Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Evgeny Konchekov | -- | 4831 | 2023-10-20 16:21:49 | | | |
| 2 | Jason Zhu | Meta information modification | 4831 | 2023-10-23 03:39:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Konchekov, E.M.; Konchekov, E.; Gusein-Zade, N.; Burmistrov, D.E.; Kolik, L.V.; Dorokhov, A.S.; Izmailov, A.Y.; Shokri, B.; Gudkov, S.V. Plasma-Activated Water Treatment in Agriculture. Encyclopedia. Available online: https://encyclopedia.pub/entry/50620 (accessed on 07 February 2026).
Konchekov EM, Konchekov E, Gusein-Zade N, Burmistrov DE, Kolik LV, Dorokhov AS, et al. Plasma-Activated Water Treatment in Agriculture. Encyclopedia. Available at: https://encyclopedia.pub/entry/50620. Accessed February 07, 2026.
Konchekov, Evgeny M., Evgeny Konchekov, Namik Gusein-Zade, Dmitriy E. Burmistrov, Leonid V. Kolik, Alexey S. Dorokhov, Andrey Yu. Izmailov, Babak Shokri, Sergey V. Gudkov. "Plasma-Activated Water Treatment in Agriculture" Encyclopedia, https://encyclopedia.pub/entry/50620 (accessed February 07, 2026).
Konchekov, E.M., Konchekov, E., Gusein-Zade, N., Burmistrov, D.E., Kolik, L.V., Dorokhov, A.S., Izmailov, A.Y., Shokri, B., & Gudkov, S.V. (2023, October 20). Plasma-Activated Water Treatment in Agriculture. In Encyclopedia. https://encyclopedia.pub/entry/50620
Konchekov, Evgeny M., et al. "Plasma-Activated Water Treatment in Agriculture." Encyclopedia. Web. 20 October, 2023.
Copy Citation
The unique properties of physical plasma and its ability to operate at atmospheric pressure make it an attractive technology for numerous scientific and industrial applications, ranging from medicine and agriculture to electronics and materials science. For example, this technology has proven to be a simple and low-cost approach for nanoparticle synthesis or an effective surface modification agent to produce superhydrophobic and superoleophilic films for oil-water separation and self-cleaning.
plants
plasma source
plasma-activated water
reactive oxygen species
reactive nitrogen species
1. Introduction
The unique properties of physical plasma and its ability to operate at atmospheric pressure make it an attractive technology for numerous scientific and industrial applications, ranging from medicine and agriculture to electronics and materials science [1]. For example, this technology has proven to be a simple and low-cost approach for nanoparticle synthesis [2] or an effective surface modification agent to produce superhydrophobic and superoleophilic films for oil-water separation and self-cleaning [3]. In medicine, plasma is widely used to solve problems of creating biocompatible materials, orthopedics [4], oncology, dentistry [5], dermatology [6], etc.
In recent years, plasma agriculture has emerged as a promising and innovative field with the potential to evaluate conventional farming practices. The application of plasma-based technologies in agriculture offers new opportunities to address various challenges faced by the agricultural industry, such as food security, environmental sustainability, and the need for increased crop yields [7].
The generation of plasma can be achieved through various methods. Each of these techniques offers distinct advantages and can be tailored to suit specific agricultural needs. Moreover, the use of plasma-activated water (PAW) has emerged as a promising technology with potential applications in agriculture. PAW is produced by subjecting water to discharges, resulting in the generation of various active species, including reactive oxygen (ROS) and nitrogen species (RNS) [8][9].
One of the key areas of focus in plasma agriculture is plant growth promotion. Plasma and PAW treatments have been shown to stimulate seed germination, enhance root development, and increase overall crop productivity. These effects are attributed to the activation of various plant growth regulators, modification of plant hormone levels, and the induction of stress tolerance mechanisms. Furthermore, plasma can also improve nutrient availability and uptake by altering the physicochemical properties of the soil, leading to increased nutrient-use efficiency and reduced fertilizer requirements.
Another aspect of plasma agriculture is its potential for pest and disease management. Plasma treatments have demonstrated efficacy in controlling pathogens, offering an environmentally friendly alternative to traditional chemical-based pesticides. The antimicrobial properties of plasma, coupled with its ability to induce systemic resistance in plants, provide an approach to disease prevention and control.
Furthermore, plasma and PAW treatments have been shown to alleviate the detrimental effects of abiotic stress factors such as drought, salinity, and extreme temperatures. By activating stress-responsive genes and enhancing antioxidant defense mechanisms, plasma can improve plant resilience and ensure sustainable crop production under challenging environmental conditions.
2. Dielectric Barrier Discharge
Ref [10] investigated the processing of fresh-cut potatoes using PAW prepared by decreasing discharge frequency. Two different frequencies, 200 Hz and 10 kHz, were used for the plasma treatment, and their effects were compared. The study suggests that the plasma-activated water prepared with a lower discharge frequency of 200 Hz is more effective in disinfecting fresh-cut potatoes and exhibits better antioxidant properties. Additionally, both 200 Hz and 10 kHz PAW can effectively inactivate enzymes that cause browning in the potatoes, contributing to their extended shelf life and improved visual quality during storage.
PAW demonstrates, especially when enriched with magnesium and zinc ions, a positive influence on the growth and development of Chinese cabbage plants [11]. The treatment results in faster seed germination, enhanced seedling growth, increased chlorophyll and protein content, and favorable gene expression patterns, which collectively lead to healthier and more robust plants.
The beneficial effect on lettuce seed germination and subsequent seedling growth is described in [12]. Short exposure times appear to be more effective for promoting germination and growth, while longer exposure times may not yield further benefits. The increased chlorophyll content and positive morphological changes in the seeds demonstrate the potential of PAW as a tool to enhance the performance of lettuce crops.
Foliar application of PAW on maize plants [13] can lead to changes in chlorophyll content, photosynthetic efficiency, and nutrient composition, which may influence the plant’s growth and physiological processes.
Treatment of soybean seeds can promote faster germination and growth [14]. Moreover, the presence of ZnO nanoparticles can reduce the uptake of heavy metals by soybean plants, which may have implications for reducing heavy metal contamination in the soil and enhancing plant health and growth in contaminated environments.
The study demonstrates that treatment with PAW can effectively preserve strawberries [15], maintaining their quality and preventing spoilage. The PAW-treated strawberries did not undergo any negative changes in their sensory attributes, and the best quality indicators were observed on the fourth day after processing. The use of PAW in treating button mushrooms [16] proved to be beneficial in reducing browning and preserving organoleptic properties.
The influence on plant calcium signaling was investigated in [17], demonstrating that the chemical composition of PAW and the frequency of treatments can significantly affect the intracellular Ca2+ signals in plants.
PAW treatment positively influenced the photo-dependent dormancy mechanisms in Nicotiana tabacum seeds [18]. It enhanced testa and endosperm rupture percentages, increased the activity of the gibberellin-3-oxidase gene, and upregulated the expansin-A4 gene, all of which contributed to improved seed germination and growth.
It was found in [19] that PAW treatments had an impact on root hair density in Arabidopsis thaliana plants, and this modulation was associated with changes in the expression of certain root developmental genes. The COBL9, XTH9, and XTH17 genes were identified as potential target genes that were influenced by the PAW treatments. These genes play essential roles in root development and root hair formation. The study observed that PAW prepared with a short duration of plasma treatment led to an up-regulation of these target genes, indicating enhanced root hair development. On the other hand, the long-exposed PAW suppressed root development.
Studying the physical and physical-chemical properties of the soil under the PAW influence [20] indicates that the application of PAW had minimal impacts on soil, except for an improvement in water retention. This suggests that PAW may be a potentially useful tool for enhancing soil water retention and moisture availability for plants without causing significant changes to other soil characteristics.
3. Atmospheric Pressure Plasma Jets and Plasma Torch
PAW showed itself as an effective tool in degrading pesticide residues on kumquat fruits [21]. Additionally, it helps preserve soluble solids, increases titratable acidity, and does not affect fruit color change.
Ref [22] shows that treatment can affect the antioxidant contents in water spinach, particularly in the presence of heavy metals in the soil. PAW treatment has the potential to increase the total content of phenols and flavonoids in water spinach plants when certain heavy metals are present in the soil. However, the effects of the treatment on antioxidant contents can vary depending on the specific heavy metal present in the soil, indicating that the interactions between PAW and heavy metals play a crucial role in modulating the antioxidant response. Ref [23] shows that treatment can be beneficial in reducing the accumulation of Cd in water spinach, but its effect on Pb uptake and overall biomass may not be as significant.
The use of PAW as a nitrate source for hydroponically grown green oak lettuces showed promising results in terms of growth and nutritional quality [24]. It did not negatively affect lettuce growth and yield and may lead to a reduction in nitrate residues and an increase in essential amino acids in the lettuce. A positive effect on the growth of lettuce plants was also observed in [25]. However, this effect diminished over time, and there were no substantial differences in the root system between the PAW-treated and control plants. Additionally, PAW treatment resulted in higher dry matter content in the lettuce plants.
Pre-treated pepper seeds [26] and pea seeds [27] had a significant positive impact on the growth and yield of plants.
The combined effects of glow discharge plasma seed treatment and foliar application of PAW on paddy plant growth and yield were studied [28]—the air plasma seed treatment involved exposing rice seeds to low-pressure plasma for 90 s. The combination of plasma seed treatment and PAW application had a significant impact on plant growth parameters. The treated plants exhibited improved growth, which can be attributed to the synergistic effects of both treatments. Additionally, the study found that the combined treatment enhanced plant defense mechanisms by increasing enzymatic activity. This indicates that the plants had a more robust defense system against potential pathogens and stressors. The concentration of total soluble protein and sugar in rice grains was increased after the combined treatment, which suggests an improvement in the nutritional quality of the rice.
Ref. [29] aimed to investigate the effects of plasma-assisted nitrogen fixation on corn plant growth and development. The experiments involved using deionized water and water enriched with magnesium (Mg), aluminum (Al), or zinc (Zn) cations. These metal cations neutralized the PAW and enhanced the reduction of nitrogen to ammonia, with additional hydrogen generated from the reaction between the acid produced by the plasma and the metal ions. The results showed that corn seeds watered with PAW had a faster germination rate and more efficient growth, especially in the presence of metal ions. Moreover, high nitrogen concentrations in the growth medium increased the chlorophyll and protein content in the green parts of the corn plants. This increase in chlorophyll and protein levels led to the formation of intensely green leaves, indicating enhanced photosynthesis and overall plant health. The paper comprehensively discussed the chemical processes in water and the role of the metal’s oxidation levels. Metals immersed in water undergo oxidation, resulting in the generation of metal ions. These metal ions subsequently convert nitrite (NO2−) and nitrate (NO3−) species into their corresponding metal nitrates and nitrites, respectively. Concurrently, the electrons from the metal species lead to the reduction of hydrogen ions (H+) to hydrogen (H), thereby inducing a substantial increase in the solution’s pH level. Hydrogen facilitates the reduction of nitrogen to yield ammonia (NH3). As a consequence, the rates of ammonia synthesis and the degree of pH elevation within various activated waters follow the sequence: Zn-PAW < Al-PAW < Mg-PAW. At restricted concentrations, metal ions such as magnesium ions (Mg2+), aluminum ions (Al3+), and zinc ions (Zn2+) serve advantageous functions in plant physiology.
The impact on cytosolic calcium levels in Arabidopsis thaliana was investigated in [30]. The results showed that exposure to PAW induced a rapid and sustained increase in cytosolic calcium concentration in the plant cells. The specific features of this response were found to be influenced by various factors, including the operating conditions of the plasma torches used to generate PAW, the duration of plasma exposure to water, the amount of PAW applied to the plants, and the temperature and time of PAW storage. When the individual components of PAW (nitrates, nitrites, and hydrogen peroxide) were administered separately at the same concentrations as PAW, no significant changes in cytosolic calcium dynamics were observed. This suggests that the combination of these components in PAW plays a unique role in triggering the calcium response.
PAW treatment indicates strong antibacterial properties [31] on suspension cells and P. fluorescence biofilms and outperforms analog mixtures of traceable ingredients commonly used for their antimicrobial effects. Ref [32] demonstrated that PAW treatment mediates environmentally transmitted pathogenic bacterial inactivation through intracellular nitrosative stress, leading to a decrease in bacterial viability and causing morphological changes in the bacterial cells. Ref [33] also suggests that PAWs have strong antibacterial properties and low cytotoxicity, making them promising candidates for various biomedical and disinfection applications, especially when the pH is carefully controlled.
Plasma-activated acidic electrolyzed water (PA-AEW) was successfully used [34] as a food disinfectant for bacterial suspension and biofilm. The results showed that PA-AEW had a significant bactericidal effect against B. subtilis, a common bacterium used as a model microorganism in research. The sterilization time for PA-AEW was only 10 s, and during this short exposure, it demonstrated a satisfactory bactericidal effect. The effectiveness of PA-AEW in killing B. subtilis was found to be higher compared to other treatments, including PAW and acidic electrolyzed water.
The application of PAW against Escherichia coli, Colletotrichum gloeosporioides, and the decontamination of pesticide residues on chili (Capsicum annuum L.) was investigated in [35]. The results showed that treatment for 30 min and 60 min effectively degraded carbendazim and chlorpyrifos. In chili, the levels of carbendazim and chlorpyrifos were significantly reduced. Moreover, the treatment demonstrated strong antimicrobial activity. The populations of Escherichia coli were reduced by 1.18 Log CFU/mL in suspension and by 2.8 Log CFU/g in chili after 60 min of treatment. Additionally, the treatment achieved 100% inhibition of fungal spore germination, indicating its potential as an effective method to control fungal pathogens such as Colletotrichum gloeosporioides.
The investigation focused on nitrate capture in PAW, and its antifungal effect on Cryptococcus pseudolongus cells was observed in [36]. The study found that enriching PAW with magnesium ions (PAW-Mg2+) resulted in the control of free nitrate through the formation of nitrate salts by magnesium ions. Both PAW and PAW-Mg2+ exhibited antifungal activity against C. pseudolongus. However, the efficacy of PAW-Mg2+ was found to be lower compared to PAW alone. This suggests that the antifungal effect of PAW can be influenced and controlled by the presence of captured nitrate.
Ref [37] described the effectiveness of treatment in inactivating Candida albicans and spoilage fungi from lemon (Citrus limon). The results showed that PAW was highly effective in reducing the population of C. albicans. Even with short treatment times, PAW achieved a reduction of more than 6 log10 CFU/mL of C. albicans. PAW treatment caused damage to the membrane of C. albicans cells. This damage led to the leakage of cellular contents and ultimately resulted in the death of the cells. Furthermore, the study demonstrated the long-term fungicidal efficacy of PAW against C. albicans and the spoilage fungi found in lemon. This suggests that PAW treatment can provide sustained protection against these fungi, making it a promising option for food preservation and hygiene applications.
Ref [38] identified two critical processes that led to the death of A. brasiliensis spores after PAW treatment. First, the structural damage to the spore cell wall allowed the ROS and RNS in the PAW to penetrate and attack the spore’s internal components. Second, the genomic DNA of the spores was degraded by the ROS and RNS present in the PAW, further contributing to cell death.
The research findings [39][40][41] indicate that when plasma-activated water is diluted to concentrations of 0.5–1.0% in distilled water, it leads to several positive effects on seed germination and plant growth for various crops, including cotton, wheat, and strawberries. Specified dilution levels have been shown to enhance germination energy. This likely results in quicker and more vigorous sprouting of seeds, which is crucial for establishing healthy and productive plant populations. The treatment provides protection against fusariosis, a fungal disease caused by Fusarium species, and hyperthermia (heat stress) in seeds. PAW demonstrates advantages over conventional seed germination stimulants such as Dalbron and Bakhor. While these traditional stimulants may have been effective, the use of plasma-activated water offers additional benefits that might include enhanced disease resistance, stress tolerance, and overall growth stimulation.
4. Corona Discharge
Longer PAW treatment times positively preserved certain vitamins and polyphenolic compounds in arugula leaves [42]. Specifically, ascorbic acid, riboflavin, nicotinic acid, and nicotinamide were better preserved in leaves treated with PAW for a longer duration (20 min). Furthermore, the longer treatment with PAW increased the content of vitamins B2 (riboflavin) and B3 (nicotinic acid), as well as some individual polyphenols. This suggests that PAW treatment can enhance the nutritional content of arugula leaves. Interestingly, PAW treatment led to a significant decrease in antioxidant activity. This could be due to the degradation or modification of certain antioxidant compounds during the treatment process. Additionally, the study found a decrease in catalase activity in arugula leaves treated with PAW. Catalase is an enzyme involved in antioxidant defense, and its decrease may be related to the observed reduction in antioxidant activity. PAW treatment also has potential benefits for fresh-cut vegetables such as arugula by preserving their nutritional properties [43], increasing total phenols and glucosinolates, and improving the redox status without inducing cytotoxicity.
PAW generated with corona discharge, as with others, had a positive impact on wheat grain germination and seedling growth [44]. PAW exposure increased the germination rate, shoot length, and the fresh and dry mass of shoots. In addition, PAW also demonstrated effective decontamination properties against bacterial and yeast pathogens on artificially infected wheat grains. Wheat grains infected with Escherichia coli were effectively decontaminated after just 1 h of exposure to PAW. On the other hand, decontamination of grains infected with Saccharomyces cerevisiae required soaking the grains in PAW for 24 h.
5. Gliding Arc Discharge
The effect of PAW on maize seed germination and growth was investigated in [45]. It was shown that treatment of corn seeds with 15 min exposed PAW resulted in significant changes to the seeds, while a 5 min exposed PAW did not cause notable alterations.
For Beta vulgaris seeds [46], the PAW treatments resulted in a higher germination rate than the treatment with sodium hypochlorite (NaClO). Although the average seedling length was slightly shorter than the NaClO treatment, it was still promising for seedling growth. However, in the case of Daucus carota seeds treated with PAW, the germination and seedling length were lower compared to using a sodium hypochlorite solution but still significantly higher than in the control group. Moreover, PAW treatment affected the composition of fungal species present in the seeds of Beta vulgaris and Daucus carota. Different fungal species responded differently to PAW treatment, and for some types of fungi, the effect was not significant.
Basil plants grown using the plasma-activated nutrient solution (PANS) exhibited significant growth improvements compared to plants treated with regular nutrient solutions [47]. Specifically, the basil plants grown with PANS were taller and had a significantly higher dry weight. The study also evaluated the organoleptic characteristics. It was observed that these plants had significantly greener leaves compared to the control group. Furthermore, the leaves of basil plants grown with PANS showed higher levels of linalool and methyleugenol, which are compounds responsible for the plant’s floral and spicy aroma. This indicates that the plasma treatment positively influenced the aromatic properties of the basil. Another significant finding of the study was related to the reduction of algae concentration in the hydroponic medium. Repeated treatment of basil plants with PANS helped mitigate algae growth, contributing to a cleaner and more controlled hydroponic environment.
Seeds treated with PAW-5 (5-min plasma treatment) and PAW-10 (10-min plasma treatment) exhibited significant enhancements in various germination parameters [48]. Specifically, they had a higher percentage of germination and germination uniformity and increased average daily germination and germination values compared to the control group. The study evaluated the effect of PAW irrigation on different types of seeds, including barley, mustard, and rayo seeds. Irrigation with PAW-10 resulted in a significant increase in germination for all three types of seeds. Moreover, seeds watered with PAW showed higher water uptake, longer roots, and longer shoots compared to the control group. This indicates that PAW irrigation positively affected seedling development and growth. Additionally, vigor analysis demonstrated that seeds irrigated with PAW exhibited stronger growth characteristics.
6. Spark and Glow Discharges
The research [49] evaluated different rice starch-phenolic complexes and assessed the contributions of different factors, including the type of phenolic compound, PAW, and ultrasonication. The addition of gallic acid to starch molecules resulted in an increase in the activity of removing 1,1-diphenyl-2-picrylhydrazyl (a common method to measure antioxidant activity). However, it was observed that the complexes with gallic acid had lower values of the complexation index and the content of resistant starch compared to the complexes with crude Mon-pu extract.
The PAW effect on the chemical compounds present in the leaves of Eruca sativa (rocket salad) was investigated in [50]. Different PAW was used to study their impact on the volatile organic compounds (VOCs) in the leaves. A total of 52 VOCs from various chemical classes were detected and quantified using gas chromatography-mass spectrometry. PAW treatment can induce chemical modifications in the VOCs. The specific changes in compound content varied with different exposure times, and some compounds showed significant increases while others decreased. The decontamination effect was achieved after washing the rocket leaves with PAW [51]. This did not lead to significant changes in the quality and nutritional parameters. There were only slight changes in color and the content of bioactive compounds. Moreover, the antibacterial effect of PAW treatment was found to be more pronounced compared to the use of hypochlorite, which is a commonly used disinfectant in the food industry.
A comparison of the effect with a chemically equivalent solution of hydrogen peroxide (H2O2) + nitrates (NO3−) on the growth and physiological parameters of lettuce plants is presented in [52]. When lettuce plants were irrigated with PAW and the chemically equivalent H2O2 + NO3− solution, they showed similar dry weight of above-ground parts and roots. However, there were notable differences in certain physiological parameters. The lettuce plants irrigated with PAW had a higher content of photosynthetic pigments (chlorophyll a + b) and exhibited a higher photosynthetic rate, and the activity of antioxidant enzymes, such as superoxide dismutase, was lower.
Ref [53] investigated that PAW treatment can positively influence the early growth stages of maize seedlings under arsenic stress. The study also highlights the complex interplay between PAW treatment and arsenic stress on various physiological and biochemical aspects of maize plants.
Application of PAW to pea seedlings [54] stimulated amylase activity, indicating enhanced starch breakdown without negatively affecting seed germination, total protein concentration, and protease activity. Furthermore, PAW had almost no oxidative stress on pea seedlings. In pea seedlings, PAW treatment resulted in a more rapid transition from anaerobic to aerobic metabolism, as evidenced by the inhibition of alcohol dehydrogenase activity. Additionally, the presence of reactive oxygen/nitrogen species in PAW did not affect DNA integrity in pea seedlings. However, the response of barley seedlings was different. They showed high levels of DNA damage, along with a decrease in the length of roots and shoots and a decrease in amylase activity.
Pea plants grown from seeds pre-treated with PAW showed enhanced growth compared to untreated seeds [55]. This improvement was particularly noticeable in the number of seeds per pod and the total number of seeds per plant. Morphological changes on the seed surfaces may include alterations in the surface structure, texture, or microstructure of the seeds. Such changes could potentially contribute to the improved growth and yield observed in the treated plants.
Significant changes in the concentration of hydrogen peroxide (H2O2) and ROS were observed in the seeds, leaves, and roots of the black gram plants [56]. Specifically, an increase in the level of catalase enzyme was observed in the roots of plants grown from seeds treated with PAW for 3 and 6 min. This increase in catalase activity is consistent with the activation of the VmCAT gene, which encodes for the catalase enzyme in black gram.
The study [57] indicates that PAW has both positive and negative effects on Triticum aestivum. While it may promote the germination of wheat grains and the growth of roots and shoots at specific concentrations and pH levels, it also demonstrated cytogenotoxic potential, indicating possible harmful effects on cell division and genetic material.
The application of PAW and the biostimulator [58] decreased the incidence of fungal diseases on the lawn, positively influenced turf density, led to a thicker and more lush lawn, and positively affected overwintering.
A reaction-discharge system optimization to continuously produce a PAW with specific physicochemical and anti-phytopathogenic properties was performed in [59]. PAW was found to have bacteriostatic and bactericidal effects against two phytopathogenic strains, Dickeya solani IFB0099 and Pectobacterium atrosepticum IFB5103.
The scalable treatment of flowing organic liquids using ambient-air glow discharge for agricultural applications was a focus in [60]. The plasma treatment of the L-phenylalanine solution had a dual effect. On one hand, it stimulated hydroponic radish seedlings, resulting in a 40% increase in growth. On the other hand, it also demonstrated a bactericidal effect on E. coli (specifically, E. coli O1:K1:H7), leading to a reduction in bacterial load.
7. Underwater Discharge
Ref. [61] highlights the beneficial effects of spraying plasma-activated water on potato plants, leading to enhanced growth, biochemical activity, nutritional composition, and yield of the potato crop.
The series of papers [62][63][64][65] highlights the diverse applications of PTS in agriculture. PTS can be utilized as both a disinfectant and a nutrient solution for various agricultural practices. It can be used either in its freshly prepared form for disinfection purposes or diluted for irrigation, hydroponics, aeroponics, and mist generation. In the case of apple fruits, PTS has been shown to impact the calcium (Ca) content of the fruits when used for irrigation. This suggests that PTS application could have a positive effect on fruit quality and potentially extend the storage duration of the fruits. PTS has also been demonstrated to be effective in enhancing sorghum seed germination. Additionally, the treated plants displayed drought resistance. The use of the technique has been extended to field experiments conducted in a saline semi-desert environment. This implies that PTS could have applications in regions with difficult growing conditions, potentially helping plants adapt to challenging soil and environmental conditions.
8. H2O2 and NOx− Generation Efficiency for Different Types of Plasma Sources
The use of PAW and PTS in agrobiological tasks and the study of plant physiology necessitates the accurate determination of the concentrations of ROS, RNS, and other bioactive compounds and nanoparticles that arise during plasma treatment. Researchers analyzed dependencies on the energy input for hydrogen peroxide generation, as well as the concentrations of nitrite and nitrate ions in different types of water. The results have been categorized based on the type of plasma source, as previously described. The results at two different scales are shown in Figure 1 and Figure 2. It is important to note that the majority of investigated studies do not focus on the energy optimization of ROS and RNS generation. Furthermore, ROS and RNS production depends on a multitude of system parameters: interaction surface area with the liquid, the distance between electrodes and liquid, gas composition in the plasma–liquid interaction region, gas flow rate (if applicable), liquid flow rate (if applicable), electrode material (for immersed electrodes), and more. Therefore, these results serve to illustrate the most general trends.
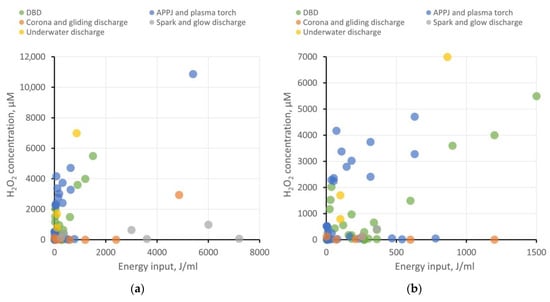
Figure 1. Production of hydrogen peroxide in water depending on energy input per milliliter of water for various types of plasma sources. For the convenience of analysis, the results are presented on two scales: (a) energy input up to 8000 J/mL and (b) energy input up to 1500 J/mL.
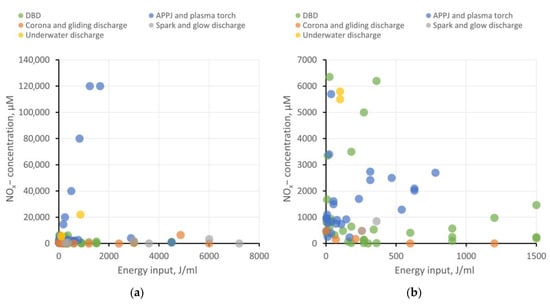
Figure 2. Production of NOx− ions in water depending on energy input per milliliter of water for various types of plasma sources. For the convenience of analysis, the results are presented on two scales: (a) energy input up to 8000 J/mL and (b) energy input up to 1500 J/mL.
The analysis, first, shows the need to standardize the description of plasma source characteristics, treatment methods, and ROS and RNS concentrations in PAW. Without this standardization, comparing experimental results and, more importantly, reproducing them using different plasma sources becomes challenging, significantly impeding the integration of plasma technologies into widespread practice.
Regarding PAW creation, all types of plasma sources demonstrate their viability. Each type has its advantages and drawbacks, which define their respective areas of application and demand.
-
DBD shows good energy efficiency when the goal is not to achieve ROS and RNS concentrations exceeding 5−6 mM and when large volumes of PAW are not required. To enhance productivity, increasing the plasma–liquid interaction surface area is necessary, transitioning from a planar electrode configuration to, for example, a coaxial one and implementing liquid flow.
-
APPJ also appears attractive in terms of energy efficiency. Using gases to create a plasma jet offers broad possibilities in enriching PAW with target active compounds. For instance, it is feasible to achieve high RNS production while keeping the liquid free from extraneous impurities. On the other hand, using a gas injection system places additional demands on workspace organization and slightly raises treatment costs. For creating larger volumes of PAW, using a microwave plasma torch as the plasma source seems more efficient.
-
Corona, gliding, and spark discharges might not initially show high ROS and RNS production, but they provide the opportunity to create “pure” PAW without side impurities, which is in demand, for example, in medicine or food processing. To improve the energy efficiency of PAW generation, increasing the plasma–liquid interaction area can be achieved through multi-spark (multi-electrode) systems. Gas injection into the discharge region is also described to enhance the production of target bioactive compounds.
-
Underwater discharges allow for the generation of large volumes of PAW with high concentrations of ROS and RNS. These discharges can be employed for creating concentrates to replace chemical fertilizers in agrobiological tasks or for disinfection purposes. However, close attention must be paid to studying electrode erosion and electrolysis processes, which significantly contribute to the chemical composition of PAW. The presence of metal nanoparticles and dissolved metal compounds can either help or hinder the benefits of PAW use.
References
- Adamovich, I.; Agarwal, S.; Ahedo, E.; Alves, L.L.; Baalrud, S.; Babaeva, N.; Bogaerts, A.; Bourdon, A.; Bruggeman, P.J.; Canal, C.; et al. The 2022 Plasma Roadmap: Low Temperature Plasma Science and Technology. J. Phys. D Appl. Phys. 2022, 55, 373001.
- Hossain, M.M.; Robinson Junior, N.A.; Mok, Y.S.; Wu, S. Investigation of Silver Nanoparticle Synthesis with Various Nonthermal Plasma Reactor Configurations. Arab. J. Chem. 2023, 16, 105174.
- Hossain, M.M.; Wu, S.; Nasir, A.; Mohotti, D.; Robinson, N.A.; Yuan, Y.; Agyekum-Oduro, E.; Akter, A.; Bhuiyan, K.A.; Ahmed, R.; et al. Superhydrophobic and Superoleophilic Surfaces Prepared by One-Step Plasma Polymerization for Oil-Water Separation and Self-Cleaning Function. Surf. Interfaces 2022, 35, 102462.
- Nonnenmacher, L.; Fischer, M.; Haralambiev, L.; Bekeschus, S.; Schulze, F.; Wassilew, G.I.; Schoon, J.; Reichert, J.C. Orthopaedic Applications of Cold Physical Plasma. EFORT Open Rev. 2023, 8, 409–423.
- Koga-Ito, C.Y.; Kostov, K.G.; Miranda, F.S.; Milhan, N.V.M.; Azevedo Neto, N.F.; Nascimento, F.; Pessoa, R.S. Cold Atmospheric Plasma as a Therapeutic Tool in Medicine and Dentistry. Plasma Chem. Plasma Process. 2023, 21, 2932.
- Lotfi, M.; Khani, M.; Shokri, B. A Review of Cold Atmospheric Plasma Applications in Dermatology and Aesthetics. Plasma Med. 2023, 13, 39–63.
- Ranieri, P.; Sponsel, N.; Kizer, J.; Rojas-Pierce, M.; Hernández, R.; Gatiboni, L.; Grunden, A.; Stapelmann, K. Plasma Agriculture: Review from the Perspective of the Plant and Its Ecosystem. Plasma Process. Polym. 2021, 18, 2000162.
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.; Cullen, P.J.; Ostrikov, K.; Bazaka, K. Plasma-Activated Water: Generation, Origin of Reactive Species and Biological Applications. J. Phys. D Appl. Phys. 2020, 53, 303001.
- Shaji, M.; Rabinovich, A.; Surace, M.; Sales, C.; Fridman, A. Physical Properties of Plasma-Activated Water. Plasma 2023, 6, 45–57.
- Aihaiti, A.; Maimaitiyiming, R.; Wang, L.; Wang, J. Processing of Fresh-Cut Potato Using Plasma-Activated Water Prepared by Decreasing Discharge Frequency. Foods 2023, 12, 2285.
- Javed, R.; Mumtaz, S.; Choi, E.H.; Han, I. Effect of Plasma-Treated Water with Magnesium and Zinc on Growth of Chinese Cabbage. Int. J. Mol. Sci. 2023, 24, 8426.
- Than, H.A.Q.; Pham, T.H.; Nguyen, D.K.V.; Pham, T.H.; Khacef, A. Non-Thermal Plasma Activated Water for Increasing Germination and Plant Growth of Lactuca sativa L. Plasma Chem. Plasma Process. 2022, 42, 73–89.
- Škarpa, P.; Klofáč, D.; Krčma, F.; Šimečková, J.; Kozáková, Z. Effect of Plasma Activated Water Foliar Application on Selected Growth Parameters of Maize (Zea mays L.). Water 2020, 12, 3545.
- Mahanta, S.; Habib, M.R.; Moore, J.M. Effect of High-Voltage Atmospheric Cold Plasma Treatment on Germination and Heavy Metal Uptake by Soybeans (Glycine max). Int. J. Mol. Sci. 2022, 23, 1611.
- Yang, X.; Zhang, C.; Li, Q.; Cheng, J.-H. Physicochemical Properties of Plasma-Activated Water and Its Control Effects on the Quality of Strawberries. Molecules 2023, 28, 2677.
- Zheng, Y.; Zhu, Y.; Zheng, Y.; Hu, J.; Chen, J.; Deng, S. The Effect of Dielectric Barrier Discharge Plasma Gas and Plasma-Activated Water on the Physicochemical Changes in Button Mushrooms (Agaricus bisporus). Foods 2022, 11, 3504.
- Cortese, E.; Galenda, A.; Famengo, A.; Cappellin, L.; Roverso, M.; Settimi, A.G.; Dabalà, M.; De Stefani, D.; Fassina, A.; Serianni, G.; et al. Quantitative Analysis of Plant Cytosolic Calcium Signals in Response to Water Activated by Low-Power Non-Thermal Plasma. Int. J. Mol. Sci. 2022, 23, 10752.
- Grainge, G.; Nakabayashi, K.; Iza, F.; Leubner-Metzger, G.; Steinbrecher, T. Gas-Plasma-Activated Water Impact on Photo-Dependent Dormancy Mechanisms in Nicotiana Tabacum Seeds. Int. J. Mol. Sci. 2022, 23, 6709.
- Ka, D.H.; Priatama, R.A.; Park, J.Y.; Park, S.J.; Kim, S.B.; Lee, I.A.; Lee, Y.K. Plasma-Activated Water Modulates Root Hair Cell Density via Root Developmental Genes in Arabidopsis thaliana L. Appl. Sci. 2021, 11, 2240.
- Šimečková, J.; Krčma, F.; Klofáč, D.; Dostál, L.; Kozáková, Z. Influence of Plasma-Activated Water on Physical and Physical–Chemical Soil Properties. Water 2020, 12, 2357.
- Wang, X.; Feng, J.; Chen, S.; Qin, S.; Zang, Y.; Huang, H.; Wei, J. Effect of Plasma Activated Water on the Degradation of Bifenazate and Spirodiclofen Residues on Cuimi Kumquat and Impact on Its Quality. Agronomy 2023, 13, 1247.
- Hsu, S.-C.; Kong, T.-K.; Chen, C.-Y.; Chen, H.-L. Plasma-Activated Water Affects the Antioxidant Contents in Water Spinach. Appl. Sci. 2023, 13, 3341.
- Hou, C.-Y.; Kong, T.-K.; Lin, C.-M.; Chen, H.-L. The Effects of Plasma-Activated Water on Heavy Metals Accumulation in Water Spinach. Appl. Sci. 2021, 11, 5304.
- Ruamrungsri, S.; Sawangrat, C.; Panjama, K.; Sojithamporn, P.; Jaipinta, S.; Srisuwan, W.; Intanoo, M.; Inkham, C.; Thanapornpoonpong, S. Effects of Using Plasma-Activated Water as a Nitrate Source on the Growth and Nutritional Quality of Hydroponically Grown Green Oak Lettuces. Horticulturae 2023, 9, 248.
- Romanjek Fajdetić, N.; Benković-Lačić, T.; Mirosavljević, K.; Antunović, S.; Benković, R.; Rakić, M.; Milošević, S.; Japundžić-Palenkić, B. Influence of Seed Treated by Plasma Activated Water on the Growth of Lactuca sativa L. Sustainability 2022, 14, 16237.
- Japundžić-Palenkić, B.; Benković, R.; Benković-Lačić, T.; Antunović, S.; Japundžić, M.; Romanjek Fajdetić, N.; Mirosavljević, K. Pepper Growing Modified by Plasma Activated Water and Growth Conditions. Sustainability 2022, 14, 15967.
- Rathore, V.; Tiwari, B.S.; Nema, S.K. Treatment of Pea Seeds with Plasma Activated Water to Enhance Germination, Plant Growth, and Plant Composition. Plasma Chem. Plasma Process. 2022, 42, 109–129.
- Rashid, M.; Rashid, M.M.; Reza, M.A.; Talukder, M.R. Combined Effects of Air Plasma Seed Treatment and Foliar Application of Plasma Activated Water on Enhanced Paddy Plant Growth and Yield. Plasma Chem. Plasma Process. 2021, 41, 1081–1099.
- Lamichhane, P.; Veerana, M.; Lim, J.S.; Mumtaz, S.; Shrestha, B.; Kaushik, N.K.; Park, G.; Choi, E.H. Low-Temperature Plasma-Assisted Nitrogen Fixation for Corn Plant Growth and Development. Int. J. Mol. Sci. 2021, 22, 5360.
- Cortese, E.; Settimi, A.G.; Pettenuzzo, S.; Cappellin, L.; Galenda, A.; Famengo, A.; Dabalà, M.; Antoni, V.; Navazio, L. Plasma-Activated Water Triggers Rapid and Sustained Cytosolic Ca2+ Elevations in Arabidopsis thaliana. Plants 2021, 10, 2516.
- Weihe, T.; Yao, Y.; Opitz, N.; Wagner, R.; Krall, J.; Schnabel, U.; Below, H.; Ehlbeck, J. Plasma-Treated Water: A Comparison with Analog Mixtures of Traceable Ingredients. Microorganisms 2023, 11, 932.
- Borkar, S.B.; Negi, M.; Kaushik, N.; Abdul Munnaf, S.; Nguyen, L.N.; Choi, E.H.; Kaushik, N.K. Plasma-Generated Nitric Oxide Water Mediates Environmentally Transmitted Pathogenic Bacterial Inactivation via Intracellular Nitrosative Stress. Int. J. Mol. Sci. 2023, 24, 1901.
- Sampaio, A.D.G.; Chiappim, W.; Milhan, N.V.M.; Botan Neto, B.; Pessoa, R.; Koga-Ito, C.Y. Effect of the pH on the Antibacterial Potential and Cytotoxicity of Different Plasma-Activated Liquids. Int. J. Mol. Sci. 2022, 23, 13893.
- Heng, Y.; Wang, M.; Jiang, H.; Gao, S.; Zhang, J.; Wan, J.; Song, T.; Ren, Z.; Zhu, Y. Plasma-Activated Acidic Electrolyzed Water: A New Food Disinfectant for Bacterial Suspension and Biofilm. Foods 2022, 11, 3241.
- Sawangrat, C.; Phimolsiripol, Y.; Leksakul, K.; Thanapornpoonpong, S.; Sojithamporn, P.; Lavilla, M.; Castagnini, J.M.; Barba, F.J.; Boonyawan, D. Application of Pinhole Plasma Jet Activated Water against Escherichia Coli, Colletotrichum Gloeosporioides, and Decontamination of Pesticide Residues on Chili (Capsicum annuum L.). Foods 2022, 11, 2859.
- Lee, G.J.; Lamichhane, P.; Ahn, S.J.; Kim, S.H.; Yewale, M.A.; Choong, C.E.; Jang, M.; Choi, E.H. Nitrate Capture Investigation in Plasma-Activated Water and Its Antifungal Effect on Cryptococcus Pseudolongus Cells. Int. J. Mol. Sci. 2021, 22, 12773.
- Rathore, V.; Patel, D.; Shah, N.; Butani, S.; Pansuriya, H.; Nema, S.K. Inactivation of Candida Albicans and Lemon (Citrus limon) Spoilage Fungi Using Plasma Activated Water. Plasma Chem. Plasma Process. 2021, 41, 1397–1414.
- Ki, S.H.; Noh, H.; Ahn, G.R.; Kim, S.H.; Kaushik, N.K.; Choi, E.H.; Lee, G.J. Influence of Nonthermal Atmospheric Plasma-Activated Water on the Structural, Optical, and Biological Properties of Aspergillus brasiliensis Spores. Appl. Sci. 2020, 10, 6378.
- Sergeichev, K.F.; Lukina, N.A.; Sarimov, R.M.; Smirnov, I.G.; Simakin, A.V.; Dorokhov, A.S.; Gudkov, S.V. Physicochemical Properties of Pure Water Treated by Pure Argon Plasma Jet Generated by Microwave Discharge in Opened Atmosphere. Front. Phys. 2021, 8, 614684.
- Ashurov, M.K.; Ashurov, E.M.; Astashev, M.E.; Baimler, I.V.; Gudkov, S.V.; Konchekov, E.M.; Lednev, V.N.; Lukina, N.A.; Matveeva, T.A.; Markendudis, A.G.; et al. Development of an Environmentally Friendly Technology for the Treatment of Aqueous Solutions with High-Purity Plasma for the Cultivation of Cotton, Wheat and Strawberries. ChemEngineering 2022, 6, 91.
- Andreev, S.N.; Apasheva, L.M.; Ashurov, M.K.; Lukina, N.A.; Sapaev, B.; Sapaev, I.B.; Sergeichev, K.F.; Shcherbakov, I.A. Production of Pure Hydrogen Peroxide Solutions in Water Activated by the Plasma of Electrodeless Microwave Discharge and Their Application to Control Plant Growth. Phys. Wave Phenom. 2019, 27, 145–148.
- Abouelenein, D.; Mustafa, A.M.; Nzekoue, F.K.; Caprioli, G.; Angeloni, S.; Tappi, S.; Castagnini, J.M.; Dalla Rosa, M.; Vittori, S. The Impact of Plasma Activated Water Treatment on the Phenolic Profile, Vitamins Content, Antioxidant and Enzymatic Activities of Rocket-Salad Leaves. Antioxidants 2022, 12, 28.
- Ramazzina, I.; Lolli, V.; Lacey, K.; Tappi, S.; Rocculi, P.; Rinaldi, M. Fresh-Cut Eruca Sativa Treated with Plasma Activated Water (PAW): Evaluation of Antioxidant Capacity, Polyphenolic Profile and Redox Status in Caco2 Cells. Nutrients 2022, 14, 5337.
- Jirešová, J.; Scholtz, V.; Julák, J.; Šerá, B. Comparison of the Effect of Plasma-Activated Water and Artificially Prepared Plasma-Activated Water on Wheat Grain Properties. Plants 2022, 11, 1471.
- Mogo, J.P.K.; Fovo, J.D.; Sop-Tamo, B.; Mafouasson, H.N.A.; Ngwem, M.C.N.; Tebu, M.J.; Youbi, G.K.; Laminsi, S. Effect of Gliding Arc Plasma Activated Water (GAPAW) on Maize (Zea mays L.) Seed Germination and Growth. Seeds 2022, 1, 230–243.
- Terebun, P.; Kwiatkowski, M.; Hensel, K.; Kopacki, M.; Pawłat, J. Influence of Plasma Activated Water Generated in a Gliding Arc Discharge Reactor on Germination of Beetroot and Carrot Seeds. Appl. Sci. 2021, 11, 6164.
- Date, M.B.; Rivero, W.C.; Tan, J.; Specca, D.; Simon, J.E.; Salvi, D.A.; Karwe, M.V. Growth of Hydroponic Sweet Basil (O. basilicum L.) Using Plasma-Activated Nutrient Solution (PANS). Agriculture 2023, 13, 443.
- Guragain, R.P.; Baniya, H.B.; Shrestha, B.; Guragain, D.P.; Subedi, D.P. Improvements in Germination and Growth of Sprouts Irrigated Using Plasma Activated Water (PAW). Water 2023, 15, 744.
- Chumsri, P.; Panpipat, W.; Cheong, L.-Z.; Nisoa, M.; Chaijan, M. Comparative Evaluation of Hydrothermally Produced Rice Starch–Phenolic Complexes: Contributions of Phenolic Type, Plasma-Activated Water, and Ultrasonication. Foods 2022, 11, 3826.
- Abouelenein, D.; Angeloni, S.; Caprioli, G.; Genovese, J.; Mustafa, A.M.; Nzekoue, F.K.; Petrelli, R.; Rocculi, P.; Sagratini, G.; Tappi, S.; et al. Effect of Plasma Activated Water on Selected Chemical Compounds of Rocket-Salad (Eruca sativa Mill.) Leaves. Molecules 2021, 26, 7691.
- Laurita, R.; Gozzi, G.; Tappi, S.; Capelli, F.; Bisag, A.; Laghi, G.; Gherardi, M.; Cellini, B.; Abouelenein, D.; Vittori, S.; et al. Effect of Plasma Activated Water (PAW) on Rocket Leaves Decontamination and Nutritional Value. Innov. Food Sci. Emerg. Technol. 2021, 73, 102805.
- Kučerová, K.; Henselová, M.; Slováková, Ľ.; Bačovčinová, M.; Hensel, K. Effect of Plasma Activated Water, Hydrogen Peroxide, and Nitrates on Lettuce Growth and Its Physiological Parameters. Appl. Sci. 2021, 11, 1985.
- Lukacova, Z.; Svubova, R.; Selvekova, P.; Hensel, K. The Effect of Plasma Activated Water on Maize (Zea mays L.) under Arsenic Stress. Plants 2021, 10, 1899.
- Kostoláni, D.; Ndiffo Yemeli, G.B.; Švubová, R.; Kyzek, S.; Machala, Z. Physiological Responses of Young Pea and Barley Seedlings to Plasma-Activated Water. Plants 2021, 10, 1750.
- Yemeli, G.B.N.; Janda, M.; Machala, Z. Non-Thermal Plasma as a Priming Tool to Improve the Yield of Pea in Outdoor Conditions. Plasma Chem. Plasma Process. 2022, 42, 1143–1168.
- Sajib, S.A.; Billah, M.; Mahmud, S.; Miah, M.; Hossain, F.; Omar, F.B.; Roy, N.C.; Hoque, K.M.F.; Talukder, M.R.; Kabir, A.H.; et al. Plasma Activated Water: The next Generation Eco-Friendly Stimulant for Enhancing Plant Seed Germination, Vigor and Increased Enzyme Activity, a Study on Black Gram (Vigna mungo L.). Plasma Chem. Plasma Process. 2020, 40, 119–143.
- Padureanu, S.; Burlica, R.; Stoleru, V.; Beniuga, O.; Dirlau, D.; Cretu, D.E.; Astanei, D.; Patras, A. Non-Thermal Plasma-Activated Water: A Cytogenotoxic Potential on Triticum aestivum. Agronomy 2023, 13, 459.
- Talar-Krasa, M.; Wolski, K.; Radkowski, A.; Khachatryan, K.; Bujak, H.; Bocianowski, J. Effects of a Plasma Water and Biostimulant on Lawn Functional Value. Agronomy 2021, 11, 254.
- Dzimitrowicz, A.; Jamroz, P.; Pohl, P.; Babinska, W.; Terefinko, D.; Sledz, W.; Motyka-Pomagruk, A. Multivariate Optimization of the FLC-Dc-APGD-Based Reaction-Discharge System for Continuous Production of a Plasma-Activated Liquid of Defined Physicochemical and Anti-Phytopathogenic Properties. Int. J. Mol. Sci. 2021, 22, 4813.
- Gamaleev, V.; Iwata, N.; Ito, G.; Hori, M.; Hiramatsu, M.; Ito, M. Scalable Treatment of Flowing Organic Liquids Using Ambient-Air Glow Discharge for Agricultural Applications. Appl. Sci. 2020, 10, 801.
- Rashid, M.; Rashid, M.M.; Alam, M.S.; Talukder, M.R. Stimulating Effects of Plasma Activated Water on Growth, Biochemical Activity, Nutritional Composition and Yield of Potato (Solanum tuberosum L.). Plasma Chem. Plasma Process. 2022, 42, 131–145.
- Belov, S.V.; Danyleiko, Y.K.; Glinushkin, A.P.; Kalinitchenko, V.P.; Egorov, A.V.; Sidorov, V.A.; Konchekov, E.M.; Gudkov, S.V.; Dorokhov, A.S.; Lobachevsky, Y.P.; et al. An Activated Potassium Phosphate Fertilizer Solution for Stimulating the Growth of Agricultural Plants. Front. Phys. 2021, 8, 616.
- Kuzin, A.; Solovchenko, A.; Khort, D.; Filippov, R.; Lukanin, V.; Lukina, N.; Astashev, M.; Konchekov, E. Effects of Plasma-Activated Water on Leaf and Fruit Biochemical Composition and Scion Growth in Apple. Plants 2023, 12, 385.
- Danilejko, Y.K.; Belov, S.V.; Egorov, A.B.; Lukanin, V.I.; Sidorov, V.A.; Apasheva, L.M.; Dushkov, V.Y.; Budnik, M.I.; Belyakov, A.M.; Kulik, K.N.; et al. Increase of Productivity and Neutralization of Pathological Processes in Plants of Grain and Fruit Crops with the Help of Aqueous Solutions Activated by Plasma of High-Frequency Glow Discharge. Plants 2021, 10, 2161.
- Belov, S.V.; Danileiko, Y.K.; Egorov, A.B.; Lukanin, V.I.; Semenova, A.A.; Lisitsyn, A.B.; Revutskaya, N.M.; Nasonova, V.V.; Yushina, Y.K.; Tolordava, E.R.; et al. Sterilizer of Knives in the Meat Industry, Working by Activating Aqueous Solutions with Glow Discharge Plasma. Processes 2022, 10, 1536.
More
Information
Subjects:
Physics, Fluids & Plasmas
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
2 times
(View History)
Update Date:
23 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




