Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giuseppe Mazzarella | -- | 3172 | 2023-10-20 15:15:05 | | | |
| 2 | Lindsay Dong | Meta information modification | 3172 | 2023-10-23 04:55:23 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Camarca, A.; Rotondi Aufiero, V.; Mazzarella, G. Regulatory T Cells in Celiac Disease Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/50618 (accessed on 08 February 2026).
Camarca A, Rotondi Aufiero V, Mazzarella G. Regulatory T Cells in Celiac Disease Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/50618. Accessed February 08, 2026.
Camarca, Alessandra, Vera Rotondi Aufiero, Giuseppe Mazzarella. "Regulatory T Cells in Celiac Disease Treatment" Encyclopedia, https://encyclopedia.pub/entry/50618 (accessed February 08, 2026).
Camarca, A., Rotondi Aufiero, V., & Mazzarella, G. (2023, October 20). Regulatory T Cells in Celiac Disease Treatment. In Encyclopedia. https://encyclopedia.pub/entry/50618
Camarca, Alessandra, et al. "Regulatory T Cells in Celiac Disease Treatment." Encyclopedia. Web. 20 October, 2023.
Copy Citation
Celiac disease (CeD) is a T-cell-mediated immune disease, in which gluten-derived peptides activate lamina propria effector CD4+ T cells. While this effector T cell subset produces proinflammatory cytokines, which cause substantial tissue injury in vivo, additional subsets of T cells exist with regulatory functions (Treg). These subsets include CD4+ type 1 regulatory T cells (Tr1) and CD4+ CD25+ T cells expressing the master transcription factor forkhead box P3 (Foxp3) that may have important implications in disease pathogenesis, as well as for the development of new therapeutic strategies for CeD patients.
celiac disease
T regulatory cells
Foxp3
type 1 regulatory T cells
cytokines
1. Introduction
Celiac disease (CeD) is an autoimmune disorder that occurs in genetically predisposed people where the ingestion of gluten leads to damage in the small intestine. It is estimated to affect 1 in 100 people worldwide. Celiac disease can develop at any age, with a large spectrum of symptoms, and the only available therapy so far is a lifelong exclusion of gluten from the diet. The histological features of CeD have always been considered to be a villous atrophy, crypt of Lieberkühn hyperplasia and an increased number of intraepithelial lymphocytes (IELs) [1][2].
In addition, IgA anti-type 2 tissue transglutaminase (TG2) autoantibodies are diagnostic for this disorder, and are only produced when celiac patients are consuming cereal gluten proteins. Beyond the anti-TG2 antibodies, other antibodies with clinical significance in CeD patients have also been described, including anti-gliadin IgG, anti-actin IgA [3], anti-neuronal antigens and anti-gangliosides autoantibodies [4][5] and also antibodies to Saccharomyces cerevisiae [6].
A distinguishing trait of CeD is its strong genetic association with HLA class II genes, with almost all patients carrying the HLA-DQ2 (DQ2.5 coded by DQA1*0501/DQB*0201 and DQ2.2 coded by DQA1*0201/DQB*0201) and/or the HLA-DQ8 (DQA1*0301/DQB*0302) haplotypes. The role of HLA-DQ predisposing molecules in CeD pathogenesis is well established since these molecules bind gluten-derived peptides and present them to T-helper (Th) 1 cells in the intestinal lamina propria. In particular, DQ2 and DQ8 molecules bind with high affinity those gluten peptides subject to deamidation by TG2.
2. Anti-Inflammatory Cytokines in CeD
In CeD, the immune response is firmly controlled by various regulatory circuits, as demonstrated by the increased expression of the anti-inflammatory cytokines that occurs concurrently with the release of the inflammatory factors [7][8]. For instance, high levels of IL-10 and IFN-γ mRNA have been reported in untreated CeD, by different groups [7][8][9][10].
Among cytokines with regulatory properties, IL-10 is an important factor that acts through different ways. It exerts its function on antigen presenting cells (APC) via inhibition of cytokine synthesis and expression of costimulatory and MHC class II molecules [11][12][13][14]. In addition, IL-10 directly interferes with T cell proliferation and differentiation [15][16] and is the crucial driving factor for Tr1 cell differentiation [17][18].
Moreover, IL-10 exerts influences relevant to inflammation outside the normal panel of immune cells and cytokines. In fact, in explant culture of human fetal small intestine, human recombinant [rh]IL-10 not only suppress T cell activation but plays an important role in the regulation of matrix metalloproteinase activity, both by inhibiting matrix metalloproteinase synthesis and by increasing the release of TIMP-1 (a tissue inhibitor of metalloproteinase), thus limiting tissue destruction [19].
In CeD, the role of IL-10 has not yet been fully clarified [7][8][20][21]. High levels of IL-10, produced by various immune cells such as T lymphocytes, macrophages, epithelial, and dendritic cells have been found in untreated celiac duodenal mucosa [7][8][9][10]. However, despite the increased levels of IL-10, which reflects a compensatory anti-inflammatory pathway, the harmful T cell immune responses to gluten in active CeD is not controlled.
Beyond IL-10, TGF-β is also an important regulatory cytokine, produced by various immune or non-immune cells in the gut, that exert a number of pleiotropic effects on cell proliferation, differentiation, adhesion, senescence, and apoptosis. Importantly, TGF-β suppresses immune responses through two ways: inhibiting the function of inflammatory cells and promoting the function of Treg cells [22].
In active CeD, large amounts of TGF-β have been observed in the intestinal mucosa, pleading against a quantitative defect [23]. However, it was shown that aberrant activation of TGF-β signaling pathways has been associated with a number of immune-mediated intestinal disorders, including inflammatory bowel disease (IBD) and celiac disease [24][25][26].
3. Regulatory T-Cell Populations in CeD
It is well known that there are many subpopulations of Treg cells, including CD4+Foxp3+ Treg cells, Tr1 cells secreting IL-10, CD8+ suppressor cells, natural killer T cells, CD4-CD8-T cells, and γδ T cells [27]. While CD4 regulatory T cells have been well characterized, the development, differentiation and activation of other T regulatory populations are still a matter of debate.
Overall, T reg cells may limit immune responses to self-antigens preventing autoimmunity as well as downregulating responses to foreign antigens (including allergens and food antigens), by a number of mechanisms that are being characterized, and that are used differently by different Treg subsets [27].
Basically, the main suppression mechanisms include:
- (1)
-
Production of inhibitory cytokines such as IL-10, TGF-β and IL-35;
- (2)
-
Direct cytotoxic activity via granzyme A/B and perforin;
- (3)
-
Inhibition by cell–cell contact through co-inhibitory receptors including CTLA4, PDL1, LAG3, TIGIT, TIM3, NKG2A;
- (4)
-
Metabolic perturbation of T effector cells by subtraction of IL-2, production of adenosine via CD73 and CD39 ATP-ectoenzymes, induction of IDO in dendritic cells (DCs) and others.
3.1. Type 1 Regulatory T Cells
Tr1 cells are one of the first CD4+ Treg cell populations described, characterized by the high expression of IL-10. They are involved in the prevention of immune responses to both foreign and autoantigens, and particularly in the maintenance of long-term tolerance [28][29][30][31].
Tr1 cells develop in the periphery and IL-10, mainly produced by tolerogenic dendritic cells [IL-10 DCs], is considered their principal generation factor [30][31].
A distinct feature of Tr1 cells is that their suppressive activity is dependent on TCR activation. After the antigen-specific TCR-binding, Tr1 cells produce predominantly IL-10 and TGF- β but, depending on the microenvironment, they can also produce intermediate amounts of IFN-γ and IL-5, and little or no IL-2, IL-4 and IL-17, with a cytokine profile distinct from those of Th1, Th2 and Th0 subsets [30][31].
In addition to the peculiar cytokine profile, Tr1 cells are identified by surface markers as LAG3+CD49b+ memory CD4 T cells; they also display high expression of CCR5, CD2, CD18, CD226, and co-inhibitory receptors such as PD-1, TIM-3, CTLA-4, and PD-L1, while the transcription factor FoxP3 is not constitutively expressed [30][31].
Tr1 mainly perform their suppressive function trough cytokine secretion but it has been demonstrated that they may also act by other inhibitory mechanisms [30][31].
Gluten-specific Tr1 suppressive cells have been described both in intestinal mucosa of celiac patients [28] and in a transgenic mouse model of CeD [32]. In a pivotal study, scholars generated gliadin-specific short-term CD4+ T cell lines from duodenal biopsies of CeD patients, in presence or absence of IL-10 [28]. As a result, IL-10-generated TCLs [IL10-iTCLs] were less responsive to gliadin stimulation in terms of IFN-γ production and cell proliferation. Further, single T cell clones were isolated from IL-10-iTCLs and characterized for their cytokine profile, to identify possible Tr1 cells. Upon activation with gliadin or polyclonal stimuli, the majority of gliadin-responsive TCCs had a Th0 phenotype (secretion of IL-2, IL-4, IL-10 and IFN-γ, and high proliferative rate), but a percentage of TCCs with the typical Tr1 cytokine profile (production of IL-10 and IFN-γ, but little or no IL-2 or IL-4 and low proliferative rate), were also expanded. Importantly, these Tr1 cell clones suppressed proliferation of pathogenic Th0 cells, in co-cultures assays (Figure 1a).

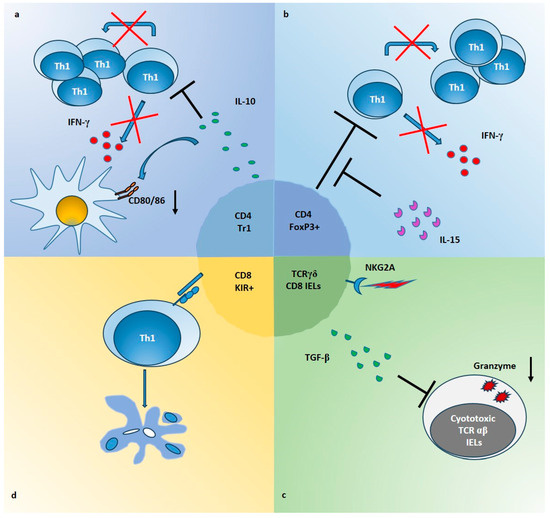
Figure 1. Mechanisms of Treg suppression in CeD intestinal mucosa. (a) CD4+ Tr1 cells producing high amounts of IL-10, inhibit the proliferation and the IFN-γ production of gliadin-responders Th1 cells (red crosses and black lines). In addition, IL-10 downregulates the expression of CD80/CD86 on APCs (black arrow), induced by gliadin stimulation in intestinal mucosa. (b) Foxp3+ Treg cells, which are expanded in CeD mucosa, are able to suppress proliferation and IFN-γ production of CD4 Tresp cells (red crosses and black lines) but IL-15 impairs their function in intestinal mucosa (black lines). (c) TCRγδ+ CD8+ IELs infiltrating the epithelium of CeD gut, increase the production of TGF-β following the ligation of NKG2A. These cells are able to inhibit expression of molecules such as granzyme B (black arrow), in cytotoxic TCRαβ+ IELs, through a mechanism partially dependent on TGF-β. (d) KIR+CD8+ T cells can block the proliferation of gliadin-specific Th1 cells by induction of apoptosis (color circles indicate apoptotic bodies), through a cell-cell contact mechanism not yet defined (blue molecules).
According to the development of gluten-specific Tr1 cells in the periphery in vivo, there are also the results of studies in transgenic mice expressing human HLA-DQ2.5 and a gliadin-specific, humanized, T-cell receptor [32][33]. In these studies, the authors found that ingestion of deamidated gliadin-induced expansion of gliadin-reactive T cells with a Tr1-like phenotype, mainly in the spleen, and not, as expected, in the mesenteric lymph nodes.
3.2. Foxp3+ Treg Cells
Another well-known CD4+ regulatory cell population, commonly referred as Treg, is characterized by high levels of IL-2 receptor α chain (CD25) and master transcription factor Foxp3 (CD4+CD25highFoxp3+ cells). This Treg family is further subdivided in two main subpopulations: thymus-derived Treg cells (tTreg, also called naturally occurring nTreg) that are early produced in the thymus and migrate into the peripheral blood to maintain tolerance toward self-antigen, and peripherally derived or induced Treg (pTreg or iTreg) that are generated in the peripheral lymphoid organs upon exposure of naïve CD4+CD25-Foxp3− T conventional (Tconv) cells to small doses of cognate antigens. Foxp3+ Treg cells can be also developed from naïve T cells in vitro, by TCR stimulation in presence of additional factors such as IL-2 and TGF-β [34].
In addition to TGF-β, playing a crucial role in the development of Foxp3+ Treg not only in vitro but also in vivo, another important Treg generation factor is retinoic acid, produced in the gut [35]. Since Foxp3 is an intracellular molecule that can be transiently expressed also by other T cells, additional markers, in particular surface molecules, are required to unequivocally identify Treg, in human. To this scope, the combination of CD4+CD25highCD127low surface markers has been documented to be effective [36].
Differently from Tr1, Foxp3+ Treg cells require antigen stimulation to expand in vivo but are able to perform their inhibitory tasks also in the absence of TCR stimulation [37][38][39]. Once activated, Foxp3+ Treg cells are able to suppress Teff cells using all the four mechanisms cited above [34].
In CeD, intestinal Foxp3 expression has been evaluated by RT-PCR in several works, and all of them reported an increased Foxp3 expression in patients with untreated CeD, compared to controls [40][41][42]. These results were confirmed also by immunohistochemistry [10][40][41][42][43][44][45] and by flow cytometry [10][40][42][44].
These data indicate that the immune system is attempting to control the persistent inflammation either by recruitment of Treg cells from blood to tissue, or through the expansion of such regulatory T cells in the mucosa.
Regardless of Treg cells frequency, some studies have reported that such cells may be impaired in their capacity to downregulate local Teff cell functions or, conversely, that Teff cells may fail to respond to Tregs.
An interesting paper by Serena et al. [46], analyzed the expression of specific Foxp3 isoforms, comparing the full length (FL) to the alternatively spliced isoform D2, since the last isoform cannot properly downregulate the Th17-driven immune response. Intestinal biopsies from patients with active CeD showed increased expression of Foxp3 D2 isoform over FL, while both isoforms were expressed similarly in control subjects, thus suggesting a possible defect in the Foxp3+ Treg function in atrophic celiac mucosa.
In line with the data that IL-15 may interfere with immune regulation, scholars have demonstrated that in active CeD patients, IL-15 was able of making Tresp cells resistant to the regulatory effects of CD4+CD25+ Treg cells [44]. In particular, in this study CD4+CD25+ T cells were directly isolated from intestinal biopsies of patients, and their suppressive ability was evaluated on autologous CD4+CD25− Tresp cells. The data showed that intestinal Treg, as well as peripheral blood Treg from celiac patients, are not functionally deficient. Nevertheless, in active CeD patients, IL-15 impaired the functions of Treg cells making Tresp cells refractory to the regulatory effects of Treg cells, in terms of proliferation and production of IFN-γ (Figure 1b).
3.3. CD8 T Lymphocytes with Regulatory Activity
In addition to CD4 Treg cells, mainly resident in intestinal lamina propria, populations of immune cells can be found within the intestinal epithelial cell layer, such as IELs, which consist mostly of CD8+T cells. Though most of those IELs express T cell receptor (TCR)-alpha beta chains (αβ), CeD is characterized by an increase in TCRγδ+ IELs that remain elevated even after removal of gluten from the diet and whose functional significance in CeD is still under investigation [47]. It has been hypothesized that CD8+TCRγδ + may play a role in the preservation of intestinal homeostasis by regulating the mucosal immune response and/or by contributing to the epithelial cell layer maintenance. Functional properties of TCRγδ+ IELs can be mediated by NK receptors (such as NKGD) expressed on a fraction of these cells [48]. At the same time, CD8+ TCRαβ+ IELs have been suggested to kill intestinal epithelial cells (IECs) in an NKG2D-MICA-dependent manner during CeD [49].
In addition to TCRγδ+ CD8+ cells, a regulatory activity has been recently proposed also for a subpopulation of TCRαβ+ CD8+ cells. A subset of regulatory CD8+ T cells that express Ly49 has been described in mice [50][51], able to reduce autoimmunity in a model of experimental autoimmune encephalomyelitis [52] but also with documented functions in several other disease settings [50]. These cells are Foxp3- but TGF-β is necessary to maintain their regulatory identity. Mechanisms of action involve IL-10 secretion, killing via granzyme/perforin, and induction of apoptosis by FAS/FASL interaction. Ly49 receptors are a family of NK receptors, including some inhibitory ones. Their functional analogues in humans are killer-cell immunoglobulin-like receptor (KIR) genes.
4. Regulatory T Cells in the Context of CeD Pathogenesis
The immune-pathogenesis of CeD is a complex mosaic, where different factors are needed to interplay for promoting the intestinal damage. Briefly, gluten is not completely digested by gastro-intestinal enzymes, and peptides that manage to cross the epithelial barrier are subjected to deamidation by tTG2, into the lamina propria. Here, peptides are taken up by DCs and presented to CD4+ T cells, in the context of HLA-DQ2 or DQ8 molecules, thus promoting the differentiation of naïve gluten-specific CD4+ T cells into Th1 effector T cells, in induction sites.
In this context, when gluten is ingested, together with expansion of pro-inflammatory lymphocytes, regulatory T cells producing large amount of IL-10 are also increased [9][10][23][53][54]. As discussed in the previous sections, at least four different T cells with suppressive activity are present in the intestinal mucosa and/or in the peripheral blood of CeD patients, which could be activated directly by gluten, or indirectly by microenvironmental signals. Such regulatory subsets may expand locally, or may be recruited from the periphery to the inflamed tissue. In spite of their origin and activation, suppressive cells may act directly or indirectly on the main orchestrators of the mucosal inflammation, including CD4+ T cells, cytotoxic IELs and DCs (Figure 1).
5. Therapeutic Applications of Treg Cells in CeD
The pathogenesis of CeD is triggered by the loss of tolerance towards gluten peptides.
On the other hand, as discussed above, several studies have suggested that the suppressive effect of Treg cells might be impaired in vivo in CeD by the inflammatory microenvironment, and their dysregulated function may contribute to sustain and expand the local inflammatory response.
Among these immunomodulating therapies, there are:
- -
-
Approaches based on in vivo administration of drugs such as rapamycin, or biologicals, such as IL-10 or low-dose IL-2, to suppress Teff cells and promote Tregs.
- -
-
Approaches resting on the administration of the autoantigen by using lentiviral vectors or antigen-specific nanoparticles, promoting tolerogenic cells and the expansion of Treg cells.
Moreover, cell-based therapies have been developed to enhance Treg cell specificity, function and number. This can be achieved by expanding Tr1 cells expressing IL-10 [55], Tregs expressing a natural repertoire of polyclonal TCRs [56] or Tregs that have been ex vivo-engineered to express a specific autoantigen receptor, such as a TCR, or a chimeric antigen receptor (CAR) [57][58].
DCs also offer a cell-based therapeutic way to restore tolerance and prevent autoimmunity [59].
Tregs can generate a tolerogenic phenotype in DCs, which can contribute to the rescue of immune tolerance. Tolerogenic DCs (TolDC), generated ex vivo, could be administrated, in vivo, to suppress autoimmunity in diseases such as T1D [60][61].
Other approaches have used mature DCs to expand antigen-specific Tregs [62], as for the generation of tolDC by genetic engineering of monocytes (CD14+) with lentiviral vectors co-encoding for immunodominant antigen-derived peptides and IL-10 [63].
Therefore, therapeutic approaches aiming to restore tolerance to gluten, and/or to correct Treg functioning, would be a great step forward to protect CeD patients from excessive immune response, to reinstate intestinal homeostasis and, possibly, to allow improvement in patients outcomes.
Other approaches have been developed using in vivo antigen-delivery to induce a tolerogenic inhibition of specific immune response [64][65].
This tolerance strategy leads to anergy within Ag-specific Teff cells and activate populations of Ag-specific regulatory T cells [64][65][66][67][68][69][70].
In this context, gliadin encapsulated in nanoparticles, namely TAK-101 (formerly TIMP-GLIA, Tolerogenic Immune Modifying nanoparticles), is under clinical development. Firstly, this approach has been shown to be effective in a mouse model of CeD [71][72], where intravenous infusion of gliadin-encapsulating nanoparticles inhibited the proliferation, and the IFN-γ and IL-17 secretion, of gliadin-specific T cells, while increasing the frequency of FoxP3+ Treg cells.
A possible cellular therapy representing a great step forward in the treatment and, possibly, the cure of chronic inflammatory conditions, is based on the use of stem cells. Both hematopoietic stem cells (HSCs) and mesenchymal stem/stromal cells (MSCs) have been employed in the treatment of refractory cases with promising results. By virtue of the lack of immunogenicity and of the ability to favor tissue regeneration and expansion of T cells with regulatory function, MSCs seem the best candidate for clinical application. MSC are multipotent non-hematopoietic cells present in different tissues, including bone marrow, amniotic fluid, umbilical cord, and placenta, which due to their immunomodulatory characteristics are considered as new therapeutic agents in the cell-based therapy of autoimmune and immune-mediated diseases [73][74][75][76][77][78][79][80].
6. Conclusions
Celiac intestinal mucosa harbors two subsets of CD4+ Treg cells, Tr1 and Foxp3+ T cells. Many factors, such as IL-15, largely expressed in the CeD mucosa, may interfere with the function of Treg cells, thus contributing to the loss of intestinal homeostasis and promoting chronic inflammation.
On the other hand, novel T cell subsets with regulatory activity are emerging, and their further characterization is of great interest. As Treg cells exist naturally in the human gut mucosa and maintain intestinal homeostasis, using methods to enhance their numbers and/or function is a possibility worthy of pursuit as a new therapeutical approach to re-establish tolerance to gluten in patients with CeD. Treg immunotherapies based on infusion of autologous TolDC or MSCs, or on enhancement of Treg numbers and function via administration of nanoparticles, remain possible strategies to be implemented in CeD.
References
- Iversen, R.; Sollid, L.M. The Immunobiology and Pathogenesis of Celiac Disease. Annu. Rev. Pathol. Mech. Dis. 2023, 18, 47–70.
- Voisine, J.; Abadie, V. Interplay Between Gluten, HLA, Innate and Adaptive Immunity Orchestrates the Development of Coeliac Disease. Front. Immunol. 2021, 12, 674313.
- Granito, A.; Muratori, P.; Cassani, F.; Pappas, G.; Muratori, L.; Agostinelli, D.; Veronesi, L.; Bortolotti, R.; Petrolini, N.; Bianchi, F.B.; et al. Anti-actin IgA antibodies in severe coeliac disease. Clin. Exp. Immunol. 2004, 137, 386–392.
- Cervio, E.; Volta, U.; Verri, M.; Boschi, F.; Pastoris, O.; Granito, A.; Barbara, G.; Parisi, C.; Felicani, C.; Tonini, M.; et al. Sera of patients with celiac disease and neurologic disorders evoke a mitochondrial-dependent apoptosis in vitro. Gastroenterology 2007, 133, 195–206.
- Schuppan, D.; Ciccocioppo, R. Coeliac disease and secondary autoimmunity. Dig. Liver Dis. 2002, 34, 13–15.
- Granito, A.; Zauli, D.; Muratori, P.; Muratori, L.; Grassi, A.; Bortolotti, R.; Petrolini, N.; Veronesi, L.; Gionchetti, P.; Bianchi, F.B.; et al. Anti-Saccharomyces cerevisiae and perinuclear anti-neutrophil cytoplasmic antibodies in coeliac disease before and after gluten-free diet. Aliment. Pharmacol. Ther. 2005, 21, 881–887.
- Forsberg, G.; Hernell, O.; Hammarström, S.; Hammarström, M.-L. Concomitant increase of IL-10 and pro-inflammatory cytokines in intraepithelial lymphocyte subsets in celiac disease. Int. Immunol. 2007, 19, 993–1001.
- Forsberg, G.; Hernell, O.; Melgar, S.; Israelsson, A.; Hammarström, S.; Hammarström, M. Paradoxical coexpression of proinflammatory and down-regulatory cytokines in intestinal T cells in childhood celiac disease. Gastroenterology 2002, 123, 667–678.
- Salvati, V.M.; Mazzarella, G.; Gianfrani, C.; Levings, M.K.; Stefanile, R.; De Giulio, B.; Iaquinto, G.; Giardullo, N.; Auricchio, S.; Roncarolo, M.G.; et al. Recombinant human interleukin 10 suppresses gliadin dependent T cell activation in ex vivo cultured coeliac intestinal mucosa. Gut 2005, 54, 46–53.
- Borrelli, M.; Salvati, V.M.; Maglio, M.; Zanzi, D.; Ferrara, K.; Santagata, S.; Ponticelli, D.; Aitoro, R.; Mazzarella, G.; Lania, G.; et al. Immunoregulatory pathways are active in the small intestinal mucosa of patients with potential celiac disease. Am. J. Gastroenterol. 2013, 108, 1775–1784.
- Fiorentino, D.F.; Zlotnik, A.; Mosmann, T.R.; Howard, M.; O’Garra, A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991, 147, 3815–3822.
- Fiorentino, D.F.; Zlotnik, A.; Vieira, P.; Mosmann, T.R.; Howard, M.; Moore, K.W.; O’Garra, A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. (Baltim. Md. 1950) 1991, 146, 3444–3451.
- de Waal Malefyt, R.; Haanen, J.; Spits, H.; Roncarolo, M.G.; te Velde, A.; Figdor, C.; Johnson, K.; Kastelein, R.; Yssel, H.; de Vries, J.E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med. 1991, 174, 915–924.
- Buelens, C.; Willems, F.; Delvaux, A.; Piérard, G.; Delville, J.P.; Velu, T.; Goldman, M. Interleukin-10 differentially regulates B7-1 (CD80) and B7-2 (CD86) expression on human peripheral blood dendritic cells. Eur. J. Immunol. 1995, 25, 2668–2672.
- de Waal Malefyt, R.; Yssel, H.; de Vries, J.E. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J. Immunol. 1993, 150, 4754–4765.
- Taga, K.; Mostowski, H.; Tosato, G. Human interleukin-10 can directly inhibit T-cell growth. Blood 1993, 81, 2964–2971.
- Groux, H.; Bigler, M.; de Vries, J.E.; Roncarolo, M.G. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J. Exp. Med. 1996, 184, 19–29.
- Groux, H.; O’Garra, A.; Bigler, M.; Rouleau, M.; Antonenko, S.; de Vries, J.E.; Roncarolo, M.G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 1997, 389, 737–742.
- Pender, S.L.; Breese, E.J.; Günther, U.; Howie, D.; Wathen, N.C.; Schuppan, D.; MacDonald, T.T. Suppression of T cell–mediated injury in human gut by interleukin 10: Role of matrix metalloproteinases. Gastroenterology 1998, 115, 573–583.
- Nilsen, E.M.; Jahnsen, F.L.; Lundin, K.E.; Johansen, F.E.; Fausa, O.; Sollid, L.M.; Jahnsen, J.; Scott, H.; Brandtzaeg, P. Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology 1998, 115, 551–563.
- Beckett, C.G.; Dell'Olio, D.; Kontakou, M.; Przemioslo, R.T.; Rosen-Bronson, S.; Ciclitira, P.J. Analysis of interleukin-4 and interleukin-10 and their association with the lymphocytic infiltrate in the small intestine of patients with coeliac disease. Gut 1996, 39, 818–823.
- Letterio, J.J.; Roberts, A.B. Regulation of immune responses by TGF-β. Annu. Rev. Immunol. 1998, 16, 137–161.
- Hansson, T.; Ulfgren, A.K.; Lindroos, E.; DannAEus, A.; Dahlbom, I.; Klareskog, L. Transforming growth factor-β (TGF-β) and tissue transglutaminase expression in the small intestine in children with coeliac disease. Scand. J. Immunol. 2002, 56, 530–537.
- Benahmed, M.; Meresse, B.; Arnulf, B.; Barbe, U.; Mention, J.; Verkarre, V.; Allez, M.; Cellier, C.; Hermine, O.; Cerf–Bensussan, N. Inhibition of TGF-β signaling by IL-15: A new role for IL-15 in the loss of immune homeostasis in celiac disease. Gastroenterology 2007, 132, 994–1008.
- Monteleone, G.; Pallone, F.; MacDonald, T.T. Smad7 in TGF-β-mediated negative regulation of gut inflammation. Trends Immunol. 2004, 25, 513–517.
- Feagins, L.A. Role of transforming growth factor-b in inflammatory bowel disease and colitis-associated colon cancer. Inflamm. Bowel Dis. 2010, 16, 1963–1968.
- Du, Y.; Fang, Q.; Zheng, S.-G. Regulatory T Cells: Concept, Classification, Phenotype, and Biological Characteristics. Adv. Exp. Med. Biol. 2021, 1278, 1–31.
- Gianfrani, C.; Levings, M.K.; Sartirana, C.; Mazzarella, G.; Barba, G.; Zanzi, D.; Camarca, A.; Iaquinto, G.; Giardullo, N.; Auricchio, S.; et al. Gliadin-specific type 1 regulatory T cells from the intestinal mucosa of treated celiac patients inhibit pathogenic T cells. J. Immunol. 2006, 177, 4178–4186.
- Bacchetta, R.; Sartirana, C.; Levings, M.; Bordignon, C.; Narula, S.; Roncarolo, M.-G. Growth and expansion of human T regulatory type 1 cells are independent from TCR activation but require exogenous cytokines. Eur. J. Immunol. 2002, 32, 2237–2245.
- Roncarolo, M.G.; Gregori, S.; Bacchetta, R.; Battaglia, M.; Gagliani, N. The Biology of T Regulatory Type 1 Cells and Their Therapeutic Application in Immune-Mediated Diseases. Immunity 2018, 49, 1004–1019.
- Freeborn, R.A.; Strubbe, S.; Roncarolo, M.G. Type 1 regulatory T cell-mediated tolerance in health and disease. Front. Immunol. 2022, 13, 1032575.
- Du Pré, M.F.; Kozijn, A.E.; van Berkel, L.A.; ter Borg, M.N.; Lindenbergh–Kortleve, D.; Jensen, L.T.; Kooy-Winkelaar, Y.; Koning, F.; Boon, L.; Nieuwenhuis, E.E.; et al. Tolerance to ingested deamidated gliadin in mice is maintained by splenic, type 1 regulatory T cells. Gastroenterology 2011, 141, 610–620.
- van Leeuwen, M.A.; Costes, L.M.M.; van Berkel, L.A.; Simons-Oosterhuis, Y.; du Pré, M.F.; Kozijn, A.E.; Raatgeep, H.C.; Lindenbergh-Kortleve, D.J.; van Rooijen, N.; Koning, F.; et al. Macrophage-mediated gliadin degradation and concomitant IL-27 production drive IL-10- and IFN-γ-secreting Tr1-like-cell differentiation in a murine model for gluten tolerance. Mucosal Immunol. 2017, 10, 635–649.
- Scheinecker, C.; Göschl, L.; Bonelli, M. Treg cells in health and autoimmune diseases: New insights from single cell analysis. J. Autoimmun. 2019, 110, 102376.
- Benson, M.J.; Pino-Lagos, K.; Rosemblatt, M.; Noelle, R.J. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 2007, 204, 1765–1774.
- Santegoets, S.J.; Dijkgraaf, E.M.; Battaglia, A.; Beckhove, P.; Britten, C.M.; Gallimore, A.; Godkin, A.; Gouttefangeas, C.; de Gruijl, T.D.; Koenen, H.J.P.M.; et al. Monitoring regulatory T cells in clinical samples: Consensus on an essential marker set and gating strategy for regulatory T cell analysis by flow cytometry. Cancer Immunol. Immunother. 2015, 64, 1271–1286.
- Tanaka, A.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 2016, 27, 109–118.
- Szymczak-Workman, A.L.; Workman, C.J.; Vignali, D.A. Cutting edge: Regulatory T cells do not require stimulation through their TCR to suppress. J. Immunol. 2009, 182, 5188–5192.
- Schmidt, A.M.; Lu, W.; Sindhava, V.J.; Huang, Y.; Burkhardt, J.K.; Yang, E.; Riese, M.J.; Maltzman, J.S.; Jordan, M.S.; Kambayashi, T. Regulatory T cells require TCR signaling for their suppressive function. J. Immunol. 2015, 194, 4362–4370.
- Hmida, N.B.; Ben Ahmed, M.; Moussa, A.; Rejeb, M.B.; Said, Y.; Kourda, N.; Meresse, B.; Abdeladhim, M.; Louzir, H.; Cerf-Bensussan, N. Impaired control of effector T cells by regulatory t cells: A clue to loss of oral tolerance and autoimmunity in celiac disease? Am. J. Gastroenterol. 2012, 107, 604–611.
- Tiittanen, M.; Westerholm-Ormio, M.; Verkasalo, M.; Savilahti, E.; Vaarala, O. Infiltration of forkhead box P3-expressing cells in small intestinal mucosa in coeliac disease but not in type 1 diabetes. Clin. Exp. Immunol. 2008, 152, 498–507.
- Vorobjova, T.; Uibo, O.; Heilman, K.; Rägo, T.; Honkanen, J.; Vaarala, O.; Tillmann, V.; Ojakivi, I.; Uibo, R. Increased FOXP3 expression in small-bowel mucosa of children with coeliac disease and type I diabetes mellitus. Scand. J. Gastroenterol. 2009, 44, 422–430.
- Brazowski, E.; Cohen, S.; Yaron, A.; Filip, I.; Eisenthal, A. FOXP3 expression in duodenal mucosa in pediatric patients with celiac disease. Pathobiology 2010, 77, 328–334.
- Zanzi, D.; Stefanile, R.; Santagata, S.; Iaffaldano, L.; Iaquinto, G.; Giardullo, N.; Lania, G.; Vigliano, I.; Vera, A.R.; Ferrara, K.; et al. IL-15 interferes with suppressive activity of intestinal regulatory T cells expanded in Celiac disease. Am. J. Gastroenterol. 2011, 106, 1308–1317.
- Vorobjova, T.; Uibo, O.; Heilman, K.; Uibo, R. Increased density of tolerogenic dendritic cells in the small bowel mucosa of celiac patients. World J. Gastroenterol. 2015, 21, 439–452.
- Serena, G.; Yan, S.; Camhi, S.; Patel, S.; Lima, R.S.; Sapone, A.; Leonard, M.M.; Mukherjee, R.; Nath, B.J.; Lammers, K.M.; et al. Proinflammatory cytokine interferon-γ and microbiome-derived metabolites dictate epigenetic switch between forkhead box protein 3 isoforms in coeliac disease. Clin. Exp. Immunol. 2017, 187, 490–506.
- Halstensen, T.S.; Scott, H.; Brandtzaeg, P. Intraepithelial Tcells of theTcRγ/δ+ CD8− andVδ1/Jδ1+ phenotypes are increased in coeliac disease. Scand. J. Immunol. 1989, 30, 665–672.
- Li, G.-Q.; Xia, J.; Zeng, W.; Luo, W.; Liu, L.; Zeng, X.; Cao, D. The intestinal γδ T cells: Functions in the gut and in the distant organs. Front. Immunol. 2023, 14, 1206299.
- Hüe, S.; Mention, J.J.; Monteiro, R.C.; Zhang, S.; Cellier, C.; Schmitz, J.; Verkarre, V.; Fodil, N.; Bahram, S.; Cerf-Bensussan, N.; et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 2004, 21, 367–377.
- Mishra, S.; Srinivasan, S.; Ma, C.; Zhang, N. CD8+ Regulatory T Cell—A Mystery to Be Revealed. Front. Immunol. 2021, 12, 708874.
- Shytikov, D.; Rohila, D.; Li, D.; Wang, P.; Jiang, M.; Zhang, M.; Xu, Q.; Lu, L. Functional Characterization of Ly49+CD8 T-Cells in Both Normal Condition and during Anti-Viral Response. Front. Immunol. 2021, 11, 602783.
- Saligrama, N.; Zhao, F.; Sikora, M.J.; Serratelli, W.S.; Fernandes, R.A.; Louis, D.M.; Yao, W.; Ji, X.; Idoyaga, J.; Mahajan, V.B.; et al. Opposing T cell responses in experimental autoimmune encephalomyelitis. Nature 2019, 572, 481–487.
- Mazzarella, G. Effector and Suppressor T cells in Celiac Disease. World J. Gastroenterol. 2015, 21, 7349–7356.
- Lahat, N.; Shapiro, S.; Karban, A.; Gerstein, R.; Kinarty, A.; Lerner, A. Cytokine profile in coeliac disease. Scand. J. Immunol. 1999, 49, 441–446.
- Raffin, C.; Vo, L.T.; Bluestone, J.A. Treg cell-based therapies: Challenges and perspectives. Nat. Rev. Immunol. 2020, 20, 158–172.
- Bluestone, J.A.; Buckner, J.H.; Fitch, M.; Gitelman, S.E.; Gupta, S.; Hellerstein, M.K.; Herold, K.C.; Lares, A.; Lee, M.R.; Li, K.; et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl. Med. 2015, 7, 315ra189.
- Harris, D.T.; Kranz, D.M. Adoptive T Cell Therapies: A Comparison of T Cell Receptors and Chimeric Antigen Receptors. Trends Pharmacol. Sci. 2016, 37, 220–230.
- Dawson, N.A.J.; Levings, M.K. Antigen-specific regulatory T cells: Are police CARs the answer? Transl. Res. 2017, 187, 53–58.
- Passeri, L.; Marta, F.; Bassi, V.; Gregori, S. Tolerogenic Dendritic Cell-Based Approaches in Autoimmunity. Int. J. Mol. Sci. 2021, 22, 8415.
- Giannoukakis, N.; Phillips, B.; Finegold, D.; Harnaha, J.; Trucco, M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care 2011, 34, 2026–2032.
- Morante-Palacios, O.; Fondelli, F.; Ballestar, E.; Martínez-Cáceres, E.M. Tolerogenic Dendritic Cells in Autoimmunity and Inflammatory Diseases. Trends Immunol. 2020, 42, 59–75.
- Yamazaki, S.; Inaba, K.; Tarbell, K.V.; Steinman, R.M. Dendritic cells expand antigen-specific Foxp3(+)CD25(+)CD4(+) regulatory T cells including suppressors of alloreactivity. Immunol. Rev. 2006, 212, 314–329.
- Passeri, L.; Andolfi, G.; Bassi, V.; Russo, F.; Giacomini, G.; Laudisa, C.; Marrocco, I.; Cesana, L.; Di Stefano, M.; Fanti, L.; et al. Tolerogenic IL-10-engineered dendritic cell-based therapy to restore antigen-specific tolerance in T cell mediated diseases. J. Autoimmun. 2023, 138, 103051.
- Getts, D.R.; Martin, A.J.; McCarthy, D.P.; Terry, R.L.; Hunter, Z.N.; Yap, W.T.; Getts, M.T.; Pleiss, M.; Luo, X.; King, N.J.; et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat. Biotechnol. 2012, 30, 1217–1224.
- Prasad, S.; Neef, T.; Xu, D.; Podojil, J.R.; Getts, D.R.; Shea, L.D.; Miller, S.D. Tolerogenic Ag-PLG nanoparticles induce tregs to suppress activated diabetogenic CD4 and CD8 T cells. J. Autoimmun. 2018, 89, 112–124.
- Hunter, Z.; McCarthy, D.P.; Yap, W.T.; Harp, C.T.; Getts, D.R.; Shea, L.D.; Miller, S.D. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano 2014, 8, 2148–2160.
- McCarthy, D.P.; Yap, J.W.; Harp, C.T.; Song, W.K.; Chen, J.; Pearson, R.M.; Miller, S.D.; Shea, L.D. An antigen-encapsulating nanoparticle platform for TH1/17 immune tolerance therapy. Nanomed. Nanotechnol. Biol. Med. 2016, 13, 191–200.
- Getts, D.R.; Shea, L.D.; Miller, S.D.; King, N.J. Harnessing nanoparticles for immune modulation. Trends Immunol. 2015, 36, 419–427.
- Getts, D.R.; Terry, R.L.; Getts, M.T.; Deffrasnes, C.; Müller, M.; van Vreden, C.; Ashhurst, T.M.; Chami, B.; McCarthy, D.; Wu, H.; et al. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci. Transl. Med. 2014, 6, 219ra7.
- Jamison, B.L.; Neef, T.; Goodspeed, A.; Bradley, B.; Baker, R.L.; Miller, S.D.; Haskins, K. Nanoparticles Containing an Insulin–ChgA Hybrid Peptide Protect from Transfer of Autoimmune Diabetes by Shifting the Balance between Effector T Cells and Regulatory T Cells. J. Immunol. (Baltim. Md. 1950) 2019, 203, 48–57.
- Freitag, T.L.; Podojil, J.R.; Pearson, R.M.; Fokta, F.J.; Sahl, C.; Messing, M.; Andersson, L.C.; Leskinen, K.; Saavalainen, P.; Hoover, L.I.; et al. Gliadin Nanoparticles Induce Immune Tolerance to Gliadin in Mouse Models of Celiac Disease. Gastroenterology 2020, 158, 1667–1681.
- Freitag, T.L.; Rietdijk, S.; Junker, Y.; Popov, Y.; Bhan, A.K.; Kelly, C.P.; Terhorst, C.; Schuppan, D. Gliadin-primed CD4+CD45RBlowCD25- T cells drive gluten-dependent small intestinal damage after adoptive transfer into lymphopenic mice. Gut 2009, 58, 1597–1605.
- Drucker, N.A.; McCulloh, C.J.; Li, B.; Pierro, A.; Besner, G.E.; Markel, T.A. Stem cell therapy in necrotizing enterocolitis: Current state and future directions. Semin. Pediatr. Surg. 2018, 27, 57–64.
- Chen, M.; Su, W.; Lin, X.; Guo, Z.; Wang, J.; Zhang, Q.; Brand, D.; Ryffel, B.; Huang, J.; Liu, Z.; et al. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum. 2013, 65, 1181–1193.
- Van Velthoven, C.T.; Sheldon, R.A.; Kavelaars, A.; Derugin, N.; Vexler, Z.S.; Willemen, H.L.; Maas, M.; Heijnen, C.J.; Ferriero, D.M. Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke 2013, 44, 1426–1432.
- Si, Y.; Zhao, Y.; Hao, H.; Liu, J.; Guo, Y.; Mu, Y.; Shen, J.; Cheng, Y.; Fu, X.; Han, W. Infusion of mesenchymal stem cells ameliorates hyperglycemia in type 2 diabetic rats: Identification of a novel role in improving insulin sensitivity. Diabetes 2012, 61, 1616–1625.
- Jones, J.; Estirado, A.; Redondo, C.; Pacheco-Torres, J.; Sirerol-Piquer, M.-S.; Garcia-Verdugo, J.M.; Martinez, S. Mesenchymal stem cells improve motor functions and decrease neurodegeneration in ataxic mice. Mol. Ther. 2015, 23, 130–138.
- Liu, C.B.; Huang, H.; Sun, P.; Ma, S.Z.; Liu, A.H.; Xue, J.; Fu, J.H.; Liang, Y.Q.; Liu, B.; Wu, D.Y.; et al. Human Umbilical Cord-Derived Mesenchymal Stromal Cells Improve Left Ventricular Function, Perfusion, and Remodeling in a Porcine Model of Chronic Myocardial Ischemia. Stem Cells Transl. Med. 2016, 5, 1004–1013.
- Rehorova, M.; Vargova, I.; Forostyak, S.; Vackova, I.; Turnovcova, K.; Kupcova Skalnikova, H.; Vodička, P.; Kubinová, Š.; Syková, E.; Jendelová, P. A combination of intrathecal and intramuscular application of human mesenchymal stem cells partly reduces the activation of necroptosis in the spinal cord of SOD1(G93A) rats. Stem Cells Transl. Med. 2019, 8, 535–547.
- Soria, B.; Martin-Montalvo, A.; Aguilera, Y.; Mellado-Damas, N.; López-Beas, J.; Herrera-Herrera, I.; López, E.; Barcia, J.A.; Alvarez-Dolado, M.; Hmadcha, A.; et al. Human Mesenchymal Stem Cells Prevent Neurological Complications of Radiotherapy. Front. Cell. Neurosci. 2019, 13, 204.
More
Information
Subjects:
Immunology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
803
Revisions:
2 times
(View History)
Update Date:
23 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




