
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | SAHAR ALTEGANI EBRAHIM NASER | -- | 4830 | 2023-10-18 16:40:02 | | | |
| 2 | Lindsay Dong | -1 word(s) | 4829 | 2023-10-20 03:03:26 | | |
Video Upload Options
Nanotechnology is one of the most active research fields in materials science. Metal-organic frameworks (MOFs) have the benefits of having a sizable specific surface area, extremely high porosity, changeable pore size, post-synthesis modification, and extreme thermal stability. Graphene oxide (GO) has attracted significant research interest due to its similar surface area to MOFs. Furthermore, oxygen-containing groups presented in graphene oxide offer the unique processing and handling advantages of amphiphilicity and dispersion in water. MOF-based GO has attracted attention due to its resemblance to metal ions and organic binding linkers. It has sparked great interest in the past few years due to its distinct characteristics and higher performance compared to MOFs or GO alone.
1. Introduction
2. The Properties of Metal-Organic Frameworks/Graphene Oxide (MOFs/GO)
3. Synthesis Methods of Metal-Organic Frameworks/Graphene Oxide (MOFs/GO)
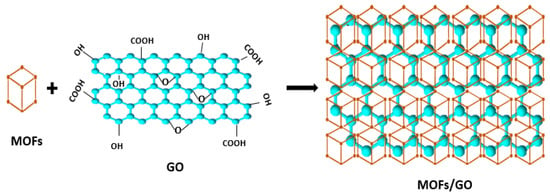
3.1. Post-Synthesis Method
3.2. Hydrothermal and Solvothermal Methods
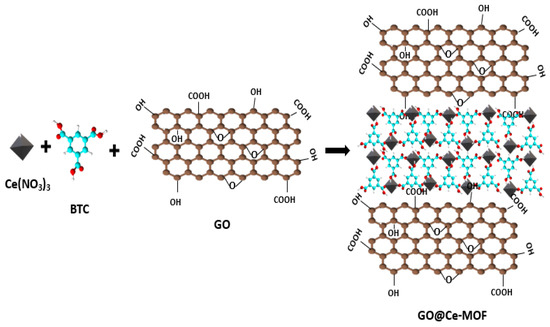
3.3. In Situ Method
3.4. Co-Precipitation Method
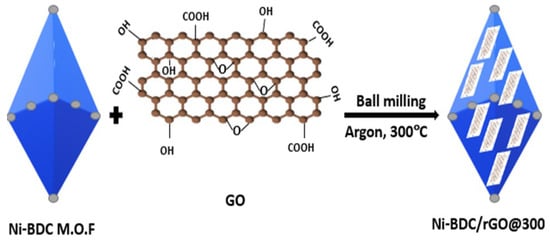
3.5. Mixing Method
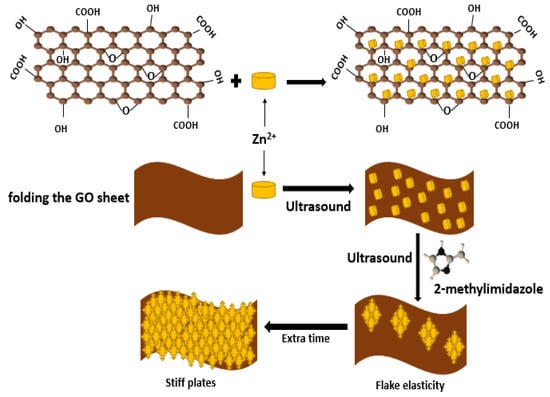
3.6. Ultrasonication Method
4. Applications of Metal-Organic Frameworks/Graphene Oxide (MOFs/GO)
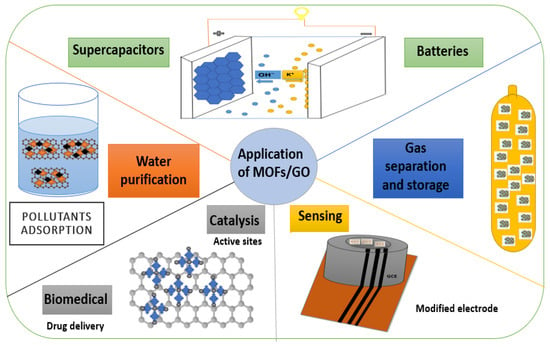
4.1. Supercapacitors
4.2. Gas Separation and Storage
4.3. Water Purification
4.4. Sensors
4.5. Catalysis
4.5.1. Electrocatalysts
4.5.2. Photocatalysts
4.6. Batteries
4.7. Other Applications (Biomedical)
5. The Advantages and Challenges of Metal-Organic Frameworks/Graphene Oxide (MOFs/GO)
References
- Gautam, S.; Agrawal, H.; Thakur, M.; Akbari, A.; Sharda, H.; Kaur, R.; Amini, M. Metal oxides and metal organic frameworks for the photocatalytic degradation: A review. J. Environ. Chem. Eng. 2020, 8, 103726.
- Čitaković, N.M. Physical properties of nanomaterials. Vojnoteh. Glas. Mil. Tech. Cour. 2019, 67, 159–171.
- Zakrzewski, W.; Dobrzyński, M.; Zawadzka-Knefel, A.; Lubojański, A.; Dobrzyński, W.; Janecki, M.; Kurek, K.; Szymonowicz, M.; Wiglusz, R.J.; Rybak, Z. Nanomaterials application in endodontics. Materials 2021, 14, 5296.
- Zhao, D.; Zhang, W.; Wu, Z.H.; Xu, H. Nanoscale metal− organic frameworks and their nanomedicine applications. Front. Chem. 2022, 9, 1243.
- Sajid, M. Nanomaterials: Types, properties, recent advances, and toxicity concerns. Curr. Opin. Environ. Sci. Health 2022, 25, 100319.
- Naghdi, S.; Shahrestani, M.M.; Zendehbad, M.; Djahaniani, H.; Kazemian, H.; Eder, D. Recent advances in application of metal-organic frameworks (MOFs) as adsorbent and catalyst in removal of persistent organic pollutants (POPs). J. Hazard. Mater. 2023, 442, 130127.
- Qu, H.J.; Huang, L.J.; Han, Z.Y.; Wang, Y.X.; Zhang, Z.J.; Wang, Y.; Chang, Q.R.; Wei, N.; Kipper, M.J.; Tang, J.G. A review of graphene-oxide/metal–organic framework composites materials: Characteristics, preparation and applications. J. Porous Mater. 2021, 28, 1837–1865.
- Hong, J.; Park, S.J.; Kim, S. Synthesis and electrochemical characterization of nanostructured Ni-Co-MOF/graphene oxide composites as capacitor electrodes. Electrochim. Acta 2019, 311, 62–71.
- Kumar, G.; Masram, D.T. Sustainable synthesis of MOF-5@ GO nanocomposites for efficient removal of rhodamine B from water. ACS Omega 2021, 6, 9587–9599.
- Heu, R.; Ateia, M.; Yoshimura, C. Photocatalytic nanofiltration membrane using Zr-MOF/GO nanocomposite with high-flux and anti-fouling properties. Catalysts 2020, 10, 711.
- Muthukumaraswamy Rangaraj, V.; Wahab, M.A.; Reddy, K.S.; Kakosimos, G.; Abdalla, O.; Favvas, E.P.; Reinalda, D.; Geuzebroek, F.; Abdala, A.; Karanikolos, G.N. Metal organic framework—based mixed matrix membranes for carbon dioxide separation: Recent advances and future directions. Front. Chem. 2020, 8, 534.
- Haeri, Z.; Ramezanzadeh, B.; Ramezanzadeh, M. Recent progress on the metal-organic frameworks decorated graphene oxide (MOFs-GO) nano-building application for epoxy coating mechanical-thermal/flame-retardant and anti-corrosion features improvement. Prog. Org. Coat. 2022, 163, 106645.
- Karamipour, M.; Fathi, S.; Safari, M. Removal of phenol from aqueous solution using MOF/GO: Synthesis, characteristic, adsorption performance and mechanism. Int. J. Environ. Anal. Chem. 2021, 6, 1–2.
- Mokhtar, N.A.; Zawawi, R.M.; Khairul, W.M.; Yusof, N.A. Electrochemical and optical sensors made of composites of metal–organic frameworks and carbon-based materials. A review. Environ. Chem. Lett. 2022, 20, 3099–3131.
- Nandihalli, N.; Gregory, D.H.; Mori, T. Energy-Saving Pathways for Thermoelectric Nanomaterial Synthesis: Hydrother-mal/Solvothermal, Microwave-Assisted, Solution-Based, and Powder Processing. Adv. Sci. 2022, 9, 2106052.
- Ahmed Malik, W.M.; Afaq, S.; Mahmood, A.; Niu, L.; Yousaf ur Rehman, M.; Ibrahim, M.; Mohyuddin, A.; Qureshi, A.M.; Ashiq, M.N.; Chughtai, A.H. A facile synthesis of CeO2 from the GO@ Ce-MOF precursor and its efficient performance in the oxygen evolution reaction. Front. Chem. 2022, 10, 996560.
- Fallatah, A.M.; Shah, H.U.; Ahmad, K.; Ashfaq, M.; Rauf, A.; Muneer, M.; Ibrahim, M.M.; El-Bahy, Z.M.; Shahzad, A.; Babras, A. Rational synthesis and characterization of highly water stable MOF@ GO composite for efficient removal of mercury (Hg2+) from water. Heliyon 2022, 8, e10936.
- Liu, K.G.; Sharifzadeh, Z.; Rouhani, F.; Ghorbanloo, M.; Morsali, A. Metal-organic framework composites as green/sustainable catalysts. Coord. Chem. Rev. 2021, 436, 213827.
- Li, H.K.; Ye, H.L.; Zhao, X.X.; Sun, X.L.; Zhu, Q.Q.; Han, Z.Y.; Yuan, R.; He, H. Artful union of a zirconium-porphyrin MOF/GO composite for fabricating an aptamer-based electrochemical sensor with superb detecting performance. Chin. Chem. Lett. 2021, 32, 2851–2855.
- Zaman, N.; Iqbal, N.; Noor, T. Advances and challenges of MOF derived carbon-based electrocatalysts and photocatalyst for water splitting: A review. Arab. J. Chem. 2022, 14, 103906.
- Xu, W.; Wang, X.; Wu, Y.; Li, W.; Chen, C. Functionalized graphene with Co-ZIF adsorbed borate ions as an effective flame retardant and smoke suppression agent for epoxy resin. J. Hazard. Mater. 2019, 363, 138–151.
- Wang, K.; Hui, K.N.; San Hui, K.; Peng, S.; Xu, Y. Recent progress in metal–organic framework/graphene-derived materials for energy storage and conversion: Design, preparation, and application. Chem. Sci. 2021, 12, 5737–5766.
- Haroon, H.; Wahid, M.; Majid, K. Structure-Activity Relationships of a Ni-MOF, a Ni-MOF-rGO, and pyrolyzed Ni/C@rGO Structures for Sodium-ion Batteries. ChemistrySelect 2022, 7, e202202011.
- Arul, P.; Gowthaman, N.S.; John, S.A.; Lim, H.N. Ultrasonic assisted synthesis of size-controlled Cu-metal–organic framework decorated graphene oxide composite: Sustainable electrocatalyst for the trace-level determination of nitrite in environmental water samples. ACS Omega 2020, 5, 14242–14253.
- Kasula, M.; Le, T.; Thomsen, A.; Esfahani, M.R. Silver metal organic frameworks and copper metal organic frameworks immobilized on graphene oxide for enhanced adsorption in water treatment. Chem. Eng. J. 2022, 439, 135542.
- Cui, H.; Cui, S.; Tian, Q.; Zhang, S.; Wang, M.; Zhang, P.; Liu, Y.; Zhang, J.; Li, X. Electrochemical Sensor for the Detection of 1-Hydroxypyrene Based on Composites of PAMAM-Regulated Chromium-Centered Metal–Organic Framework Nanoparticles and Graphene Oxide. ACS Omega 2021, 6, 31184–31195.
- Yang, K.; Dai, Y.; Zheng, W.; Ruan, X.; Li, H.; He, G. ZIFs-modified GO plates for enhanced CO2 separation performance of ethyl cellulose based mixed matrix membranesf. Sep. Purif. Technol. 2019, 214, 87–94.
- Zhu, L.; Meng, L.; Shi, J.; Li, J.; Zhang, X.; Feng, M. Metal-organic frameworks/carbon-based materials for environmental remediation: A state-of-the-art mini-review. J. Environ. Manag. 2019, 232, 964–977.
- Yang, W.; Li, X.; Li, Y.; Zhu, R.; Pang, H. Applications of metal–organic-framework-derived carbon materials. Adv. Mater. 2019, 31, 1804740.
- Wang, D.G.; Liang, Z.; Gao, S.; Qu, C.; Zou, R. Metal-organic framework-based materials for hybrid supercapacitor application. Coord. Chem. Rev. 2020, 404, 213093.
- Huang, S.; Shi, X.R.; Sun, C.; Duan, Z.; Ma, P.; Xu, S. The application of metal–organic frameworks and their derivatives for supercapacitors. Nanomaterials 2020, 10, 2268.
- Li, S.; Shi, C.; Pan, Y.; Wang, Y. 2D/2D NiCo-MOFs/GO hybrid nanosheets for high-performance asymmetrical supercapacitor. Diam. Relat. Mater. 2021, 115, 108358.
- Chen, T.; Yang, A.; Zhang, W.; Nie, J.; Wang, T.; Gong, J.; Wang, Y.; Ji, Y. Architecting Nanostructured Co-BTC@ GO Composites for Supercapacitor Electrode Application. Nanomaterials 2022, 12, 3234.
- Roohollahi, H.; Zeinalzadeh, H.; Kazemian, H. Recent Advances in Adsorption and Separation of Methane and Carbon Dioxide Greenhouse Gases Using Metal–Organic Framework-Based Composites. Ind. Eng. Chem. Res. 2022, 61, 10555–10586.
- Ventura, K.; Arrieta, R.A.; Marcos-Hernández, M.; Jabbari, V.; Powell, C.D.; Turley, R.; Lounsbury, A.W.; Zimmerman, J.B.; Gardea-Torresdey, J.; Wong, M.S.; et al. Superparamagnetic MOF@ GO Ni and Co based hybrid nanocomposites as efficient water pollutant adsorbents. Sci. Total Environ. 2020, 738, 139213.
- Evans, H.A.; Mullangi, D.; Deng, Z.; Wang, Y.; Peh, S.B.; Wei, F.; Wang, J.; Brown, C.M.; Zhao, D.; Canepa, P.; et al. Aluminum formate, Al (HCOO)3: An earth-abundant, scalable, and highly selective material for CO2 capture. Sci. Adv. 2022, 8, eade1473.
- Zhao, Y.; Ge, H.; Miao, Y.; Chen, J.; Cai, W. CO2 capture ability of Cu-based metal-organic frameworks synergized with amino acid-functionalized layered materials. Catal. Today 2020, 356, 604–612.
- Hooriabad Saboor, F.; Nasirpour, N.; Shahsavari, S.; Kazemian, H. The effectiveness of MOFs for the removal of pharmaceuticals from aquatic environments: A review focused on antibiotics removal. Chem. Asian J. 2022, 17, e202101105.
- Chen, J.Q.; Sharifzadeh, Z.; Bigdeli, F.; Gholizadeh, S.; Li, Z.; Hu, M.L.; Morsali, A. MOF Composites as high Potential Materials for Hazardous Organic Contaminants Removal in Aqueous Environments. J. Environ. Chem. Eng. 2023, 11, 109469.
- Yang, F.; Du, M.; Yin, K.; Qiu, Z.; Zhao, J.; Liu, C.; Zhang, G.; Gao, Y.; Pang, H. Applications of Metal-Organic Frameworks in Water Treatment: A Review. Small 2022, 18, 2105715.
- Jun, B.M.; Al-Hamadani, Y.A.; Son, A.; Park, C.M.; Jang, M.; Jang, A.; Kim, N.C.; Yoon, Y. Applications of metal-organic framework based membranes in water purification: A review. Sep. Purif. Technol. 2020, 247, 116947.
- Jafarian, H.; Firouzjaei, M.D.; Aktij, S.A.; Aghaei, A.; Khomami, M.P.; Elliott, M.; Wujcik, E.K.; Sadrzadeh, M.; Rahimpour, A. Synthesis of heterogeneous metal organic Framework-Graphene oxide nanocomposite membranes for water treatment. Chem. Eng. J. 2023, 455, 140851.
- Venkadesh, A.; Mathiyarasu, J.; Radhakrishnan, S. Voltammetric Sensing of Caffeine in Food Sample Using Cu-MOF and Graphene. Electroanalysis 2021, 33, 1007–1013.
- Chang, Y.; Lou, J.; Yang, L.; Liu, M.; Xia, N.; Liu, L. Design and Application of Electrochemical Sensors with Metal–Organic Frameworks as the Electrode Materials or Signal Tags. Nanomaterials 2022, 12, 3248.
- Gao, J.; He, P.; Yang, T.; Wang, X.; Zhou, L.; He, Q.; Jia, L.; Deng, H.; Zhang, H.; Jia, B.; et al. Short rod-like Ni-MOF anchored on graphene oxide nanosheets: A promising voltammetric platform for highly sensitive determination of p-chloronitrobenzene. J. Electroanal. Chem. 2020, 861, 113954.
- Zhou, M.; Liu, M.; Jiang, H.; Chen, R. Controllable synthesis of Pd-ZIF-L-GO: The role of drying temperature. Ind. Eng. Chem. Res. 2021, 60, 4847–4859.
- Miceli, M.; Frontera, P.; Macario, A.; Malara, A. Recovery/reuse of heterogeneous supported spent catalysts. Catalysts 2021, 11, 591.
- Andrade, M.A.; Martins, L.M. Sustainability in catalytic cyclohexane oxidation: The contribution of porous support materials. Catalysts 2019, 10, 2.
- Alamgholiloo, H.; Rostamnia, S.; Zhang, K.; Lee, T.H.; Lee, Y.S.; Varma, R.S.; Jang, H.W.; Shokouhimehr, M. Boosting aerobic oxidation of alcohols via synergistic effect between TEMPO and a composite Fe3O4/Cu-BDC/GO nanocatalyst. ACS Omega 2020, 5, 5182–5191.
- Su, C.; Wang, B.; Li, S.; Wie, Y.; Wang, Q.; Li, D. Fabrication of Pd@ ZnNi-MOF/GO Nanocomposite and Its Application for H2O2 Detection and Catalytic Degradation of Methylene Blue Dyes. ChemistrySelect 2021, 6, 8480–8489.
- Chen, Y.; Huang, N.; Liang, Y. Preparation of CeO2/Cu-MOF/GO composite for efficient electrocatalytic oxygen evolution reaction. Ionics 2021, 27, 4347–4360.
- Tang, B.; Wang, S.; Li, R.; Gou, X.; Long, J. Urea treated metal organic frameworks-graphene oxide composites derived N-doped Co-based materials as efficient catalyst for enhanced oxygen reduction. J. Power Sources 2019, 425, 76–86.
- Radwan, A.; Jin, H.; He, D.; Mu, S. Design engineering, synthesis protocols, and energy applications of MOF-derived electrocatalysts. Nano-Micro Lett. 2021, 13, 1–32.
- Gopi, S.; Panda, A.; Ramu, A.G.; Theerthagiri, J.; Kim, H.; Yun, K. Bifunctional electrocatalysts for water splitting from a bimetallic (V doped-NixFey) Metal–Organic framework MOF@ Graphene oxide composite. Int. J. Hydrogen Energy 2022, 47, 42122–42135.
- Bagherzadeh, S.B.; Kazemeini, M.; Mahmoodi, N.M. Preparation of novel and highly active magnetic ternary structures (metal-organic framework/cobalt ferrite/graphene oxide) for effective visible-light-driven photocatalytic and photo-Fenton-like degradation of organic contaminants. J. Colloid Interface Sci. 2021, 602, 73–94.
- Qian, Y.; Zhang, F.; Pang, H. A review of MOFs and their composites-based photocatalysts: Synthesis and applications. Adv. Funct. Mater. 2021, 31, 2104231.
- Jin, J.; Kim, J.P.; Wan, S.; Kim, K.H.; Choi, Y.; Li, P.; Kang, J.; Ma, Z.; Lee, J.H.; Kwon, O.; et al. Hierarchical pore enhanced adsorption and photocatalytic performance of graphene oxide/Ti-based metal-organic framework hybrid for toluene removal. Appl. Catal. B Environ. 2022, 317, 121751.
- Heu, R.; Ateia, M.; Awfa, D.; Punyapalakul, P.; Yoshimura, C. Photocatalytic degradation of organic micropollutants in water by Zr-MOF/GO composites. J. Compos. Sci. 2020, 4, 54.
- Xiao, X.; Zou, L.; Pang, H.; Xu, Q. Synthesis of micro/nanoscaled metal–organic frameworks and their direct electrochemical applications. Chem. Soc. Rev. 2020, 49, 301–331.
- Teffu, D.M.; Ramoroka, M.E.; Makhafola, M.D.; Makgopa, K.; Maponya, T.C.; Seerane, O.A.; Hato, M.J.; Iwuoha, E.I.; Modibane, K.D. High-performance supercabattery based on reduced graphene oxide/metal organic framework nanocomposite decorated with palladium nanoparticles. Electrochim. Acta 2022, 412, 140136.
- Zhang, L.; Liu, W.; Shi, W.; Xu, X.; Mao, J.; Li, P.; Ye, C.; Yin, R.; Ye, S.; Liu, X.; et al. Boosting lithium storage properties of MOF derivatives through a wet-spinning assembled fiber strategy. Chem. A Eur. J. 2018, 24, 13792–13799.
- Li, Z.; Ma, Q.; Zhang, H.; Zhang, Q.; Zhang, K.; Mei, H.; Xu, B.; Sun, D. Self-Assembly of Metal–Organic Frameworks on Graphene Oxide Nanosheets and In Situ Conversion into a Nickel Hydroxide/Graphene Oxide Battery-Type Electrode. Inorg. Chem. 2022, 61, 12129–12137.
- Liu, J.; Wu, D.; Zhu, N.; Wu, Y.; Li, G. Antibacterial mechanisms and applications of metal-organic frameworks and their derived nanomaterials. Trends Food Sci. Technol. 2021, 109, 413–434.
- Ge, X.; Wong, R.; Anisa, A.; Ma, S. Recent development of metal-organic framework nanocomposites for biomedical applications. Biomaterials 2022, 281, 121322.
- Sontakke, A.D.; Tiwari, S.; Purkait, M.K. A comprehensive review on graphene oxide-based nanocarriers: Synthesis, functionalization and biomedical applications. FlatChem 2023, 38, 100484.
- Asl, E.A.; Pooresmaeil, M.; Namazi, H. Chitosan coated MOF/GO nanohybrid as a co-anticancer drug delivery vehicle: Synthesis, characterization, and drug delivery application. Mater. Chem. Phys. 2023, 293, 126933.
- Pooresmaeil, M.; Asl, E.A.; Namazi, H. A new pH-sensitive CS/Zn-MOF@ GO ternary hybrid compound as a biofriendly and implantable platform for prolonged 5-Fluorouracil delivery to human breast cancer cells. J. Alloys Compd. 2021, 885, 160992.
- Zhang, X.; Zhang, S.; Tang, Y.; Huang, X.; Pang, H. Recent advances and challenges of metal–organic framework/graphene-based composites. Compos. Part B Eng. 2022, 230, 109532.
- Wang, S.; Ye, B.; An, C.; Wang, J.; Li, Q.; Guo, H.; Zhang, J. Exploring the coordination effect of GO@ MOF-5 as catalyst on thermal decomposition of ammonium perchlorate. Nanoscale Res. Lett. 2019, 14, 1–11.
- Wang, D.; Zhao, Y. Single-atom engineering of metal-organic frameworks toward healthcare. Chem 2021, 7, 2635–2671.
- Li, C.; Ji, Y.; Wang, Y.; Liu, C.; Chen, Z.; Tang, J.; Hong, Y.; Li, X.; Zheng, T.; Jiang, Q.; et al. Applications of Metal–Organic Frameworks and Their Derivatives in Electrochemical CO2 Reduction. Nano-Micro Lett. 2023, 15, 113.
- Zadehahmadi, F.; Eden, N.T.; Mahdavi, H.; Konstas, K.; Mardel, J.I.; Shaibani, M.; Banerjee, P.C.; Hill, M.R. Removal of metals from water using MOF-based composite adsorbents. Environ. Sci. Water Res. Technol. 2023, 9, 1305–1330.
- Mazlan, N.A.; Butt, F.S.; Lewis, A.; Yang, Y.; Yang, S.; Huang, Y. The Growth of Metal–Organic Frameworks in the Presence of Graphene Oxide: A Mini Review. Membranes 2022, 12, 501.




