
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fumitoshi Ishino | -- | 3542 | 2023-10-19 13:24:06 | | | |
| 2 | Fumitoshi Ishino | Meta information modification | 3542 | 2023-10-19 13:46:14 | | | | |
| 3 | Lindsay Dong | + 6 word(s) | 3548 | 2023-10-24 10:17:46 | | | | |
| 4 | Fumitoshi Ishino | Meta information modification | 3548 | 2023-10-24 15:15:09 | | | | |
| 5 | Lindsay Dong | Meta information modification | 3548 | 2023-10-25 10:51:40 | | |
Video Upload Options
Eutherians have 11 retrotransposon Gag-like (RTL)/sushi-ichi retrotransposon homolog (SIRH) genes presumably derived from a certain retrovirus. Accumulating evidence indicates that the RTL/SIRH genes play a variety of roles in the mammalian developmental system, such as in the placenta, brain, and innate immune system, in a eutherian-specific manner. It has been shown that the functional role of Paternally Expressed 10 (PEG10) in placental formation is unique to the therian mammals, as are the eutherian-specific roles of PEG10 and PEG11/RTL1 in maintaining the fetal capillary network and the endocrine regulation of RTL7/SIRH7 (aka Leucine Zipper Down-Regulated in Cancer 1 (LDOCK1)) in the placenta. In the brain, PEG11/RTL1 is expressed in the corticospinal tract and hippocampal commissure, mammalian-specific structures, and in the corpus callosum, a eutherian-specific structure. Unexpectedly, at least three RTL/SIRH genes, RTL5/SIRH8, RTL6/SIRH3, and RTL9/SIRH10, play important roles in combating a variety of pathogens, namely viruses, bacteria, and fungi, respectively, suggesting that the innate immunity system of the brain in eutherians has been enhanced by the emergence of these new components.
1. Introduction
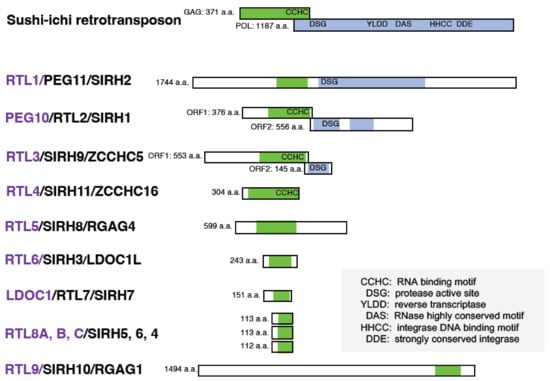
2. Discovery of PEG10 and PEG11/RTL1 from Genomic Imprinting Research
2.1. Roles of PEG10 and PEG11/RTL1 in Placental Evolution in Mammals
2.2. Roles of PEG11/RTL1 in Muscle Development
2.3. PEG10 and PEG11/RTL1 in Neurological Disorders
3. Retrovirus-Derived RTL/SIRH Genes as Eutherian-Specific Genes
3.1. RTL7/SIRH7 in the Placenta
3.2. RTL4/SIRH11 in the Brain
3.3. RTL8A, 8B, 8C/SIRH5, 6, 4 in the Brain
3.4. RTL6/SIRH3, RTL5/SIRH8 and RTL9/SIRH10 Are Microglial Genes in the Brain
Using the same approach of combining Venus KI mice and KO mice, RTL9/SIRH10 (aka RGAG1) was demonstrated to be another microglial gene that is actively protective against fungi by reacting to zymosan, the cell wall of fungi [86]. It encodes a large protein comprising two herpes virus-derived domains in addition to the GAG-like domain. The role of the first two regions remains unknown, but the latter is essential for zymosan removal. Unlike RTL6/SIRH3 and RTL5/SIRH8, RTL9/SIRH10 is restricted to the lysosomes of microglia, where the zymosan is ultimately taken up and degraded. Thus, at least three RLT/SIRH genes are involved in the clearance of bacterial, viral, and fungal pathogens from the brain, suggesting that these genes must have critically contributed to the evolution of the innate immune system in eutherians [87][86].
3.5. Relationship between RTL/SIRH Genes and Extraembryonic Tissues
4. Two Types of Exapted Genes: Acquired RTL/SIRH Genes and Captured Syncytin Genes
References
- Ono, R.; Kobayashi, S.; Wagatsuma, H.; Aisaka, K.; Kohda, T.; Kaneko-Ishino, T.; Ishino, F. A retrotransposon-derived gene, PEG10, is a novel imprinted gene located on human chromosome 7q21. Genomics 2001, 73, 232–237.
- Charlier, C.; Segers, K.; Wagenaar, D.; Karim, L.; Berghmans, S.; Jaillon, O.; Shay, T.; Weissenbach, J.; Cockett, N.; Gyapay, G.; et al. Human-ovine comparative sequencing of a 250-kb imprinted domain encompassing the callipyge (clpg) locus and identification of six imprinted transcripts: DLK1, DAT, GTL2, PEG11, antiPEG11, and MEG8. Genome Res. 2001, 11, 850–862.
- Blond, J.-L.; Lavillette, D.; Cheynet, V.; Bouton, O.; Oriol, G.; Chapel-Fernandes, S.; Mandrand, B.; Mallet, F.; Cosset, F.-L. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J. Virol. 2000, 74, 3321–3329.
- Mi, S.; Lee, X.; Li, X.-P.; Veldman, G.M.; Finnerty, H.; Racie, L.; LaVallie, E.; Tang, X.-Y.; Edouard, P.; Howes, S.; et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 2000, 403, 785–789.
- Ono, R.; Nakamura, K.; Inoue, K.; Naruse, M.; Usami, T.; Wakisaka-Saito, N.; Hino, T.; Suzuki-Migishima, R.; Ogonuki, N.; Miki, H.; et al. Deletion of Peg10, an imprinted gene acquired from a retrotransposon, causes early embryonic lethality. Nat. Genet. 2006, 38, 101–106.
- Sekita, Y.; Wagatsuma, H.; Nakamura, K.; Ono, R.; Kagami, M.; Wakisaka, N.; Hino, T.; Suzuki-Migishima, R.; Kohda, T.; Ogura, A.; et al. Role of retrotransposon-derived imprinted gene, Rtl1, in the feto-maternal interface of mouse placenta. Nat. Genet. 2008, 40, 243–248.
- Dupressoir, A.; Vernochet, C.; Bawa, O.; Harper, F.; Pierron, G.; Opolon, P.; Heidmann, T. Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc. Natl. Acad. Sci. USA 2009, 106, 12127–12132.
- Dupressoir, A.; Vernochet, C.; Harper, F.; Guegan, J.; Dessen, P.; Pierron, G.; Heidmann, T. A pair of co-opted retroviral envelope syncytin genes is required for formation of the two-layered murine placental syncytiotrophoblast. Proc. Natl. Acad. Sci. USA 2011, 108, 1164–1173.
- Blaise, S.; de Parseval, N.; Bénit, L.; Heidmann, T. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc. Natl. Acad. Sci. USA 2003, 100, 13013–13018.
- Mallet, F.; Bouton, O.; Prudhomme, S.; Cheynet, V.; Oriol, G.; Bonnaud, B.; Lucotte, G.; Duret, L.; Mandrand, B. The endogenous retroviral locus ERVWE1 is a bona fide gene involved in hominoid placental physiology. Proc. Natl. Acad. Sci. USA 2004, 101, 1731–1736.
- Heidmann, O.; Vernochet, C.; Dupressoir, A.; Heidemann, T. Identification of an endogenous retroviral envelope gene with fusogenic activity and placenta-specific expression in the rabbit: A new “syncytin” in a third order of mammals. Retrovirology 2009, 6, 107.
- Cornelis, G.; Heidmann, O.; Degrelle, S.A.; Vernochet, C.; Lavialle, C.; Letzelter, C.; Bernard-Stoecklin, S.; Hassanin, A.; Mulot, B.; Guillomot, M.; et al. Captured retroviral envelope syncytin gene associated with the unique placental structure of higher ruminants. Proc. Natl. Acad. Sci. USA 2013, 110, E828–E837.
- Cornelis, G.; Vernochet, C.; Malicorne, S.; Souquere, S.; Tzika, A.C.; Goodman, S.M.; Catzeflis, F.; Robinson, T.J.; Milinkovitch, M.C.; Pierron, G.; et al. Retroviral envelope syncytin capture in an ancestrally diverged mammalian clade for placentation in the primitive Afrotherian tenrecs. Proc. Natl. Acad. Sci. USA 2014, 111, E4332–E4341.
- Cornelis, G.; Vernochet, C.; Carradec, Q.; Souquere, S.; Mulot, B.; Catzeflis, F.; Nilsson, M.A.; Menzies, B.R.; Renfree, M.B.; Pierron, G.; et al. Retroviral envelope gene captures and syncytin exaptation for placentation in marsupials. Proc. Natl. Acad. Sci. USA 2015, 112, E487–E496.
- Nakaya, Y.; Koshi, K.; Nakagawa, S.; Hashizume, K.; Miyazawa, T. Fematrin-1 is involved in fetomaternal cell-to-cell fusion in Bovinae placenta and has contributed to diversity of ruminant placentation. J. Virol. 2013, 87, 10563–10572.
- Sugimoto, J.; Sugimoto, M.; Bernstein, H.; Jinno, Y.; Schust, D. A novel human endogenous retroviral protein inhibits cell-cell fusion. Sci. Rep. 2013, 3, 1462.
- Sugimoto, J.; Schust, D.J.; Kinjo, T.; Aoki, Y.; Jinno, Y.; Kudo, Y. Suppressyn localization and dynamic expression patterns in primary human tissues support a physiologic role in human placentation. Sci. Rep. 2019, 9, 19502.
- Kitao, K.; Shoji, H.; Miyazawa, T.; Nakagawa, S. Dynamic Evolution of Retroviral Envelope Genes in Egg-Laying Mammalian Genomes. Mol. Biol. Evol. 2023, 40, msad090.
- Kagami, M.; Sekita, Y.; Nishimura, G.; Irie, M.; Kato, F.; Okada, M.; Yamamori, S.; Kishimoto, H.; Nakayama, M.; Tanaka, Y.; et al. Deletions and epimutations affecting the human chromosome 14q32.2 imprinted region in individuals with paternal and maternal upd(14)-like phenotypes. Nat. Genet. 2008, 40, 237–242.
- Brandt, J.; Schrauth, S.; Veith, A.-M.; Froschauer, A.; Haneke, T.; Schultheis, C.; Gessler, M.; Leimeister, C.; Volff, J.-N. Transposable elements as a source of genetic innovation: Expression and evolution of a family of retrotransposon-derived neogenes in mammals. Gene 2005, 345, 101–111.
- Youngson, N.A.; Kocialkowski, S.; Peel, N.; Ferguson-Smith, A.C. A small family of sushi-class retrotransposon-derived genes in mammals and their relation to genomic imprinting. J. Mol. Evol. 2005, 61, 481–490.
- Kaneko-Ishino, T.; Ishino, F. The role of genes domesticated from LTR retrotransposons and retroviruses in mammals. Front. Microbiol. 2012, 3, 262.
- Poulter, R.; Butler, M. A retrotransposon family from the pufferfish (fugu) Fugu rubripes. Gene 1998, 215, 241–249.
- Butler, M.; Goodwin, T.; Simpson, M.; Singh, M.; Poulter, R. Vertebrate LTR retrotransposons of the Tf1/Sushi Group. J. Mol. Evol. 2001, 52, 260–274.
- Llorens, C.; Muñoz-Pomer, A.; Bernad, L.; Botella, H.; Moyam, A. Network dynamics of eukaryotic LTR retroelements beyond phylogenetic trees. Biol. Direct 2009, 4, 41.
- Surani, M.A.; Barton, S.C.; Norris, M.L. Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature 1984, 308, 548–550.
- McGrath, J.; Solter, D. Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell 1984, 37, 179–183.
- Mann, J.R.; Lovell-Badge, R.H. Inviability of parthenogenones is determined by pronuclei, not egg cytoplasm. Nature 1984, 310, 66–67.
- Cattanach, B.M.; Kirk, M. Differential activity of maternally and paternally derived chromosome regions in mice. Nature 1985, 315, 496–498.
- Cattanach, B.M.; Beechey, C.V. Autosomal and X-chromosome imprinting. Development 1990, 108, 63–72.
- Kaneko-Ishino, T.; Kohda, T.; Ishino, F. The regulation and biological significance of genomic imprinting in mammals. J. Biochem. 2003, 133, 699–711.
- Bartolomei, M.S.; Ferguson-Smith, A.C. Mammalian genomic imprinting. Cold Spring Harb. Perspect. Biol. 2011, 3, a002592.
- Barlow, D.P.; Bartolomei, M.S. Genomic imprinting in mammals. Cold Spring Harb. Perspect. Biol. 2014, 6, a018382.
- Kaneko-Ishino, T.; Ishino, F. Mammalian-specific genomic functions: Newly acquired traits generated by genomic imprinting and LTR retrotransposon-derived genes in mammals. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2015, 91, 511–538.
- Kaneko-Ishino, T.; Ishino, F. Evolution of viviparaity in mammals: What genomic imprinting tells us about mammalian placental evolution. Reprod. Fert. Dev. 2019, 31, 1219–1227.
- Ono, R.; Shiura, H.; Abratani, Y.; Kohda, T.; Kaneko-Ishino, T.; Ishino, F. Identification of a large novel imprinted gene cluster on mouse proximal chromosome 6. Genome Res. 2003, 13, 1696–1705.
- Cockett, N.E.; Jackson, S.P.; Shay, T.L.; Nielsen, D.; Moore, S.S.; Steele, M.R.; Barendse, W.; Green, R.D.; Georges, M. Chromosomal localization of the callipyge gene in sheep (Ovis aries) using bovine DNA markers. Proc. Natl. Acad. Sci. USA 1994, 91, 3019–3023.
- Kotzot, D. Maternal uniparental disomy 14 dissection of the phenotype with respect to rare autosomal recessively inherited traits, trisomy mosaicism, and genomic imprinting. Ann. Genet. 2004, 47, 251–260.
- Kagami, M.; Nishimura, G.; Okuyama, T.; Hayashidani, M.; Takeuchi, T.; Tanaka, S.; Ishino, F.; Kurosawa, K.; Ogata, T. Segmental and full paternal isodisomy for chromosome 14 in three patients: Narrowing the critical region and implication for the clinical features. Am. J. Med. Genet. 2005, 138A, 127–132.
- Ioannides, Y.; Lokulo-Sodipe, K.; Mackay, D.J.; Davies, J.H.; Temple, I.K. Temple syndrome: Improving the recognition of an underdiagnosed chromosome 14 imprinting disorder: An analysis of 51 published cases. J. Med. Genet. 2014, 51, 495–501.
- Kagami, M.; Kurosawa, K.; Miyazaki, O.; Ishino, F.; Matsuoka, K.; Ogata, T. Comprehensive clinical studies in 34 patients with molecularly defined UPD (14)pat and related conditions (Kagami Ogata syndrome). Eur. J. Hum. Genet. 2015, 23, 1488–1498.
- Kagami, M.; Nagasaki, K.; Kosaki, R.; Horikawa, R.; Naiki, Y.; Saitoh, S.; Tajima, T.; Yorifuji, T.; Numakura, C.; Mizuno, S.; et al. Temple syndrome: Comprehensive molecular and clinical findings in 32 Japanese patients. Genet. Med. 2017, 19, 1356–1366.
- Kitazawa, M.; Hayashi, S.; Imamura, M.; Takeda, S.; Oishi, Y.; Kaneko-Ishino, T.; Ishino, F. Deficiency and overexpression of Rtl1 in the mouse cause distinct muscle abnormalities related to Temple and Kagami-Ogata syndromes. Development 2020, 147, dev185918.
- Kitazawa, M.; Sutani, A.; Kaneko-Ishino, T.; Ishino, F. The role of eutherian-specific RTL1 in the nervous system and its implications for the Kagami-Ogata and Temple syndromes. Genes Cells 2021, 26, 165–179.
- Kitazawa, M.; Tamura, M.; Kaneko-Ishino, T.; Ishino, F. Severe damage to the placental fetal capillary network causes mid- to late fetal lethality and reductionin placental size in Peg11/Rtl1 KO mice. Genes Cells 2017, 22, 174–188.
- Dauber, A.; Cunha-Silva, M.; Macedo, D.B.; Brito, V.N.; Abreu, A.P.; Roberts, S.A.; Montenegro, L.R.; Andrew, M.; Kirby, A.; Weirauch, M.T.; et al. Paternally inherited DLK1 deletion associated with familial central precocious puberty. J. Clin. Endocrinol. Metab. 2017, 102, 1557–1567.
- Macedo, D.B.; Kaiser, U.B. DLK1, Notch signaling and the timing of puberty. Semin. Reprod. Med. 2019, 37, 174–181.
- Volff, J.; Körting, C.; Schartl, M. Ty3/Gypsy retrotransposon fossils in mammalian genomes: Did they evolve into new cellular functions? Mol. Biol. Evol. 2001, 18, 266–270.
- Irie, M.; Itoh, J.; Matsuzawa, A.; Ikawa, M.; Kiyonari, H.; Kihara, M.; Suzuki, T.; Hiraoka, Y.; Ishino, F.; Kaneko-Ishino, T. Retrovirus-derived acquired genes, RTL5 and RTL6, are novel constituents of the innate immune system in the eutherian brain. Development 2022, 149, dev200976.
- Imakawa, K.; Kusama, K.; Kaneko-Ishino, T.; Nakagawa, S.; Kitao, K.; Miyazawa, T.; Ishino, F. Endogenous retroviruses and placental evolution, development, and diversity. Cells 2022, 11, 2458.
- Suzuki, S.; Ono, R.; Narita, T.; Pask, A.J.; Shaw, G.; Wang, C.; Kohda, T.; Alsop, A.E.; Graves, J.A.M.; Kohara, Y.; et al. Retrotransposon Silencing by DNA Methylation Can Drive Mammalian Genomic Imprinting. PLoS Genet. 2007, 3, e55.
- Edwards, C.A.; Mungall, A.J.; Matthews, L.; Ryder, E.; Gray, D.J.; Pask, A.J.; Shaw, G.; Graves, J.A.; Rogers, J.; Dunham, I.; et al. The evolution of the DLK1-DIO3 imprinted domain in mammals. PLoS Biol. 2008, 6, e135.
- Kim, A.; Terzian, C.; Santamaria, P.; Pelisson, A.; Purd’homme, N.; Bucheton, A. Retroviruses in invertebrates: The gypsy retrotransposon is apparently an infectious retrovirus of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1994, 91, 1285–1289.
- Song, S.U.; Gerasimova, T.; Kurkulos, M.; Boeke, J.D.; Corces, V.G. An env-like protein encoded by a Drosophila retroelement: Evidence that gypsy is an infectious retrovirus. Genes Dev. 1994, 8, 2046–2057.
- Seitz, H.; Youngson, N.; Lin, S.-P.; Dalbert, S.; Paulsen, M.; Bachellerie, J.-P.; Ferguson-Smith, A.C.; Cavaillé, J. Imprinted microRNA genes transcribed antisense to a reciprocally imprinted retrotransposon-like gene. Nat. Genet. 2003, 34, 261–262.
- Davis, E.; Caiment, F.; Tordoir, X.; Cavaillé, J.; Ferguson-Smith, A.; Cockett, N.; Georges, M.; Charlier, C. RNAi-mediated allelic trans-interaction at the imprinted Rtl1/Peg11 locus. Curr. Biol. 2005, 15, 743–749.
- Ito, M.; Sferruzzi-Perri, A.N.; Edwards, C.A.; Adalsteinsson, B.T.; Allen, S.E.; Loo, T.-H.; Kitazawa, M.; Kaneko-Ishino, T.; Ishino, F.; Stewart, C.L.; et al. A trans-homologue interaction between reciprocally imprinted miR-127 and Rtl1 regulates placenta development. Development 2015, 142, 2425–2430.
- Xu, X.; Ectors, F.; Davis, E.E.; Pirottin, D.; Cheng, H.; Farnir, F.; Hadfield, T.; Cockett, N.; Charlier, C.; Georges, M.; et al. Ectopic expression of retrotransposon-derived PEG11/RTL1 contributes to the callipyge muscular hypertrophy. PLoS ONE 2015, 10, e0140594.
- Abdallah, B.M.; Ditzel, N.; Mahmood, A.; Isa, A.; Traustadottir, G.A.; Schilling, A.F.; Ruiz-Hidalgo, M.-J.; Laborda, J.; Amling, M.; Kassem, M. DLK1 is a novel regulator of bone mass that mediates estrogen deficiency–induced bone loss in mice. J. Bone Miner. Res. 2011, 26, 1457–1471.
- Byrne, K.; Colgrave, M.L.; Vuocolo, T.; Pearson, R.; Bidwell, C.A.; Cockett, N.E.; Lynn, D.J.; Fleming-Waddell, J.N.; Tellam, R.L. The imprinted retrotransposon-like gene PEG11 (RTL1) is expressed as a full-length protein in skeletal muscle from Callipyge sheep. PLoS ONE 2010, 5, e8638.
- Mihrshahi, R. The corpus callosum as an evolutionary innovation. J. Exp. Zool. 2006, 306B, 8–17.
- Suárez, R.; Gobius, I.; Richards, L.J. Evolution and development of interhemispheric connections in the vertebrate forebrain. Front. Hum. Neurosci. 2014, 14, 497.
- Fame, R.M.; MacDonald, J.L.; Macklis, J.D. Development, specification, and diversity of callosal projection neurons. Trends Neurosci. 2011, 34, 41–50.
- Aboitiz, F.; Montiel, J. One hundred million years of interhemispheric communication: The history of the corpus callosum. Braz. J. Med. Biol. Res. 2003, 36, 409–420.
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172.
- Deng, H.X.; Chen, W.; Hong, S.T.; Boycott, K.M.; Gorrie, G.H.; Siddique, N.; Yang, Y.; Fecto, F.; Shi, Y.; Zhai, H.; et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature 2011, 477, 211–215.
- Whiteley, A.M.; Prado, M.A.; de Poot, S.A.; Paulo, J.A.; Ashton, M.; Dominguez, S.; Weber, M.; Ngu, H.; Szpyt, J.; Jedrychowski, M.P.; et al. Global proteomics of Ubqln2-based murine models of ALS. J. Biol. Chem. 2021, 296, 100153.
- Black, H.H.; Hanson, J.L.; E Roberts, J.; Leslie, S.N.; Campodonico, W.; Ebmeier, C.C.; Holling, G.A.; Tay, J.W.; Matthews, A.M.; Ung, E.; et al. UBQLN2 restrains the domesticated retrotransposon PEG10 to maintain neuronal health in ALS. Elife 2023, 12, e79452.
- Malassine, A.; Frendo, J.-L.; Evain-Brion, D. A comparison of placental development and endocrine functions between the human and mouse model. Hum. Reprod. Update 2003, 9, 531–539.
- Arensburg, J.; Payne, A.H.; Orly, J. Expression of steroidogenic genes in maternal and extraembryonic cells during early pregnancy in mice. Endocrinology 1999, 140, 5220–5232.
- Simmons, D.G.; Rawn, S.; Davies, A.; Hughes, M.; Cross, J.C. Spatial and temporal expression of the 23 murine Prolactin/Placental Lactogen-related genes is not associated with their position in the locus. BMC Genom. 2008, 9, 352.
- Naruse, M.; Ono, R.; Irie, M.; Nakamura, K.; Furuse, T.; Hino, T.; Oda, K.; Kashimura, M.; Yamada, I.; Wakana, S.; et al. Sirh7/Ldoc1 knockout mice exhibit placental P4 overproduction and delayed parturition. Development 2014, 141, 4763–4771.
- Lyon, M.F. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature 1961, 190, 372–373.
- Takagi, N.; Sasaki, M. Preferential inactivation of the paternally derived X chromosome in the extraembryonic membranes of the mouse. Nature 1975, 256, 640–642.
- Murr, S.M.; Stabenfeldt, G.H.; Bradford, G.E.; Geschwind, I.I. Plasma progesterone during pregnancy in the mouse. Endocrinology 1974, 94, 1209–1211.
- Virgo, B.B.; Bellward, G.D. Serum progesterone levels in the pregnant and postpartum laboratory mouse. Endocrinology 1974, 95, 1486–1490.
- Lim, E.T.; Raychaudhuri, S.; Sanders, S.J.; Stevens, C.; Sabo, A.; MacArthur, D.G.; Neale, B.M.; Kirby, A.; Ruderfer, D.M.; Fromer, M.; et al. Rare complete knockouts in humans: Population distribution and significant role in autism spectrum disorders. Neuron 2013, 77, 235–242.
- Irie, M.; Yoshikawa, M.; Ono, R.; Iwafune, H.; Furuse, T.; Yamada, I.; Wakana, S.; Yamashita, Y.; Abe, T.; Ishino, F.; et al. Cognitive function related to the Sirh11/Zcchc16 gene acquired from an LTR retrotransposon in eutherians. PLoS Genet. 2015, 11, e1005521.
- Aston-Jones, G.; Rajkowski, J.; Cohen, J. Role of locus coeruleus in attention and behavioral flexibility. Biol. Psychiatry 1999, 46, 1309–1320.
- Berridge, C.W.; Waterhouse, B.D. The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev. 2003, 42, 33–84.
- Sara, S.J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009, 10, 211–223.
- Bouret, S.; Sara, S.J. Network reset: A simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005, 28, 574–582.
- Pandya, N.J.; Wang, C.; Costa, V.; Lopatta, P.; Meier, S.; Zampeta, F.I.; Punt, A.M.; Mientjes, E.; Grossen, P.; Distler, T.; et al. Secreted retrovirus-like GAG-domain-containing protein PEG10 is regulated by UBE3A and is involved in Angelman syndrome pathophysiology. Cell Rep. Med. 2021, 2, 100360.
- Nicholls, R.D.; Knepper, J.L. Genome organization, function, and imprinting in Prader-Willi and Angelman syndromes. Annu. Rev. Genom. Hum. Genet. 2001, 2, 153–175.
- Buiting, K.; Williams, C.; Horsthemke, B. Angelman syndrome—Insights into a rare neurogenetic disorder. Nat. Rev. Neurol. 2016, 12, 584–593.
- Ishino, F.; Itoh, J.; Irie, M.; Matsuzawa, A.; Naruse, M.; Suzuki, T.; Hiraoka, Y.; Kaneko-Ishino, T. Retrovirus-derived RTL9 plays an important role in innate antifungal immunity in the eutherian brain. bioRxiv 2023, 2023-06.
- Irie, M.; Itoh, J.; Matsuzawa, A.; Ikawa, M.; Kiyonari, H.; Kihara, M.; Suzuki, T.; Hiraoka, Y.; Ishino, F.; Kaneko-Ishino, T. Retrovirus-derived acquired genes, RTL5 and RTL6, are novel constituents of the innate immune system in the eutherian brain. Development 2022, 149, dev200976.
- Imakawa, K.; Nakagawa, S.; Miyazawa, T. Baton pass hypothesis: Successive incorporation of unconserved endogenous retroviral genes for placentation during mammalian evolution. Genes Cells 2005, 20, 771–788.
- Lavialle, C.; Cornelis, G.; Dupressoir, A.; Esnault, C.; Heidmann, O.; Vernochet, C.; Heidmann, T. Paleovirology of ‘syncytins’, retroviral env genes exapted for a role in placentation. Phil. Trans. R. Soc. B 2013, 368, 20120507.




