Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yongjun Wei and Version 2 by Jessie Wu.
Epimedium is a classical Chinese herbal medicine, which has been used extensively to treat various diseases, such as sexual dysfunction, osteoporosis, cancer, rheumatoid arthritis, and brain diseases. Flavonoids, such as icariin, baohuoside I, icaritin, and epimedin C, are the main active ingredients with diverse pharmacological activities.
- flavonoids
- pharmacological activities
- extraction methods
- biotransformation
- biosynthesis
1. Introduction
The Epimedium genus, belonging to the Berberidaceae family, contains 68 species worldwide, with 58 of them (85.3%) distributed in China [1]. China is the center of geographical distribution and varieties of Epimedium. Over 15 Epimedium species have a long history of use in Traditional Chinese Medicine (TCM) and are believed to have kidney-nourishing and Yang-reinforcing properties [2]. Tao Hongjing, a renowned medical scientist, learned from shepherds that male sheep consuming a certain plant experienced significantly increased penile erections and mating frequency. Tao believed that this plant could enhance “Yang” energy, and named it “Yin-Yang-Huo” in Chinese [3]. It was later discovered that this was an Epimedium plant. Epimedium was first mentioned over 2000 years ago in the “Shen Nong Ben Cao Jing”. It was later listed as a medium-grade herb in the “Ben Cao Gang Mu” by Li Shizhen during the Ming Dynasty [4]. In the Chinese Pharmacopoeia (2020 edition), Epimedii Folium (EF) refers to the dried leaves of four Epimedium plants, namely E. brevicornum Maxim, E. sagittatum (Sieb. et Zucc.) Maxim, E. pubescens Maxim, and E. koreanum Nakai [5]. EF is a classical herbal medicine. Alone, or combined within diverse prescriptions, it has been used to treat various diseases, including sexual dysfunction [6][7][6,7], osteoporosis [8], cancer [9], rheumatoid arthritis [10], and brain diseases [11]. Additionally, EF has been used in functional food production, and is available in alcoholic health beverages, health tea, and medicated gruel and noodle diets [2][12][2,12].2. The Pharmacological Activities of Major Epimedium Flavonoids
More than 379 compounds have been detected in EF, including flavonoids, lignans, organic acids, terpenoids, dihydrophenanthrene derivatives, alkaloids, and other constituents [13]. Flavonoids, such as epimedin A, epimedin B, epimedin C, icariin, baohuoside I (also known as icariside II), icariside I, and icaritin have been recognized as major phytochemical and pharmacological active ingredients (Figure 1) [13][14][13,14]. These compounds differ in varying degrees of glycosylation at the C-3 and C-7 of icaritin [2][14][2,14]. There were great variations among the flavonoid contents in Epimedium from different species, collection and/or storage times and/or locations [15][16][15,16]. Icariin was the most abundant component in E. brevicornum Maxim and E. koreanum Nakai, followed by epimedin B, epimedin C, and epimedin A. However, epimedin C was the most abundant component in E. sagittatum (Sieb. et Zucc.) Maxim, E. pubescens Maxim, and E. wushanense T.S. Ying, followed by icariin, epimedin B, and epimedin A [15][16][15,16]. The average proportions of the total contents of epimedin A, B, C, and icariin to the 15 investigated flavonoid contents were 85.6%, 82.3%, 68.8%, 74.9%, and 69.8% in E. brevicornum Maxim, E. koreanum Nakai, E. sagittatum (Sieb. et Zucc.) Maxim, E. pubescens Maxim, and E. wushanense T.S. Ying, respectively [15][16][15,16]. In another study, epimedin A, B, C, and icariin accounted for over 52% of the total flavonoid contents in E. brevicornum Maxim [17]. In the Chinese Pharmacopoeia (2020 edition), the total amount of epimedin A, B, C and icariin was identified as the quality control indicator for the EF herb [5]. The major flavonoids in EF exhibit significant and diverse pharmacological activities (Figure 1).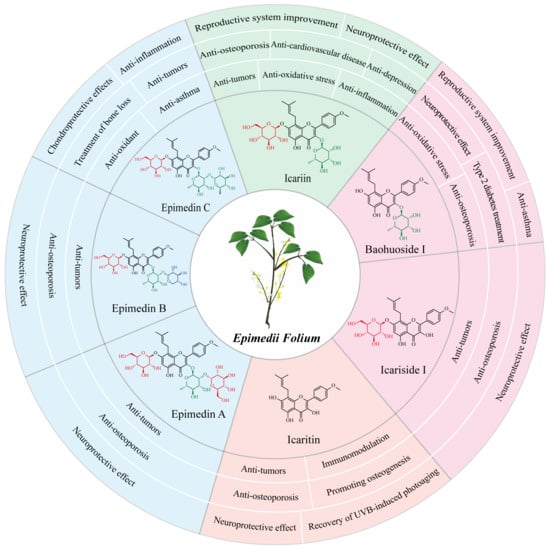
Figure 1.
Chemical structures and pharmaceutical properties of the major flavonoids from
Epimedii Folium
.
2.1. Icariin and Its Pharmaceutical Effects
Icariin, the major bioactive component in EF (about 1%) [17], has been found to possess various pharmacological effects. These include improved reproductive system function, a neuroprotective effect, an anti-osteoporosis effect, protective effects from cardiovascular disease, an anti-inflammation effect, an anti-oxidative stress effect, an anti-depressive effect, and an anti-tumor effect [18][19][20][21][18,19,20,21]. In ancient China, EF was commonly used to treat sexual dysfunction [3]. Icariin can enhance erectile function in spontaneously hypertensive rats by reducing endothelial microparticle levels in the blood and inhibiting platelet activation [22]. In male mice, icariin can improve sexual function through the PI3K/AKT/eNOS/NO signaling pathway [23]. In the female reproductive system, icariin promotes estrogen biosynthesis in human ovarian granulosa-like KGN cells, and upregulates the expression of aromatase, which is responsible for the conversion of androgens to estrogens in vertebrates [24]. Additionally, icariin has exhibited protective effects in various nervous system disorders, including Alzheimer’s disease, Parkinson’s disease, and depressive disorder [20][25][26][27][20,25,26,27]. Moreover, icariin is regarded as a potential drug for osteoporosis treatment. Recent studies have demonstrated that icariin could prevent bone loss in ovariectomized rat models by modulating gut microbiota and regulating metabolite alterations [28] or by activating autophagy [29], as well as protect against iron overload-induced bone loss via suppressing oxidative stress [30].2.2. Baohuoside I and Its Pharmaceutical Effects
Baohuoside I, although presents in low contents (<0.15%) in the raw material of EF compared to icariin, exhibits a wider range of pharmacological activities [31][32][31,32]. Baohuoside I has better bioavailability in vivo than icariin, as it is more easily absorbed by the capillaries of intestinal epithelial cells because of its lower polarity [33]. Cheng et al. found that 91.2% of icariin was converted to baohuoside I after oral administration in rats [34]. Similarly, human intestinal microflora metabolized most icariin to baohuoside I in a short time before absorption in the human intestine [35]. Baohuoside I has been proved to have a significant therapeutic effect on various diseases, such as sexual dysfunction, osteoporosis, and cancers [9][36][37][38][9,36,37,38]. For improving erectile dysfunction, baohuoside I could facilitate the differentiation of adipose-derived stem cells into Schwann cells and preserve the erectile function of bilateral cavernous nerve injury (BCNI) in rats [36][39][36,39]. The anti-osteoporotic activity of baohuoside I was suggested to be associated with its ability to induce bone marrow stromal cell differentiation into osteoblasts while inhibiting adipocyte formation, regulating immune functions, and providing antioxidant activity [40]. Baohuoside I could inhibit osteoclastogenesis and protect against ovariectomy-induced bone loss in mice, surpassing the effects of icariin [38]. Current studies have shown that baohuoside I exhibits promising anti-tumor effects on lung cancer cells [41], melanoma cells [42], breast cancer cells [43], prostate cancer cells [44], and osteosarcoma cells [45]. Furthermore, baohuoside I has shown its potential application in type 2 diabetes treatment [46], neuroprotection [47], and asthma inhibition [48].2.3. Icaritin and Its Pharmaceutical Effects
Icaritin is a flavonoid aglycone in EF [14], which can be generated by hydrolytic reactions that remove the glycone parts of icariin, baohuoside I, and icariside I [49][50][49,50]. Icaritin possesses diverse pharmacological activities [51], including protection of neurons against amyloid-induced neurotoxicity [52], promotion of differentiation from embryonic stem cells into cardiomyocytes [53], anti-osteoporosis effects and osteogenesis promotion [54][55][54,55], immunomodulation [56][57][56,57], and recovery of UVB-induced photoaging of human keratinocytes [58]. Moreover, icaritin is considered as a promising candidate for the treatment of various cancers [59][60][59,60], including hepatocellular carcinoma [61][62][63][61,62,63], breast cancer [64], lung cancer [65], ovarian cancer [66], endometrial cancer [67], human oral squamous cell carcinoma [68], and multiple hematological malignancies [59][69][70][71][59,69,70,71]. In the treatment of hepatocellular carcinoma, icaritin can suppress cell growth and promote cell apoptosis via down-regulating alpha-fetoprotein gene expression in hepatocellular carcinoma [62] and inducing anti-tumor immune responses [61][63][61,63]. In 2022, an icaritin soft capsule was marketed as a small molecule immunomodulatory drug, providing a solution for patients with advanced hepatocellular carcinoma with poor prognosis, and significantly improving the life quality of patients with hepatocellular carcinoma [72][73][72,73].2.4. Epimedin C and Its Pharmaceutical Effects
Epimedin C is a trioglycoside ingredient in EF, with the highest content among all flavonol glycosides in certain Epimedium species, such as E. brevicornu Maxim [74], E. wushanense T.S. Ying, and E. sagittatum Maxim [75]. Epimedin C is considered as the quality control standard for evaluating the quality of E. wushanense T.S. Ying in the Chinese Pharmacopoeia (2020 edition) [5]. The pharmacological activities of epimedin C mainly include treatment of bone loss, anti-oxidant effects, and anti-inflammation. Epimedin C has shown significant anti-inflammatory and chondroprotective effects by increasing the expression of extracellular matrix components in osteoarthritis chondrocytes [76]. Epimedin C could alleviate the suppressive impact of dexamethasone on the osteogenesis of larval zebrafish and MC3T3-E1 cells via triggering the PI3K/AKT/RUNX2 signaling pathway [77]. Notably, epimedin C has a stronger anti-osteoporosis effect than icariin at the same dose on dexamethasone-induced osteoporosis in a mouse model [78]. Furthermore, epimedin C has been found to protect against H2O2-induced peroxidation injury by enhancing the function of endothelial progenitor human umbilical vein endothelial cells, which plays an important role in repairing endothelial cell vascular injury [79]. In an ovalbumin-induced murine asthma model, epimedin C was demonstrated to dose-dependently decrease the protein levels of p52 and RelB, and the phosphorylation of ERK1/2, and p38 MAPK, which are pivotal in the development of Th9 cells and Treg cells, thereby inhibiting airway inflammation [80].2.5. Other Flavonoids and Their Pharmaceutical Effects
Other flavonoids presented in EF include epimedin A and B, icariside I, and sagittatoside A, B, and C [2][13][2,13]. Their contents in EF are very low, and limited pharmacological research is available on them. However, similar to the major flavonoids described above, icariside I, epimedin A, and epimedin B also exhibit anti-osteoporosis, neuroprotective, and anti-cancer effects. Epimedin A has shown excellent efficacy against senile osteoporosis [8], and in vitro and in vivo experiments demonstrated that a complex epimedin A drug significantly enhances bone regeneration [81]. In addition, epimedin A could ameliorate 2,4-dinitrofluorobenzene (DNFB)-induced allergic contact dermatitis in mice, due to its ability to suppress the NF-κB/NLRP3 pathway, enhance the Nrf2 pathway, and modulate local inflammation [82]. Diao et al. provided evidence that epimedin B ameliorates osteoporosis in male mice via regulating PI3K-Akt, MAPK, and PPAR signaling pathways [83]. Additionally, epimedin B can exert a neuroprotective effect against Parkinson’s disease in an MPTP-induced mouse model [84]. Chen et al. suggested that icariside I performed tumor immunotherapy activity by blocking the kynurenine-AhR pathway and tumor immune escape [85]. Icariside I could significantly inhibit B16F10 melanoma growth in vivo through regulation of gut microbiota and host immunity [85]. Moreover, icariside I also effectively ameliorated estrogen deficiency-induced osteoporosis in an ovariectomy mouse model [86]. In addition to the various beneficial effects of EF flavonoids, it is important to note that EF can potentially cause drug-induced liver injury (Table 1). In clinical applications, there are increasing evidences indicate that Zhuangguguanjie pills and Xianlinggubao capsules have toxic effects, leading to liver injury in humans [87][88][87,88]. Both medicines contain EF as their major components, and are used to treat rheumatism, bone pain, arthritis, osteoporosis, and other diseases. Recently, animal studies have indicated that EF extracts can cause liver toxicity in mice and rats, with the severity of hepatotoxic effects increasing with higher dosages and prolonged exposure [89][90][89,90]. However, the exact compound(s) and the underlying mechanisms contributing to the observed liver toxicity remain unclear. Zhang et al. suggested that icariside I and sagittatoside A are the most relevant compounds related to the hepatotoxicity of EF extracts [91]. Epimedin C has been reported to have potential hepatotoxicity. Song et al. revealed that mRNA methylation might be associated with epimedin C-induced liver injury by the UPLC-MS/MS method [92]. When treated with the normal human liver cell line (HL-7702) and human hepatocellular carcinoma cell line (HepG2), 2″-O-Rhamnosyl icariside II, baohuoside I, and baohuoside II showed significant dose-toxic effects, and baohuoside I was more likely to be involved in the hepatotoxicity of EF [93]. Therefore, the hepatotoxicity of EF, like other TCMs, is probably due to the combined effects of multiple components. Further investigations are needed to fully understand the hepatotoxicity mechanism in order to avoid EF-induced liver injury.Table 1.
The published mechanisms of the hepatotoxicity effects of
Epimedium
flavonoids.
| Epimedium Flavonoids | Research Systems | Mechanisms | Reference |
|---|---|---|---|
| Alcohol extracts of E. koreanum Nakai and E. wushanense T.S. Ying | SD rats | Compared with the normal group, animal groups treated with EF extracts showed severer hepatotoxicity, which was positively correlated with the dose and course. Additionally, the females experienced more significant damage compared to the males. | [90] |
| Icariside I and sagittatoside A | HL-7702 and HepG2 cells | Icariside I could destroy the cell structure and cause oxidative stress. Sagittatoside A could cause oxidative stress and damage to mitochondria. | [91] |
| Epimedin C | Male Balb/c mice | Epigenetic modification changed in mouse liver after epimedin C treatment with a test dose, and the m6A and m5C may be associated with epimedin C-induced liver injury. | [92] |
| Baohuoside I | HL-7702 and HepG2 cells | The toxicity mechanism(s) of baohuoside I may be involved in increasing oxidative stress and inducing apoptosis. | [93] |
| E. koreanum Nakai ethanol extract | Male Sprague Dawley rats | The mechanism of hepatotoxicity of E. koreanum Nakai was probably related to the induction of ferroptosis in hepatocytes. | [94] |
3. Extraction Methods of Epimedium Flavonoids
Currently, commercially available Epimedium flavonoids are extracted from Epimedium plants. Several techniques have been developed for isolating flavonoids from Epimedium (Figure 23), including hot water extraction, alcohol extraction, ultrasonic extraction, microwave-assisted extraction, and ultra-high-pressure extraction. Among these techniques, hot water extraction and alcohol extraction have been implemented in industrial production, while others are at the lab-scale stage.
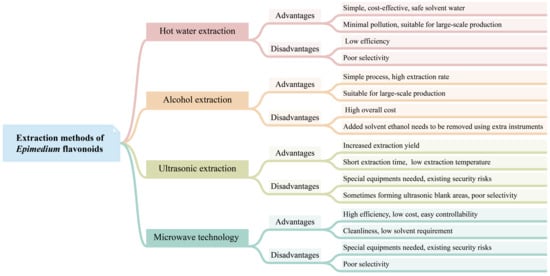
Figure 23.
The advantages and disadvantages of different extraction methods of
Epimedium
flavonoids.
3.1. Hot Water Extraction
Hot water extraction is a traditional method used for decocting Chinese herbs. In this method, the crushed herbs are immersed in water in a container for an appropriate amount of time, then heated and gently boiled for a certain period of time. The liquid is subsequently filtered, and the process of decoction is repeated 2–3 times. The decocted liquids from each iteration are mixed and concentrated to achieve the desired flavonoid concentration. Wang et al. optimized the hot water extraction procedure with an orthogonal test [95][101]. The results showed that the optimized extraction procedure was 2% Na2CO3, 15 times the water volume of the weight of dried material, with three 1.5 h extractions. The final extracting ratio of the total flavonoids was 97.92%. Other new technologies, such as microwave technology, have been used to enhance hot water extraction. Compared to the conventional hot water extraction method, microwave-assisted extraction offers higher extraction efficiency and is time-saving [96][102]. The hot water extraction process is simple and cost-effective, and utilizes water as a safe solvent. The whole process generates minimal pollution. Therefore, hot water extraction is suitable for the large-scale production of flavonoids. However, the efficiency of hot water extraction for flavonoid extraction is low, and it lacks selectivity in capturing specific flavonoids.
3.2. Alcohol Extraction
The alcohol extraction method is the most commonly used technique for extracting flavonoids, adopted by the Chinese Pharmacopoeia (2020 edition) [5]. In this method, ethanol is generally employed as the extraction solvent. The process of the alcohol extraction method is relatively simple, and well-suited for industrial applications. However, a large amount of ethanol is added to the extraction reactor, which subsequently needs to be removed using extra instruments. As a result, the overall cost of this method is higher compared to hot water extraction. Zhang et al. demonstrated that the extraction rate of icariin using the alcohol extraction method was significantly higher than that of the water extraction method [75]. The optimal extraction parameters were determined to be 50% ethanol, 1:10 solid–liquid ratio, 60 °C extraction temperature, 2 h extraction time, and two extraction cycles. In addition, an ultrasonic-assisted ethanol extraction procedure has shown to increase the extraction yield of epimedin A, epimedin B, epimedin C, and icariin from Herba Epimedii, when compared to the conventional ethanol boiling extraction method [75].
3.3. Other Extraction Methods
Ultrasonic extraction utilizes the effects of strong vibrations, cavitation, and thermal energy generated by ultrasound to extract the active components of plants into solvents. Ultrasonic extraction is regarded as a powerful tool for extracting flavonoids from plant biomass, offering several advantages, such as increased extraction yield, shorter extraction time, and lower extraction temperature [97][103]. Microwave technology utilizes the ability to generate heat within cells and vaporize water to break down the cell walls, allowing for better release of active ingredients in plant cells. The microwave technique presents numerous benefits, including high efficiency, low energy consumption, short processing time, low cost, cleanliness, easy controllability, and low solvent requirement [98][104]. Both ultrasonic extraction and the microwave technique are often used to assist common extraction methods, such as water extraction and alcohol extraction, to improve the efficiency of extracting Epimedium flavonoids [75][96][99][100][75,102,105,106]. Furthermore, ultra-high-pressure extraction has also been utilized for extracting flavonoids from E. sagittatum. Compared to heating extraction and ultrasonic-assisted extraction, ultra-high-pressure extraction presents distinctive advantages in superior extraction yield and a higher percentage of marker compounds [101][107].
