
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vadivel Ganapathy | -- | 4456 | 2023-10-18 18:18:48 | | | |
| 2 | Peter Tang | + 3 word(s) | 4459 | 2023-10-19 04:29:15 | | |
Video Upload Options
Sigma receptors are non-opiate/non-phencyclidine receptors that bind progesterone and/or heme and also several unrelated xenobiotics/chemicals. They reside in the plasma membrane and in the membranes of the endoplasmic reticulum, mitochondria, and nucleus. The biology/pharmacology of these proteins focused primarily on their role in neuronal functions in the brain/retina. However, there have been developments in the field with the discovery of unexpected roles for these proteins in iron/heme homeostasis. Sigma receptor 1 (S1R) regulates the oxidative stress-related transcription factor NRF2 and protects against ferroptosis, an iron-induced cell death process. Sigma receptor 2 (S2R), which is structurally unrelated to S1R, complexes with progesterone receptor membrane components PGRMC1 and PGRMC2. S2R, PGRMC1, and PGRMC2, either independently or as protein–protein complexes, elicit a multitude of effects with a profound influence on iron/heme homeostasis. This includes the regulation of the secretion of the iron-regulatory hormone hepcidin, the modulation of the activity of mitochondrial ferrochelatase, which catalyzes iron incorporation into protoporphyrin IX to form heme, chaperoning heme to specific hemoproteins thereby influencing their biological activity and stability, and protection against ferroptosis.
1. Introduction
|
S1R (%) |
S2R (%) |
PGRMC1 (%) |
PGRMC2 (%) |
|
|---|---|---|---|---|
|
S1R |
100 |
21 |
24 |
25 |
|
S2R |
21 |
100 |
21 |
21 |
|
PGRMC1 |
24 |
21 |
100 |
58 |
|
PGRMC2 |
25 |
21 |
58 |
100 |
2. Sigma Receptor 1 (S1R)
2.1. Amino Acid Sequence and Structure of S1R
2.2. Role of S1R in Protection against Neurodegeneration
2.3. Functional Relationship of S1R to Transcription Factor NRF2
2.4. Protection against Ferroptosis by S1R and Its Relationship to Hemochromatosis and Cancer
3. Sigma Receptor 2 (S2R)
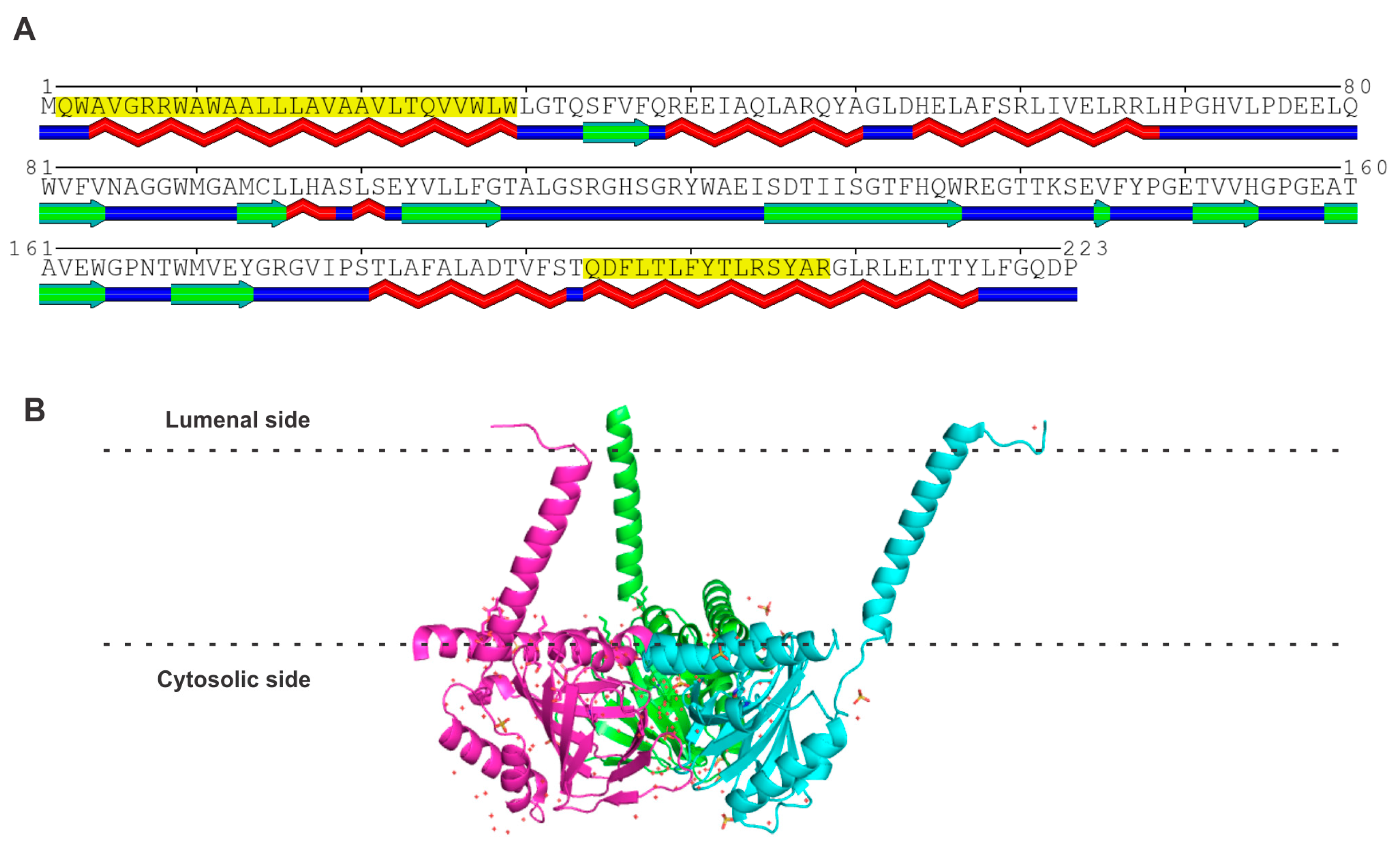
3.1. Amino Acid Sequence and Structure of S2R
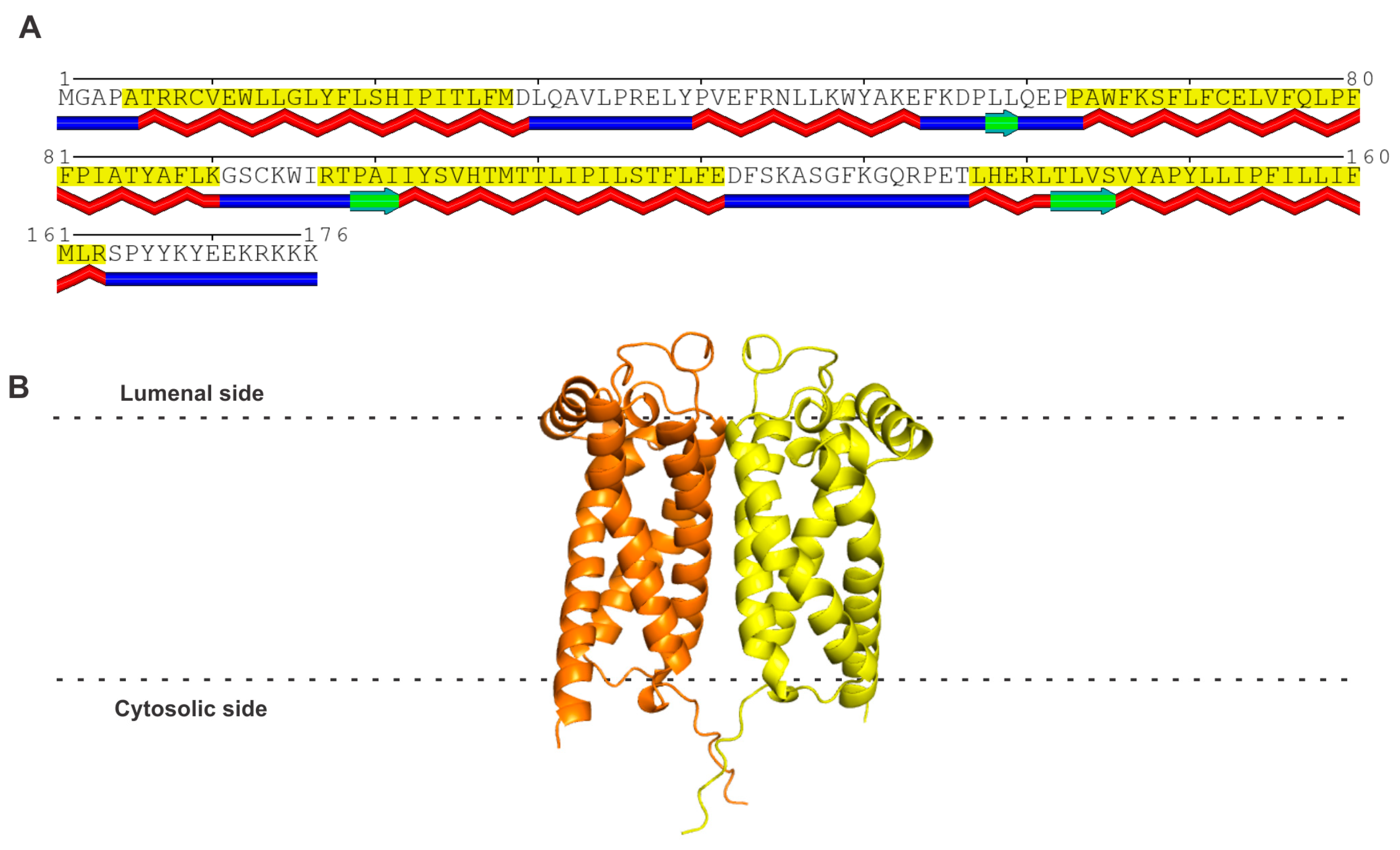
4. Progesterone Receptor Membrane Components 1 and 2 (PGRMC1 and PGRMC2)
4.1. Amino Acid Sequences and Structures of PGRMC1 and PGRMC2
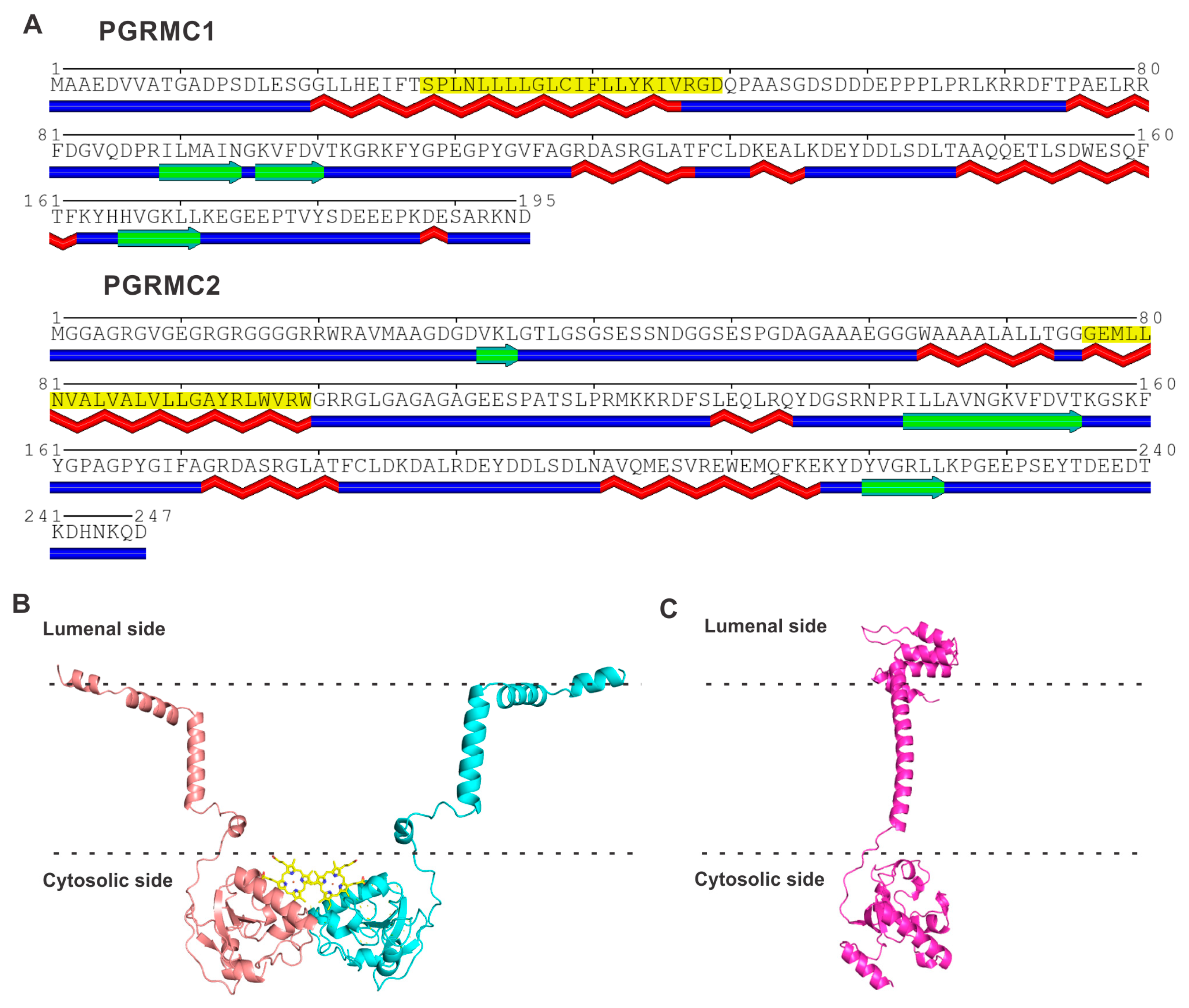
4.2. Common Structural Features in PGRMC1 and PGRMC2
References
- Martin, W.R.; Eades, C.G.; Thompson, J.A.; Huppler, R.E.; Gilbert, P.E. The effects of morphine and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976, 197, 517–532.
- Vaupel, D.B. Naltrexone fails to antagonize the sigma effects of PCP and SKF10,047 in the dog. Eur. J. Pharmacol. 1983, 92, 269–274.
- Largent, B.L.; Gundlach, A.L.; Snyder, S.H. Pharmacological and autoradiographic discrimination of sigma and phencyclidine receptor binding sites in brain with (+)-SKF 10,047, (+)--3--N-(1-propyl)piperidine and -1-piperidine. J. Pharmacol. Exp. Ther. 1986, 238, 739–748.
- Su, T.P. Pharmacologic characterizations of sigma receptors. NIDA Res. Monogr. 1993, 133, 41–53.
- Quirion, R.; Bowen, W.D.; Itzhak, Y.; Junien, J.L.; Musacchio, J.M.; Rothman, R.B.; Su, T.P.; Tam, S.W.; Taylor, D.P. A proposal for the classification of sigma binding sites. Trends Pharmacol. Sci. 1992, 13, 85–86.
- Bowen, W.D. Sigma receptors: Recent advances and new clinical potentials. Pharm. Acta Helv. 2000, 74, 211–218.
- Fishback, J.A.; Robsen, M.J.; Xu, Y.T.; Matsumoto, R.R. Sigma receptors: Potential targets for a new class of antidepressant drug. Pharmacol. Ther. 2010, 127, 271–282.
- Weng, C.C.; Riad, A.; Lieberman, B.P.; Xu, K.; Peng, X.; Mikitsh, J.L.; Mach, R.H. Characterization of sigma-2 receptor-specific binding sites using DTG and RHM-4. Pharmaceuticals 2022, 15, 1564.
- Schmidt, H.R.; Kruse, A.C. The molecular function of σ receptors: Past, present, and future. Trends Pharmacol. Sci. 2019, 40, 636–654.
- Pergolizzi, J.; Varrassi, G.; Coleman, M.; Breve, F.; Christo, D.K.; Christo, P.J.; Moussa, C. The sigma enigma: A narrative review of sigma receptors. Cureus 2023, 15, e35756.
- Lizama, B.N.; Kahle, J.; Catalano, S.M.; Caggiano, A.O.; Grundman, M.; Hamby, M.E. Sigma-2 receptors—From basic biology to therapeutic target: A focus on age-related degenerative diseases. Int. J. Mol. Sci. 2023, 24, 6251.
- Hasegawa, S.; Kasubuchi, M.; Terasawa, K.; Kimura, I. Perspectives on membrane-associated progesterone receptors as prospective therapeutic targets. Curr. Drug Targets 2016, 17, 1189–1197.
- Ryu, C.S.; Klein, K.; Zanger, U.M. Membrane associated progesterone receptors: Promiscuous proteins with pleiotropic functions—Focus on interactions with cytochrome P450. Front. Pharmacol. 2017, 8, 159.
- Cahill, M.A. Unde venisti PGRMC? Grand-scale biology from early eukaryotes and eumetazoan animal origins. Front. Biosci. (Landmark Ed.) 2022, 27, 317.
- Cahill, M.A. Quo vadis PGRMC? Grand-scale biology in human health and disease. Front. Biosci. (Landmark Ed.) 2022, 27, 318.
- Cahill, M.A. Progesterone receptor membrane component 1: An integrative review. J. Steroid Biochem. Mol. Biol. 2007, 105, 16–36.
- Pru, J.K.; Clark, N.C. PGRMC1 and PGRMC2 in uterine physiology and disease. Front. Neurosci. 2013, 7, 168.
- Smith, S.B.; Wang, J.; Cui, X.; Mysona, B.A.; Zhao, J.; Bollinger, K.E. Sigma 1 receptor: A novel therapeutic target in retinal disease. Prog. Retin. Eye Res. 2018, 67, 130–149.
- Malar, D.S.; Thitilertdecha, P.; Ruckvongacheep, K.S.; Brimson, S.; Tencomnao, T.; Brimson, J.M. Targeting sigma receptors for the treatment of neurodegenerative and neurodevelopmental disorders. CNS Drugs 2023, 37, 399–440.
- Wang, T.; Jia, H. The sigma receptors in Alzheimer’s disease: New potential targets for diagnosis and therapy. Int. J. Mol. Sci. 2023, 24, 12025.
- Yang, K.; Zeng, C.; Wang, C.; Sun, M.; Yin, D.; Sun, T. Sigma-2 receptor—A potential target for cancer/Alzheimer’s disease treatment via its regulation of cholesterol homeostasis. Molecules 2020, 25, 5439.
- Hanner, M.; Moebius, F.F.; Flandorfer, A.; Knaus, H.G.; Striessnig, J.; Kempner, R.; Glossmann, H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc. Natl. Acad. Sci. USA 1996, 93, 8072–8077.
- Kekuda, R.; Prasad, P.D.; Fei, Y.J.; Leibach, F.H.; Ganapathy, V. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1). Biochem. Biophys. Res. Commun. 1996, 229, 553–558.
- Seth, P.; Fei, Y.J.; Li, H.W.; Huang, W.; Leibach, F.H.; Ganapathy, V. Cloning and functional characterization of a sigma receptor from rat brain. J. Neurochem. 1998, 70, 922–931.
- Seth, P.; Leibach, F.H.; Ganapathy, V. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem. Biophys. Res. Commun. 1997, 241, 535–540.
- Prasad, P.D.; Li, H.W.; Fei, Y.J.; Ganapathy, M.E.; Fujita, T.; Plumley, L.H.; Yang-Feng, T.L.; Leibach, F.H.; Ganapathy, V. Exon-intron structure, analysis of promoter region, and chromosomal localization of the human type 1 sigma receptor gene. J. Neurochem. 1998, 70, 443–451.
- Cao, B.; Porollo, A.; Adamczak, R.; Jarrell, M.; Meller, J. Enhanced recognition of protein transmembrane domains with prediction-based structural profiles. Bioinformatics 2006, 22, 303–309.
- Schmidt, H.R.; Zheng, S.; Gurpinar, E.; Koehl, A.; Manglik, A.; Kruse, A.C. Crystal structure of the human σ1 receptor. Nature 2016, 532, 527–530.
- Schmidt, H.R.; Betz, R.M.; Dror, R.O.; Kruse, A.C. Structural basis for σ1 receptor ligand recognition. Nat. Struct. Mol. Biol. 2018, 25, 981–987.
- Meng, F.; Xiao, Y.; Ji, Y.; Sun, Z.; Zhou, X. An open-like conformation of the sigma-1 receptor reveals its ligand entry pathway. Nat. Commun. 2022, 13, 1267.
- Georgiadis, M.O.; Karoutzou, O.; Foscolos, A.S.; Papanastasiou, I. Sigma receptor (σR) ligands with antiproliferative and anticancer activity. Molecules 2017, 22, 1408.
- Ye, N.; Qin, W.; Tian, S.; Xu, Q.; Wold, E.A.; Zhou, J.; Zhen, X.C. Small molecules selectively targeting sigma-1 receptor for the treatment of neurological diseases. J. Med. Chem. 2020, 63, 15187–15217.
- Su, T.P.; London, E.D.; Jaffe, J.H. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science 1988, 240, 219–221.
- Yamada, M.; Nishigami, T.; Nakasho, K.; Nishimoto, Y.; Miyaji, H. Relationship between sigma-like site and progesterone-binding site of adult male rat liver microsomes. Hepatology 1994, 20, 1271–1280.
- Ramamoorthy, J.D.; Ramamoorthy, S.; Mahesh, V.B.; Leibach, F.H.; Ganapathy, V. Cocaine-sensitive sigma receptor and its interaction with steroid hormones in the human placental syncytiotrophoblast and in choriocarcinoma cells. Endocrinology 1995, 136, 924–932.
- Ganapathy, M.E.; Prasad, P.D.; Huang, W.; Seth, P.; Leibach, F.H.; Ganapathy, V. Molecular and ligand-binding characterization of the sigma receptor in the Jurkat human T lymphocyte cell line. J. Pharmacol. Exp. Ther. 1999, 289, 251–260.
- Maurice, T.; Urani, A.; Phan, V.L.; Romieu, P. The interaction between neuroactive steroids and the sigma1 receptor function: Behavioral consequences and therapeutic opportunities. Brain Res. Brain Res. Rev. 2001, 37, 116–132.
- Maurice, T. Neurosteroids and sigma1 receptors, biochemical and behavioral relevance. Pharmacopsychiatry 2004, 37 (Suppl. 3), S171–S182.
- Mysona, B.A.; Kansara, N.; Zhao, J.; Bollinger, K. The role of sigma 1 receptor as a neuroprotective target in glaucoma. Adv. Exp. Med. Biol. 2017, 964, 299–307.
- Ryskamp, D.A.; Korban, S.; Zhemkov, V.; Kraskovskaya, N.; Bezprozvanny, I. Neuronal sigma-1 receptors: Signaling functions and protective roles in neurodegenerative diseases. Front. Neurosci. 2019, 13, 862.
- Wu, N.H.; Ye, Y.; Wan, B.B.; Yu, Y.D.; Liu, C.; Chen, Q.J. Emerging benefits: Pathophysiological functions and target drugs of the sigma-1 receptor in neurodegenerative diseases. Mol. Neurobiol. 2021, 58, 5649–5666.
- Couly, S.; Yasui, Y.; Su, T.P. SIGMAR1 confers innate resilience against neurodegeneration. Int. J. Mol. Sci. 2023, 24, 7767.
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426.
- Ganapathy, V.; Thangaraju, M.; Prasad, P.D. Nutrient transporters in cancer: Relevance to Warburg hypothesis and beyond. Pharmacol. Ther. 2009, 121, 29–40.
- Lewerenz, J.; Hewett, S.J.; Huang, Y.; Lambros, M.; Gout, P.W.; Kalivas, P.W.; Massie, A.; Smolders, I.; Methner, A.; Pergande, M.; et al. The cystine/glutamate antiporter system xc− in health and disease: From molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox. Signal. 2013, 18, 522–555.
- Bhutia, Y.D.; Babu, E.; Ramachandran, S.; Ganapathy, V. Amino acid transporters in cancer and their relevance to “glutamine addiction”: Novel targets for the design of a new class of anticancer drugs. Cancer Res. 2015, 75, 1782–1788.
- Wang, J.; Zhao, J.; Cui, X.; Mysona, B.A.; Navneet, S.; Saul, A.; Ahuja, M.; Lambert, N.; Gazaryan, I.G.; Thomas, B.; et al. The molecular chaperone sigma 1 receptor mediates rescue of retinal cone photoreceptor cells via modulation of NRF2. Free Radic. Biol. Med. 2019, 134, 604–616.
- Barwick, S.R.; Siddiq, M.S.; Wang, J.; Xiao, H.; Marshall, B.; Perry, E.; Smith, S.B. Sigma 1 receptor co-localizes with NRF2 in retinal photoreceptor cells. Antioxidants 2021, 10, 981.
- Wang, J.; Shanmugam, A.; Markand, S.; Zorrilla, E.; Ganapathy, V.; Smith, S.B. Sigma 1 receptor regulates the oxidative stress response in primary retinal Muller glial cells via NRF2 signaling and system xc−, the Na+-independent glutamate-cystine exchanger. Free Radic. Biol. Med. 2015, 86, 25–36.
- Bai, T.; Lei, P.; Zhou, H.; Liang, R.; Zhu, R.; Wang, W.; Zhou, L.; Sun, Y. Sigma-1 receptor protects against ferroptosis in hepatocellular carcinoma cells. J. Cell. Mol. Med. 2019, 23, 7349–7359.
- Bai, T.; Wang, S.; Zhao, Y.; Zhu, R.; Wang, W.; Sun, Y. Haloperidol, a sigma receptor 1 antagonist, promotes ferroptosis in hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2017, 491, 919–925.
- Babitt, J.L.; Lin, H.Y. The molecular pathogenesis of hereditary hemochromatosis. Semin. Liver Dis. 2011, 31, 280–292.
- Adams, P.C.; Jeffrey, G.; Ryan, J. Haemochromatosis. Lancet 2023, 401, 1811–1821.
- Merryweather-Clarke, A.T.; Pointon, J.J.; Jouanolle, A.M.; Rochette, J.; Robson, K.J.H. Geography of HFE C282Y and H63D mutations. Genet. Test. 2000, 4, 183–198.
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355.
- Rodriguez, R.; Schreiber, S.L.; Conrad, M. Persister cancer cells: Iron addiction and vulnerability to ferroptosis. Mol. Cell 2022, 82, 728–740.
- Gnanaprakasam, J.P.; Thangaraju, M.; Liu, K.; Ha, Y.; Martin, P.M.; Smith, S.B.; Ganapathy, V. Absence of iron-regulatory protein Hfe results in hyperproliferation of retinal pigment epithelium. Role of cystine-glutamate exchanger. Biochem. J. 2009, 424, 243–252.
- Bhutia, Y.D.; Ogura, J.; Grippo, P.J.; Torres, C.; Sato, T.; Wachtel, M.; Ramachandran, S.; Babu, E.; Sivaprakasam, S.; Rajasekaran, D.; et al. Chronic exposure to excess iron promotes EMT and cancer via p53 loss in pancreatic cancer. Asian J. Pharm. Sci. 2020, 15, 237–251.
- Kim, F.J.; Maher, C.M. Sigma1 pharmacology in the context of cancer. Handb. Exp. Pharmacol. 2017, 244, 237–308.
- Robinson, T.S.; Osman, M.A. An emerging role for sigma receptor 1 in personalized treatment of breast cancer. Cancers 2023, 15, 3464.
- Ahmed, I.S.A.; Chamberlain, C.; Craven, R.J. S2R(PGRMC1): The cytochrome-related sigma-2 receptor that regulates lipid and drug metabolism and hormone signaling. Expert Opin. Drug Metab. Toxicol. 2012, 8, 361–370.
- Kabe, Y.; Nakane, T.; Koike, I.; Yamamoto, T.; Sugiura, Y.; Harada, E.; Sugase, K.; Shimamura, T.; Ohmura, M.; Muraoka, K.; et al. Haem-dependent dimerization of PGRMC1/sigma-2 receptor facilitates cancer proliferation and chemoresistance. Nat. Commun. 2016, 7, 11030.
- Xu, J.; Zeng, C.; Chu, W.; Pan, F.; Rothfuss, J.M.; Zhang, F.; Tu, Z.; Zhou, D.; Zeng, D.; Vangveravong, S.; et al. Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nat. Commun. 2011, 2, 380.
- Alon, A.; Schmidt, H.; Wood, M.D.; Sahn, J.J.; Martin, S.F.; Kruse, A.C. Identification of the gene that codes for the σ2 receptor. Proc. Natl. Acad. Sci. USA 2017, 114, 7160–7165.
- Porollo, A.A.; Adamczak, R.; Meller, J. POLYVIEW: A flexible visualization tool for structural and functional annotations of proteins. Bioinformatics 2004, 20, 2460–2462.
- Alon, A.; Lyu, J.; Braz, J.M.; Tummino, T.A.; Craik, V.; O’Meara, M.J.; Webb, C.M.; Radchenko, D.S.; Moroz, Y.S.; Huang, X.P.; et al. Structures of the σ2 receptor enable docking for bioactive ligand discovery. Nature 2021, 600, 759–764.
- Lomize, M.A.; Pogozheva, I.D.; Joo, H.; Mosberg, H.I.; Lomize, A.L. OPM database and PPM web server: Resources for positioning of proteins in membranes. Nucleic Acids Res. 2012, 40, D370–D376.
- Izzo, N.J.; Colom-Cadena, M.; Riad, A.A.; Xu, J.; Singh, M.; Abate, C.; Cahill, M.A.; Spires-Jones, T.L.; Bowen, W.D.; Mach, R.H.; et al. Proceedings from the fourth international symposium on σ-2 receptors: Role in health and disease. eNeuro 2020, 7, ENEURO.0317-20.2020.
- Guo, L.; Zhen, X. Sigma-2 receptor ligands: Neurobiological effects. Curr. Med.Chem. 2015, 22, 989–1003.
- Cheng, Y.S.; Zhang, T.; Ma, X.; Pratuangtham, S.; Zhang, G.C.; Ondrus, A.A.; Mafi, A.; Lomenick, B.; Jones, J.J.; Ondrus, A.E. A proteome-wide map of 20(S)hydroxycholesterol interactors in cell membranes. Nat. Chem. Biol. 2021, 17, 1271–1280.
- Shen, H.; Li, J.; Xie, X.; Yang, H.; Zhang, M.; Wang, B.; Kent, K.C.; Plutzky, J.; Guo, L.W. BRD2 regulation of sigma-2 receptor upon cholesterol deprivation. Life Sci. Alliance 2020, 4, e201900540.
- Kabe, Y.; Koike, I.; Yamamoto, T.; Hirai, M.; Kanai, A.; Furuhata, R.; Tsugawa, H.; Harada, E.; Sugase, K.; Hanadate, K.; et al. Glycyrrhizin derivatives suppress cancer chemoresistance by inhibiting progesterone receptor membrane component 1. Cancers 2021, 13, 3265.
- Kimura, I.; Nakayama, Y.; Konishi, M.; Terasawa, K.; Ohta, M.; Itoh, N.; Fujimoto, M. Functions of MAPR (membrane-associated progesterone receptor) family members as heme/steroid-binding proteins. Curr. Protein Pept. Sci. 2012, 13, 687–696.
- Hehenberger, E.; Eitel, M.; Fortunato, S.A.V.; Miller, D.J.; Keeling, P.J.; Cahill, M.A. Early eukaryotic origins and metazoan elaboration of MAPR family proteins. Mol. Phylogenet. Evol. 2020, 148, 106814.




