effects, leading to liver injury in humans [87][88]. Both medicines contain EF as their major components, and are used to treat rheumatism, bone pain, arthritis, osteoporosis, and other diseases. Recently, animal studies have indicated that EF extracts can cause liver toxicity in mice and rats, with the severity of hepatotoxic effects increasing with higher dosages and prolonged exposure [89][90]. However, the exact compound(s) and the underlying mechanisms contributing to the observed liver toxicity remain unclear. Zhang et al. suggested that icariside I and sagittatoside A are the most relevant compounds related to the hepatotoxicity of EF extracts [91]. Epimedin C has been reported to have potential hepatotoxicity. Song et al. revealed that mRNA methylation might be associated with epimedin C-induced liver injury by the UPLC-MS/MS method [92]. When treated with the normal human liver cell line (HL-7702) and human hepatocellular carcinoma cell line (HepG2), 2″-O-Rhamnosyl icariside II, baohuoside I, and baohuoside II showed significant dose-toxic effects, and baohuoside I was more likely to be involved in the hepatotoxicity of EF [93]. Therefore, the hepatotoxicity of EF, like other TCMs, is probably due to the combined effects of multiple components. Further investigations are needed to fully understand the hepatotoxicity mechanism in order to avoid EF-induced liver injury.
Table 1. The published mechanisms of the hepatotoxicity effects of Epimedium flavonoids.
| Epimedium Flavonoids | Research Systems | Mechanisms | Reference |
|---|---|---|---|
| Alcohol extracts of E. koreanum Nakai and E. wushanense T.S. Ying | SD rats | Compared with the normal group, animal groups treated with EF extracts showed severer hepatotoxicity, which was positively correlated with the dose and course. Additionally, the females experienced more significant damage compared to the males. | [90] |
| Icariside I and sagittatoside A | HL-7702 and HepG2 cells | Icariside I could destroy the cell structure and cause oxidative stress. Sagittatoside A could cause oxidative stress and damage to mitochondria. | [91] |
| Epimedin C | Male Balb/c mice | Epigenetic modification changed in mouse liver after epimedin C treatment with a test dose, and the m6A and m5C may be associated with epimedin C-induced liver injury. | [92] |
| Baohuoside I | HL-7702 and HepG2 cells | The toxicity mechanism(s) of baohuoside I may be involved in increasing oxidative stress and inducing apoptosis. | [93] |
| E. koreanum Nakai ethanol extract | Male Sprague Dawley rats | The mechanism of hepatotoxicity of E. koreanum Nakai was probably related to the induction of ferroptosis in hepatocytes. | [94] |
3. Extraction Methods of Epimedium Flavonoids
Currently, commercially available Epimedium flavonoids are extracted from Epimedium plants. Several techniques have been developed for isolating flavonoids from Epimedium (Figure 2), including hot water extraction, alcohol extraction, ultrasonic extraction, microwave-assisted extraction, and ultra-high-pressure extraction. Among these techniques, hot water extraction and alcohol extraction have been implemented in industrial production, while others are at the lab-scale stage.
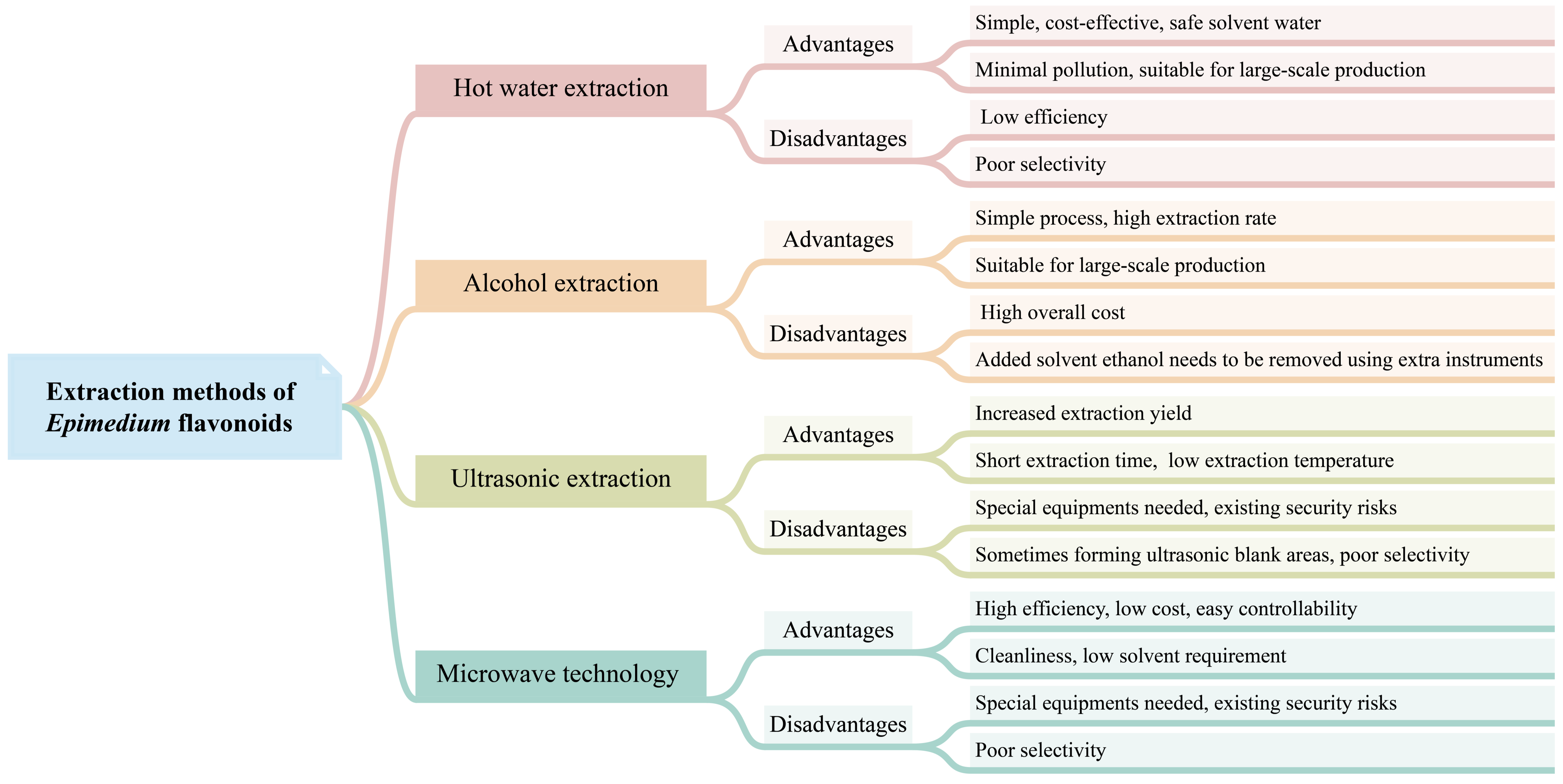
Figure 2. The advantages and disadvantages of different extraction methods of Epimedium flavonoids.
3.1. Hot Water Extraction
Hot water extraction is a traditional method used for decocting Chinese herbs. In this method, the crushed herbs are immersed in water in a container for an appropriate amount of time, then heated and gently boiled for a certain period of time. The liquid is subsequently filtered, and the process of decoction is repeated 2–3 times. The decocted liquids from each iteration are mixed and concentrated to achieve the desired flavonoid concentration. Wang et al. optimized the hot water extraction procedure with an orthogonal test [95]. The results showed that the optimized extraction procedure was 2% Na2CO3, 15 times the water volume of the weight of dried material, with three 1.5 h extractions. The final extracting ratio of the total flavonoids was 97.92%. Other new technologies, such as microwave technology, have been used to enhance hot water extraction. Compared to the conventional hot water extraction method, microwave-assisted extraction offers higher extraction efficiency and is time-saving [96]. The hot water extraction process is simple and cost-effective, and utilizes water as a safe solvent. The whole process generates minimal pollution. Therefore, hot water extraction is suitable for the large-scale production of flavonoids. However, the efficiency of hot water extraction for flavonoid extraction is low, and it lacks selectivity in capturing specific flavonoids.
3.2. Alcohol Extraction
The alcohol extraction method is the most commonly used technique for extracting flavonoids, adopted by the Chinese Pharmacopoeia (2020 edition) [5]. In this method, ethanol is generally employed as the extraction solvent. The process of the alcohol extraction method is relatively simple, and well-suited for industrial applications. However, a large amount of ethanol is added to the extraction reactor, which subsequently needs to be removed using extra instruments. As a result, the overall cost of this method is higher compared to hot water extraction. Zhang et al. demonstrated that the extraction rate of icariin using the alcohol extraction method was significantly higher than that of the water extraction method [75]. The optimal extraction parameters were determined to be 50% ethanol, 1:10 solid–liquid ratio, 60 °C extraction temperature, 2 h extraction time, and two extraction cycles. In addition, an ultrasonic-assisted ethanol extraction procedure has shown to increase the extraction yield of epimedin A, epimedin B, epimedin C, and icariin from Herba Epimedii, when compared to the conventional ethanol boiling extraction method [75].
3.3. Other Extraction Methods
Ultrasonic extraction utilizes the effects of strong vibrations, cavitation, and thermal energy generated by ultrasound to extract the active components of plants into solvents. Ultrasonic extraction is regarded as a powerful tool for extracting flavonoids from plant biomass, offering several advantages, such as increased extraction yield, shorter extraction time, and lower extraction temperature [97]. Microwave technology utilizes the ability to generate heat within cells and vaporize water to break down the cell walls, allowing for better release of active ingredients in plant cells. The microwave technique presents numerous benefits, including high efficiency, low energy consumption, short processing time, low cost, cleanliness, easy controllability, and low solvent requirement [98]. Both ultrasonic extraction and the microwave technique are often used to assist common extraction methods, such as water extraction and alcohol extraction, to improve the efficiency of extracting Epimedium flavonoids [75][96][99][100]. Furthermore, ultra-high-pressure extraction has also been utilized for extracting flavonoids from E. sagittatum. Compared to heating extraction and ultrasonic-assisted extraction, ultra-high-pressure extraction presents distinctive advantages in superior extraction yield and a higher percentage of marker compounds [101].
References
- Xu, Y.Q.; Jiang, Y.; Huang, H.; Li, R.Q.; Li, F.Q.; Liu, Y.; Huang, X.F. Taxonomic study of Epimedium L.: Status, issues and prospect. Guihaia 2020, 40, 601–617.
- Ma, H.; He, X.; Yang, Y.; Li, M.; Hao, D.; Jia, Z. The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011, 134, 519–541.
- Niu, Y.; Lin, G.; Pan, J.; Liu, J.; Xu, Y.; Cai, Q.; Wang, T.; Luan, Y.; Chen, Y.; Feng, Y.; et al. Deciphering the myth of icariin and synthetic derivatives in improving erectile function from a molecular biology perspective: A narrative review. Transl. Androl. Urol. 2022, 11, 1007–1022.
- Seyedi, Z.; Amiri, M.S.; Mohammadzadeh, V.; Hashemzadeh, A.; Haddad-Mashadrizeh, A.; Mashreghi, M.; Qayoomian, M.; Hashemzadeh, M.R.; Simal-Gandara, J.; Taghavizadeh Yazdi, M.E. Icariin: A promising natural product in biomedicine and tissue engineering. J. Funct. Biomater. 2023, 14, 44.
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; Volume 1.
- Punyawudho, B.; Puttilerpong, C.; Wirotsaengthong, S.; Aramwit, P. A randomized, double-blind, placebo-controlled crossover study of Cappra® for the treatment of mild or mild to moderate erectile dysfunction in Thai male. Afr. J. Tradit. Complement. Altern. Med. 2012, 10, 310–315.
- Zhang, H.; Sun, Z.X.; Men, B.; Fu, X.J.; Chen, J.S. Clinical and mechanism research on functional erectile dysfunction treated with moxibustion and qiangshen shugan qiwei decoction. Zhongguo Zhen Jiu 2021, 41, 1325–1330.
- Wang, L.; Li, Y.; Guo, Y.; Ma, R.; Fu, M.; Niu, J.; Gao, S.; Zhang, D. Herba Epimedii: An ancient Chinese herbal medicine in the prevention and treatment of osteoporosis. Curr. Pharm. Des. 2016, 22, 328–349.
- Chen, M.; Wu, J.; Luo, Q.; Mo, S.; Lyu, Y.; Wei, Y.; Dong, J. The anticancer properties of Herba Epimedii and its main bioactive components icariin and icariside II. Nutrients 2016, 8, 563.
- Zhang, L.B.; Yan, Y.; He, J.; Wang, P.P.; Chen, X.; Lan, T.Y.; Guo, Y.X.; Wang, J.P.; Luo, J.; Yan, Z.R.; et al. Epimedii Herba: An ancient Chinese herbal medicine in the prevention and treatment of rheumatoid arthritis. Front. Chem. 2022, 10, 1023779.
- Cho, J.H.; Jung, J.Y.; Lee, B.J.; Lee, K.; Park, J.W.; Bu, Y. Epimedii Herba: A promising herbal medicine for neuroplasticity. Phytother. Res. 2017, 31, 838–848.
- Zhang, H.F.; Yang, X.H. Application of Herba Epimedii in food industry: Current status and prospect. Sci. Technol. Food Ind. 2010, 31, 390–393.
- Qian, H.Q.; Wu, D.C.; Li, C.Y.; Liu, X.R.; Han, X.K.; Peng, Y.; Zhang, H.; Zhao, B.Y.; Zhao, Y. A systematic review of traditional uses, phytochemistry, pharmacology and toxicity of Epimedium koreanum Nakai. J. Ethnopharmacol. 2024, 318, 116957.
- Lin, Y.; Chen, W.W.; Ding, B.; Guo, M.; Liang, M.; Pang, H.; Wei, Y.T.; Huang, R.B.; Du, L.Q. Highly efficient bioconversion of icariin to icaritin by whole-cell catalysis. Microb. Cell Fact. 2023, 22, 64.
- Chen, X.J.; Ji, H.; Zhang, Q.W.; Tu, P.F.; Wang, Y.T.; Guo, B.L.; Li, S.P. A rapid method for simultaneous determination of 15 flavonoids in Epimedium using pressurized liquid extraction and ultra-performance liquid chromatography. J. Pharm. Biomed. Anal. 2008, 46, 226–235.
- Chen, X.J.; Guo, B.L.; Li, S.P.; Zhang, Q.W.; Tu, P.F.; Wang, Y.T. Simultaneous determination of 15 flavonoids in Epimedium using pressurized liquid extraction and high-performance liquid chromatography. J. Chromatogr. A 2007, 1163, 96–104.
- Gao, Y.; Shi, W.; Tu, C.; Li, P.; Zhao, G.; Xiao, X.; Wang, J.; Bai, Z. Immunostimulatory activity and structure-activity relationship of epimedin B from Epimedium brevicornu Maxim. Front. Pharmacol. 2022, 13, 1015846.
- He, C.; Wang, Z.; Shi, J. Pharmacological effects of icariin. Adv. Pharmacol. 2020, 87, 179–203.
- Li, Z.; Li, D.; Chen, R.; Gao, S.; Xu, Z.; Li, N. Cell death regulation: A new way for natural products to treat osteoporosis. Pharmacol. Res. 2023, 187, 106635.
- Wang, S.; Ma, J.; Zeng, Y.; Zhou, G.; Wang, Y.; Zhou, W.; Sun, X.; Wu, M. Icariin, an up-and-coming bioactive compound against neurological diseases: Network pharmacology-based study and literature review. Drug Des. Devel. Ther. 2021, 15, 3619–3641.
- Zeng, Y.; Xiong, Y.; Yang, T.; Wang, Y.; Zeng, J.; Zhou, S.; Luo, Y.; Li, L. Icariin and its metabolites as potential protective phytochemicals against cardiovascular disease: From effects to molecular mechanisms. Biomed. Pharmacother. 2022, 147, 112642.
- Li, X.; Yang, H.F.; Chen, Y.; Pei, L.J.; Jiang, R. Effect of the icariin on endothelial microparticles, endothelial progenitor cells, platelets, and erectile function in spontaneously hypertensive rats. Andrology 2022, 10, 576–584.
- Ding, J.; Tang, Y.; Tang, Z.; Zu, X.; Qi, L.; Zhang, X.; Wang, G. Icariin improves the sexual function of male mice through the PI3K/AKT/eNOS/NO signalling pathway. Andrologia 2018, 50, e12802.
- Yang, L.; Lu, D.; Guo, J.; Meng, X.; Zhang, G.; Wang, F. Icariin from Epimedium brevicornum Maxim promotes the biosynthesis of estrogen by aromatase (CYP19). J. Ethnopharmacol. 2013, 145, 715–721.
- Angeloni, C.; Barbalace, M.C.; Hrelia, S. Icariin and its metabolites as potential protective phytochemicals against Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 271.
- Wang, G.Q.; Li, D.D.; Huang, C.; Lu, D.S.; Zhang, C.; Zhou, S.Y.; Liu, J.; Zhang, F. Icariin reduces dopaminergic neuronal loss and microglia-mediated inflammation in vivo and in vitro. Front. Mol. Neurosci. 2017, 10, 441.
- Wei, K.; Xu, Y.; Zhao, Z.; Wu, X.; Du, Y.; Sun, J.; Yi, T.; Dong, J.; Liu, B. Icariin alters the expression of glucocorticoid receptor, FKBP5 and SGK1 in rat brains following exposure to chronic mild stress. Int. J. Mol. Med. 2016, 38, 337–344.
- Wang, S.; Wang, S.; Wang, X.; Xu, Y.; Zhang, X.; Han, Y.; Yan, H.; Liu, L.; Wang, L.; Ye, H.; et al. Effects of icariin on modulating gut microbiota and regulating metabolite alterations to prevent bone loss in ovariectomized rat model. Front. Endocrinol. 2022, 13, 874849.
- Liang, X.; Hou, Z.; Xie, Y.; Yan, F.; Li, S.; Zhu, X.; Cai, L. Icariin promotes osteogenic differentiation of bone marrow stromal cells and prevents bone loss in OVX mice via activating autophagy. J. Cell Biochem. 2019, 120, 13121–13132.
- Jing, X.; Du, T.; Chen, K.; Guo, J.; Xiang, W.; Yao, X.; Sun, K.; Ye, Y.; Guo, F. Icariin protects against iron overload-induced bone loss via suppressing oxidative stress. J. Cell Physiol. 2019, 234, 10123–10137.
- Lu, S.; Zou, K.; Guo, B.; Pei, J.; Wang, Z.; Xiao, W.; Zhao, L. One-step purification and immobilization of thermostable β-glucosidase on Na-Y zeolite based on the linker and its application in the efficient production of baohuoside I from icariin. Bioorg. Chem. 2022, 121, 105690.
- Xie, J.; Xu, H.; Jiang, J.; Zhang, N.; Yang, J.; Zhao, J.; Wei, M. Characterization of a novel thermostable glucose-tolerant GH1 β-glucosidase from the hyperthermophile Ignisphaera aggregans and its application in the efficient production of baohuoside I from icariin and total epimedium flavonoids. Bioorg. Chem. 2020, 104, 104296.
- Yan, H.; Song, J.; Jia, X.; Zhang, Z. Hyaluronic acid-modified didecyldimethylammonium bromide/ d-a-tocopheryl polyethylene glycol succinate mixed micelles for delivery of baohuoside I against non-small cell lung cancer: In vitro and in vivo evaluation. Drug Deliv. 2017, 24, 30–39.
- Cheng, T.; Zhang, Y.; Zhang, T.; Lu, L.; Ding, Y.; Zhao, Y. Comparative pharmacokinetics study of icariin and icariside II in rats. Molecules 2015, 20, 21274–21286.
- Wu, H.; Kim, M.; Han, J. Icariin metabolism by human intestinal microflora. Molecules 2016, 21, 1158.
- Ge, P.; Guo, Y.; Shen, J. Icariside II facilitates the differentiation of ADSCs to SCs via let-7i/STAT3 axis to preserve erectile function. Biol. Res. 2019, 52, 54.
- Khan, M.; Maryam, A.; Qazi, J.I.; Ma, T. Targeting apoptosis and multiple signaling pathways with icariside II in cancer cells. Int. J. Biol. Sci. 2015, 11, 1100–1112.
- Ma, M.; Fan, A.Y.; Liu, Z.; Yang, L.Q.; Huang, J.M.; Pang, Z.Y.; Yin, F. Baohuoside I inhibits osteoclastogenesis and protects against ovariectomy-induced bone loss. Front. Pharmacol. 2022, 13, 874952.
- Zheng, T.; Zhang, T.; Zhang, W.; Lv, K.; Jia, D.; Yang, F.; Sun, Y.; Lian, J.; Wang, R. Icariside II facilitates the differentiation of ADSCs to schwann cells and restores erectile dysfunction through regulation of miR-33/GDNF axis. Biomed. Pharmacother. 2020, 125, 109888.
- Xi, Y.; Jiang, T.; Yu, J.; Xue, M.; Xu, N.; Wen, J.; Wang, W.; He, H.; Ye, X. Preliminary studies on the anti-osteoporosis activity of Baohuoside I. Biomed. Pharmacother. 2019, 115, 108850.
- Kong, Q.; Ma, M.; Zhang, L.; Liu, S.; He, S.; Wu, J.; Liu, B.; Dong, J. Icariside II potentiates the anti-PD-1 antitumor effect by reducing chemotactic infiltration of myeloid-derived suppressor cells into the tumor microenvironment via ROS-mediated inactivation of the SRC/ERK/STAT3 signaling pathways. Phytomedicine 2023, 110, 154638.
- Peng, Y.G.; Zhang, L. Baohuoside-I suppresses cell proliferation and migration by up-regulating miR-144 in melanoma. Pharm. Biol. 2018, 56, 43–50.
- Wang, S.; Wang, N.; Huang, X.; Yang, B.; Zheng, Y.; Zhang, J.; Wang, X.; Lin, Y.; Wang, Z. Baohuoside i suppresses breast cancer metastasis by downregulating the tumor-associated macrophages/C-X-C motif chemokine ligand 1 pathway. Phytomedicine 2020, 78, 153331.
- Li, S.; Zhan, Y.; Xie, Y.; Wang, Y.; Liu, Y. The impact of icariside II on human prostate cancer cell proliferation, mobility, and autophagy via PI3K-AKT-mTOR signaling pathway. Drug Des. Devel. Ther. 2020, 14, 4169–4178.
- Choi, H.J.; Eun, J.S.; Kim, D.K.; Li, R.H.; Shin, T.Y.; Park, H.; Cho, N.P.; Soh, Y. Icariside II from Epimedium koreanum inhibits hypoxia-inducible factor-1alpha in human osteosarcoma cells. Eur. J. Pharmacol. 2008, 579, 58–65.
- Kim, D.H.; Jung, H.A.; Sohn, H.S.; Kim, J.W.; Choi, J.S. Potential of icariin metabolites from Epimedium koreanum Nakai as antidiabetic therapeutic agents. Molecules 2017, 22, 986.
- Gao, J.; Ma, C.; Xia, D.; Chen, N.; Zhang, J.; Xu, F.; Li, F.; He, Y.; Gong, Q. Icariside II preconditioning evokes robust neuroprotection against ischaemic stroke, by targeting Nrf2 and the OXPHOS/NF-κB/ferroptosis pathway. Br. J. Pharmacol. 2023, 180, 308–329.
- Zhou, Y.; Huang, X.; Yu, H.; Shi, H.; Chen, M.; Song, J.; Tang, W.; Teng, F.; Li, C.; Yi, L.; et al. TMT-based quantitative proteomics revealed protective efficacy of Icariside II against airway inflammation and remodeling via inhibiting LAMP2, CTSD and CTSS expression in OVA-induced chronic asthma mice. Phytomedicine 2023, 118, 154941.
- Wang, Z.; Liu, C.; Yu, H.; Wu, B.; Huai, B.; Zhuang, Z.; Sun, C.; Xu, L.; Jin, F. Icaritin preparation from icariin by a special Epimedium flavonoid-glycosidase from Aspergillus sp. y848 strain. J. Microbiol. Biotechnol. 2022, 32, 437–446.
- Zhang, S.; Luo, J.; Dong, Y.; Wang, Z.; Xiao, W.; Zhao, L. Biotransformation of the total flavonoid extract of epimedium into icaritin by two thermostable glycosidases from Dictyoglomus thermophilum DSM3960. Process Biochem. 2021, 105, 8–18.
- Huong, N.T.; Son, N.T. Icaritin: A phytomolecule with enormous pharmacological values. Phytochemistry 2023, 213, 113772.
- Wang, Z.; Zhang, X.; Wang, H.; Qi, L.; Lou, Y. Neuroprotective effects of icaritin against beta amyloid-induced neurotoxicity in primary cultured rat neuronal cells via estrogen-dependent pathway. Neuroscience 2007, 145, 911–922.
- Zhu, D.Y.; Lou, Y.J. Inducible effects of icariin, icaritin, and desmethylicaritin on directional differentiation of embryonic stem cells into cardiomyocytes in vitro. Acta Pharmacol. Sin. 2005, 26, 477–485.
- Gao, L.; Zhang, S.Q. Antiosteoporosis effects, pharmacokinetics, and drug delivery systems of icaritin: Advances and prospects. Pharmaceuticals 2022, 15, 397.
- Wei, Q.; Wang, B.; Hu, H.; Xie, C.; Ling, L.; Gao, J.; Cao, Y. Icaritin promotes the osteogenesis of bone marrow mesenchymal stem cells via the regulation of sclerostin expression. Int. J. Mol. Med. 2020, 45, 816–824.
- Bi, Z.; Zhang, W.; Yan, X. Anti-inflammatory and immunoregulatory effects of icariin and icaritin. Biomed. Pharmacother. 2022, 151, 113180.
- Liao, J.; Liu, Y.; Wu, H.; Zhao, M.; Tan, Y.; Li, D.; Long, H.; Dai, Y.; Yung, S.; Chan, T.M.; et al. The role of icaritin in regulating Foxp3/IL17a balance in systemic lupus erythematosus and its effects on the treatment of MRL/lpr mice. Clin. Immunol. 2016, 162, 74–83.
- Hwang, E.; Lin, P.; Ngo, H.T.T.; Gao, W.; Wang, Y.S.; Yu, H.S.; Yi, T.H. Icariin and icaritin recover UVB-induced photoaging by stimulating Nrf2/ARE and reducing AP-1 and NF-κB signaling pathways: A comparative study on UVB-irradiated human keratinocytes. Photochem. Photobiol. Sci. 2018, 17, 1396–1408.
- Yang, X.J.; Xi, Y.M.; Li, Z.J. Icaritin: A novel natural candidate for hematological malignancies therapy. Biomed. Res. Int. 2019, 2019, 4860268.
- Zhang, C.; Sui, X.; Jiang, Y.; Wang, X.; Wang, S. Antitumor effects of icaritin and the molecular mechanisms. Discov. Med. 2020, 29, 5–16.
- Fan, Y.; Li, S.; Ding, X.; Yue, J.; Jiang, J.; Zhao, H.; Hao, R.; Qiu, W.; Liu, K.; Li, Y.; et al. First-in-class immune-modulating small molecule Icaritin in advanced hepatocellular carcinoma: Preliminary results of safety, durable survival and immune biomarkers. BMC Cancer 2019, 19, 279.
- Li, H.; Liu, Y.; Jiang, W.; Xue, J.; Cheng, Y.; Wang, J.; Yang, R.; Zhang, X. Icaritin promotes apoptosis and inhibits proliferation by down-regulating AFP gene expression in hepatocellular carcinoma. BMC Cancer 2021, 21, 318.
- Tao, H.; Liu, M.; Wang, Y.; Luo, S.; Xu, Y.; Ye, B.; Zheng, L.; Meng, K.; Li, L. Icaritin induces anti-tumor immune responses in hepatocellular carcinoma by inhibiting splenic myeloid-derived suppressor cell generation. Front. Immunol. 2021, 12, 609295.
- Wang, X.; Zheng, N.; Dong, J.; Wang, X.; Liu, L.; Huang, J. Estrogen receptor-α36 is involved in icaritin induced growth inhibition of triple-negative breast cancer cells. J. Steroid Biochem. Mol. Biol. 2017, 171, 318–327.
- Zheng, Q.; Liu, W.W.; Li, B.; Chen, H.J.; Zhu, W.S.; Yang, G.X.; Chen, M.J.; He, G.Y. Anticancer effect of icaritin on human lung cancer cells through inducing S phase cell cycle arrest and apoptosis. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2014, 34, 497–503.
- Gao, L.; Chen, M.; Ouyang, Y.; Li, R.; Zhang, X.; Gao, X.; Lin, S.; Wang, X. Icaritin induces ovarian cancer cell apoptosis through activation of p53 and inhibition of Akt/mTOR pathway. Life Sci. 2018, 202, 188–194.
- Jin, Y.B.; Liang, X.C.; Cai, J.H.; Wang, K.; Wang, C.Y.; Wang, W.H.; Chen, X.L.; Bao, S. Mechanism of action of icaritin on uterine corpus endometrial carcinoma based on network pharmacology and experimental evaluation. Front. Oncol. 2023, 13, 1205604.
- Yang, J.G.; Lu, R.; Ye, X.J.; Zhang, J.; Tan, Y.Q.; Zhou, G. Icaritin reduces oral squamous cell carcinoma progression via the inhibition of STAT3 signaling. Int. J. Mol. Sci. 2017, 18, 132.
- Wu, T.; Wang, S.; Wu, J.; Lin, Z.; Sui, X.; Xu, X.; Shimizu, N.; Chen, B.; Wang, X. Icaritin induces lytic cytotoxicity in extranodal NK/T-cell lymphoma. J. Exp. Clin. Cancer Res. 2015, 34, 17.
- Zhu, J.; Li, Z.; Zhang, G.; Meng, K.; Kuang, W.; Li, J.; Zhou, X.; Li, R.; Peng, H.; Dai, C.; et al. Icaritin shows potent anti-leukemia activity on chronic myeloid leukemia in vitro and in vivo by regulating MAPK/ERK/JNK and JAK2/STAT3/AKT signalings. PLoS ONE 2011, 6, e23720.
- Zhu, S.; Wang, Z.; Li, Z.; Peng, H.; Luo, Y.; Deng, M.; Li, R.; Dai, C.; Xu, Y.; Liu, S.; et al. Icaritin suppresses multiple myeloma, by inhibiting IL-6/JAK2/STAT3. Oncotarget 2015, 6, 10460–10472.
- Liu, X.; Yang, F.; Jia, D.; Dong, X.; Zhang, Y.; Wu, Z. Case report: A case study on the treatment using icaritin soft capsules in combination with lenvatinib achieving impressive PR and stage reduction in unresectable locally progressive pancreatic cancer and a literature review. Front. Genet. 2023, 14, 1167470.
- Tang, X.; Zhang, Y.; Dong, X.; Jiang, G.; Hong, D.; Liu, X. The synergy of gene targeting drug icaritin soft capsule with immunomodulator and TACE brings new hope for drug combination in patients with advanced liver cancer: A case report and literature review. Cancer Manag. Res. 2023, 15, 707–717.
- Guo, Y.; Wang, X.; Gao, J. Simultaneous preparation and comparison of the osteogenic effects of epimedins A–C and icariin from Epimedium brevicornu. Chem. Biodivers. 2018, 15, e1700578.
- Zhang, H.F.; Yang, T.S.; Li, Z.Z.; Wang, Y. Simultaneous extraction of epimedin A, B, C and icariin from Herba Epimedii by ultrasonic technique. Ultrason. Sonochem. 2008, 15, 376–385.
- Ziadlou, R.; Barbero, A.; Martin, I.; Wang, X.; Qin, L.; Alini, M.; Grad, S. Anti-inflammatory and chondroprotective effects of vanillic acid and epimedin C in human osteoarthritic chondrocytes. Biomolecules 2020, 10, 932.
- Xu, Y.; Chen, S.; Huang, L.; Han, W.; Shao, Y.; Chen, M.; Zhang, Y.; He, R.; Xie, B. Epimedin C alleviates glucocorticoid-induced suppression of osteogenic differentiation by modulating PI3K/AKT/RUNX2 signaling pathway. Front. Pharmacol. 2022, 13, 894832.
- Liu, Y.L.; Huang, M.; Feng, J.; Xia, P.; Wang, Y.; Wei, X.; Qiu, L. Effects of icariin and epimedium C on microstructure of bone tissue in glucocorticoid osteoporosis model mice based on Micro-CT technique. Drug Eval. Res. 2020, 43, 1733–1739.
- Wei, D.H.; Deng, J.L.; Shi, R.Z.; Ma, L.; Shen, J.M.; Hoffman, R.; Hu, Y.H.; Wang, H.; Gao, J.L. Epimedin C protects H2O2-induced peroxidation injury by enhancing the function of endothelial progenitor HUVEC populations. Biol. Pharm. Bull. 2019, 42, 1491–1499.
- Huang, M.; Wei, Y.; Dong, J. Epimedin C modulates the balance between Th9 cells and Treg cells through negative regulation of noncanonical NF-κB pathway and MAPKs activation to inhibit airway inflammation in the ovalbumin-induced murine asthma model. Pulm. Pharmacol. Ther. 2020, 65, 102005.
- Liu, Y.; Bi, Y.; Chai, L.; Song, L.; Huang, J.; Wang, Q.; Li, Y.; Zhou, K. Development of epimedin A complex drugs for treating the osteoporosis. J. Mater. Sci. Mater. Med. 2021, 32, 17.
- Balaha, M.F.; Ahmed, N.J.; Almalki, Z.S.; Alahmari, A.K.; Alshehri, A.M.; Soliman, G.A.; Hamad, A.M. Epimedin A ameliorates DNFB-induced allergic contact dermatitis in mice: Role of NF-κ B/NLRP3-driven pyroptosis, Nrf2/HO-1 pathway, and inflammation modulation. Life Sci. 2022, 302, 120653.
- Diao, X.; Wang, L.; Zhou, Y.; Bi, Y.; Zhou, K.; Song, L. The mechanism of Epimedin B in treating osteoporosis as revealed by RNA sequencing-based analysis. Basic Clin. Pharmacol. Toxicol. 2021, 129, 450–461.
- Zhang, M.; Hu, Z.F.; Dong, X.L.; Chen, W.F. Epimedin B exerts neuroprotective effect against MPTP-induced mouse model of Parkinson’s disease: GPER as a potential target. Biomed. Pharmacother. 2022, 156, 113955.
- Chen, G.; Huang, J.; Lei, H.; Wu, F.; Chen, C.; Song, Y.; Cao, Z.; Zhang, C.; Zhang, C.; Ma, Y.; et al. Icariside I—A novel inhibitor of the kynurenine-AhR pathway with potential for cancer therapy by blocking tumor immune escape. Biomed. Pharmacother. 2022, 153, 113387.
- Chen, C.; Wu, M.; Lei, H.; Cao, Z.; Wu, F.; Song, Y.; Zhang, C.; Qin, M.; Zhang, C.; Du, R.; et al. A novel prenylflavonoid icariside I ameliorates estrogen deficiency-induced osteoporosis via simultaneous regulation of osteoblast and osteoclast differentiation. ACS Pharmacol. Transl. Sci. 2023, 6, 270–280.
- Cheng, J.; Cai, H. Adverse reactions to Zhuangguguanjie Wan and cause analysis. Advers. Drug React. J. 2000, 2, 15–19.
- Du, Q.; Wang, Z.; Yun, N.R.; Huang, Y.H.; Xu, Q.; Wang, B.H. Literature analysis of 185 cases of ADR induced by Xianling Gubao capsule. China Pharm. 2017, 28, 3785–3787.
- Wang, Q.; Zhang, P.Y.; Yuan, X.M.; Bi, Y.N.; Zhou, K.; Zhang, Y. Long-term toxicity of different extracts of Epimedium brevicornu maxim in mice. Chin. J. Pharmacovigil. 2018, 15, 65–69.
- Zhang, L.; Zhang, J.X.; Fan, Q.Y.; Su, Z.Q.; Chen, C.; Peng, L.; Wang, T. Hepatoxicity of Epimedii folium in rat model based on uniform design and regression analysis. Chin. J. Exp. Tradit. Med. Formulae. 2018, 24, 189–197.
- Zhang, L.; Xu, A.L.; Yang, S.; Zhao, B.S.; Wang, T. In vitro screening and toxic mechanism exploring of leading components with potential hepatotoxicity of Herba Epimedii extracts. Toxicol. In Vitro 2020, 62, 104660.
- Song, Z.; Li, Z.; Wen, X.; Liu, R.; Tian, X. UPLC-MS/MS method for simultaneously determining nucleosides and methyl-nucleosides in liver mRNA of Epimedin C-induced liver injury mouse model. Chin. Med. 2021, 16, 91.
- Zhang, L.; Wang, T.; Zhao, B.S.; Zhang, J.X.; Yang, S.; Fan, C.L.; Li, P. Effect of 2″-O-rhamnosyl icariside II, baohuoside I and baohuoside II in Herba Epimedii on cytotoxicity indices in HL-7702 and HepG2 Cells. Molecules 2019, 24, 1263.
- Li, P.; Zhang, L.; Guo, Z.; Kang, Q.; Chen, C.; Liu, X.; Ma, Q.; Zhang, J.; Hu, Y.; Wang, T. Epimedium koreanum Nakai-induced liver injury-A mechanistic study using untargeted metabolomics. Front. Pharmacol. 2022, 13, 934057.
- Wang, Q.; Li, Z.; Ren, X.X. Optimization of the water-extraction method of total flavonoids from Epimedium sagittatum Maxin. Tianjin Agric. Sci. 2012, 18, 3.
- Huang, R.H.; Zhou, Y.C.; Han, W.; Deng, X. Study on water extraction process of Herba epimedii with microwave technology. Zhongguo Zhong Yao Za Zhi. 2005, 30, 107–110.
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S. Eggplant peel as a high potential source of high methylated pectin: Ultrasonic extraction optimization and characterization. LWT 2019, 105, 182–189.
- Spinei, M.; Oroian, M. Microwave-assisted extraction of pectin from grape pomace. Sci. Rep. 2022, 12, 12722.
- Yang, X.H.; Li, L.; Xue, Y.B.; Zhou, X.X.; Tang, J.H. Flavonoids from Epimedium pubescens: Extraction and mechanism, antioxidant capacity and effects on CAT and GSH-Px of Drosophila melanogaster. PeerJ 2020, 8, e8361.
- Karbuz, P.; Tugrul, N. Microwave and ultrasound assisted extraction of pectin from various fruits peel. J. Food Sci. Technol. 2021, 58, 641–650.
- Zhang, R.; Su, D.; Hou, F.; Liu, L.; Huang, F.; Dong, L.; Deng, Y.; Zhang, Y.; Wei, Z.; Zhang, M. Optimized ultra-high-pressure-assisted extraction of procyanidins from lychee pericarp improves the antioxidant activity of extracts. Biosci. Biotechnol. Biochem. 2017, 81, 1576–1585.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
23 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




