Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yong Teng | -- | 2619 | 2023-10-18 14:05:18 | | | |
| 2 | Lindsay Dong | + 3 word(s) | 2622 | 2023-10-20 02:12:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Teng, Y.; Gao, L.; Mäkitie, A.A.; Florek, E.; Czarnywojtek, A.; Saba, N.F.; Ferlito, A. Iron, Ferroptosis, and Head and Neck Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/50466 (accessed on 07 February 2026).
Teng Y, Gao L, Mäkitie AA, Florek E, Czarnywojtek A, Saba NF, et al. Iron, Ferroptosis, and Head and Neck Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/50466. Accessed February 07, 2026.
Teng, Yong, Lixia Gao, Antti A. Mäkitie, Ewa Florek, Agata Czarnywojtek, Nabil F. Saba, Alfio Ferlito. "Iron, Ferroptosis, and Head and Neck Cancer" Encyclopedia, https://encyclopedia.pub/entry/50466 (accessed February 07, 2026).
Teng, Y., Gao, L., Mäkitie, A.A., Florek, E., Czarnywojtek, A., Saba, N.F., & Ferlito, A. (2023, October 18). Iron, Ferroptosis, and Head and Neck Cancer. In Encyclopedia. https://encyclopedia.pub/entry/50466
Teng, Yong, et al. "Iron, Ferroptosis, and Head and Neck Cancer." Encyclopedia. Web. 18 October, 2023.
Copy Citation
Ferroptosis is an iron-dependent regulatory form of cell death characterized by the accumulation of intracellular reactive oxygen species and lipid peroxidation. It plays a critical role not only in promoting drug resistance in tumors, but also in shaping therapeutic approaches for various malignancies.
ferroptosis
head and neck cancer
iron
cell death
antitumor strategy
1. Introduction
Iron is an essential trace element in mammals, which plays an important role in DNA synthesis, electron transport, oxygen transport, and other metabolic processes [1][2]. Under normal conditions, cell iron metabolism is in a dynamic balance of constant absorption, utilization, storage, and circulation. This process is called iron balance and plays an important role in maintaining the normal physiological function of cells. However, when the iron balance in cells is disrupted, it can lead to intracellular iron overload. Excess iron produces reactive oxygen species (ROS) through the Fenton reaction [3], which can induce ferroptosis, which is different from other forms of cell death, such as apoptosis, paraptosis, and necrosis (Figure 1) [4]. Reactive oxygen species (ROS) is mainly produced by iron-dependent Fenton reactions, mitochondria, or enzymes from the nicotinamide adenine dinucleotide phosphate oxidase (NADPH) family [5]. Because iron is an essential element for all cell growth, the rapid proliferation of tumor cells is usually more dependent on iron than normal cells are. As a result, tumor cells are more sensitive to the damage associated with iron excess [6].
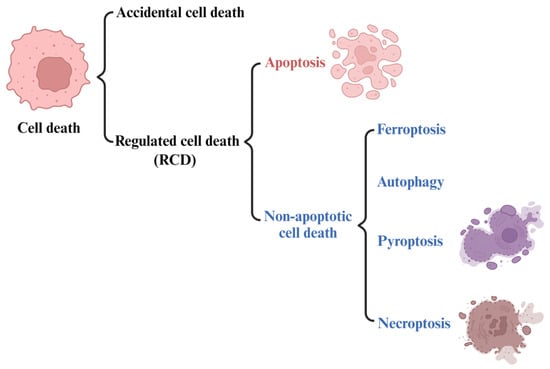
Figure 1. The types of cell death. Regulated non-apoptotic forms of cell death include ferroptosis, autophagy, pyroptosis, and necroptosis.
Ferroptosis is a newly defined form of iron-dependent cell death with excessive accumulation of lipid peroxidation [7][8]. Due to their potential for damaging biofilms and trigger a cascade of lipid peroxidation, excess ROS play a significant role in various forms of cell death, including ferroptosis and autophagy [9]. Some common tumor chemotherapy drugs, such as sorafenib, 5-FU, and paclitaxel, can cause cytotoxicity by triggering ROS accumulation and lipid peroxidation to kill tumor cells. Accumulation of iron ions has been demonstrated in various tumor cells [10][11][12]. Therefore, regulating ferroptosis through iron ion homeostasis provides a novel strategy for killing tumor cells [13].
2. Ferroptosis
2.1. The Development of the Concept of Ferroptosis
For a long time, cell death has been divided into three forms based on morphological characteristics: apoptosis, autophagy, and necrosis [14]. With the advancement in research, new types of cell death are emerging, and each type shows different characteristics in terms of molecular mechanisms and regulatory signals. Ferroptosis was originally discovered by targeting drugs against RAS mutations. RAS is the first identified and the most common human oncogene [15]. It is the most conserved family of oncogenes known to date and plays an important role in cell growth, proliferation, differentiation, regulation, and malignant transformation [16][17]. Ferroptosis was originally identified and named in cancer cell experiments with RAS mutations, and ferroptosis agonists are capable of killing such tumor cells in vitro [18]. Two small molecule compounds (erastin and RSL3) were initially found to have specific killing effects on cancer cells expressing oncogenic RAS compared with wild-type cells [19].
In 2018, the cell death committee made an updated recommendation on cell death from morphological, biochemical, and functional perspectives [20]. The types of cell death can be classified into accidental cell death (ACD) and regulated cell death (RCD) (Figure 1). RCD is a homeostasis mechanism in multicellular organisms, which is essential for maintaining the morphology and function of cells and can be regulated at the genetic and pharmacological levels [21]. The RCD category at least includes five high-profile forms, including apoptosis, pyroptosis, autophagy, necroptosis and ferroptosis (Figure 1). However, ferroptosis, as a newly identified cell death mechanism, is attracting increasing attention. The concept of ferroptosis was first proposed by Dr. Brent R Stock in 2012 [19][22][23] as a form of iron-dependent regulatory cell death that occurs through the accumulation of toxic lipid ROS and the consumption of polyunsaturated fatty acids, resulting in mechanical damage to cells. It also can be defined as being triggered by oxidative perturbation of the intracellular microenvironment, controlled by glutathione peroxidase 4 (GPX4), which can be inhibited by iron-chelating agents and lipophilic antioxidants [24].
2.2. Features and Components of Ferroptosis
Programmed cell death is an active and orderly cell death determined by genes and regulated by molecular mechanisms. It is closely related to the maintenance of homeostasis and the occurrence of diseases, and mainly includes pyroptosis, necrosis, and autophagy. As a newly discovered form of programmed cell death, ferroptosis is characterized by iron-dependent lipid peroxidation [25]. The typical morphological features of ferroptosis are reduced mitochondrial volume, increased double membrane density, reduced or disappeared mitochondrial ridge, broken mitochondrial outer membrane, normal nuclear size, and lack of chromatin aggregation. The biochemical characteristics of ferroptosis are different from those of apoptosis, autophagy, and necrosis.
Lipid peroxidation and ROS are two significant markers of ferroptosis. Lipid peroxidation, which often leads to lipid hydroperoxide formation, occurs in response to oxidative stress. Phospholipid peroxidation represents a critical stage within the ferroptosis process, which is closely associated with a variety of human diseases. In addition, the process of ferroptosis is accompanied by changes in related genes and proteins, such as ACSL4, TP53, GPX4, HSPB1, ACSL4, TFRC, ACSF2, and others [26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41].
2.3. The Mechanism and Regulation of Ferroptosis
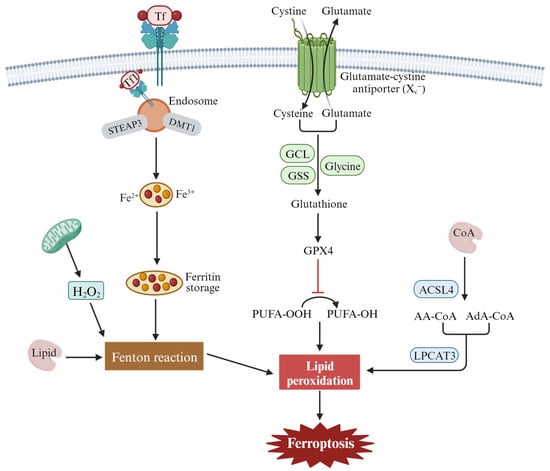
Ferroptosis can be divided into extrinsic and intrinsic pathways. The extrinsic pathway refers mainly to transporter-dependent pathways, while the intrinsic pathway refers to pathways regulated by various enzymes [26][42][43][44] (Figure 2). Extrinsic pathways are initiated by the inhibition of cell membrane transporters such as cystine/glutamate reverse transporters (System xc−) or by the activation of the iron transporters’ serum transferrin and lactotransferrin. System xc− exchanges intracellular glutamate for extracellular cystine (Cys2), which is catalyzed by glutamate-cysteine ligase (GCL) and glutathione synthetase (GSS) to synthesize glutathione (GSH). Inhibiting the activity of System xc- can inhibit the absorption of cystine, affect the synthesis of GSH, lead to a decrease in activity of the membrane lipid repair enzyme GPX4, reduce the antioxidant capacity of cells, and eventually induce ferroptosis of cells. In addition, increased iron accumulation through increased iron absorption, reduced iron storage, and limited iron outflow can also promote ferroptosis. Under pathological conditions, Fe2+ accumulates in the cell and produces a large number of ROS, which occurs in the Haber–Weiss and Fenton reactions. A series of peroxidation reactions occurs with the polyunsaturated fatty acid PUFA on the cell membrane to generate lipid peroxides, which destroy the cell membrane structure and cause ferroptosis in the cell. Transferrin (serum transferrin or lactoferrin, Tf) mediates iron uptake through the transferrin receptor (TFRC), and FTH1/FTL (ferritin components) increases iron levels through autophagy degradation, all of which can contribute to ferroptosis.

Figure 2. The main regulatory mechanisms of ferroptosis. These mechanisms include the inhibition of the cystine/glutamate antiporter system (system xc−), leading to depletion of intracellular glutathione and the accumulation of lipid peroxidation products due to the oxidation of polyunsaturated fatty acids (PUFAs) by reactive oxygen species (ROS) and the dysregulation of iron metabolism through transferrin receptor 1 (Tf1).
The intrinsic pathway of ferroptosis is activated by enzymes, mainly through lipid metabolism and other metabolism [44]. The central mechanism in ferroptosis is the iron-dependent lipid oxidation metabolism disorder, and PUFAs are the key substances of lipid peroxide accumulation in ferroptosis. Under normal circumstances, PUFAs are important substrates for lipid metabolism, containing diallyl hydrogen atoms, especially arachidonoyl (AA) and renal/supra-adenoid/adrenoyl (AdA), which easily react with ROS and cause lipid peroxidation. Long-chain fatty acyl-CoA synthetase 4 (ACSL4) catalyzes free AA or AdA to combine with coenzyme A (CoA) to form the derivative AA-CoA or ADA-CoA, which is then esterified into membrane phosphatidyl ethyl/alcohol/amine (PEs) by lysophosphatidyllecithin acyltransferase 3(LPCAT3). After oxidation through lipoxygenase (ALOXs) or cytochrome P450 oxidoreductase (POR), harmful lipid peroxidation products are formed, which induce cell ferroptosis.
Fatty acid synthesis mediated by acetyl-CoA carboxylase (ACAC) and fatty acid release mediated by lipophagy can induce the accumulation of free fatty acids in cells and promote ferroptosis [45][46]. p53 can induce ferroptosis by downregulating the expression of the system xc− component SLC7A11 and inhibiting cystine uptake. At the same time, p53 can inhibit the activity of dipeptidyl peptidase-4 (DPP4) and block erastin-induced ferroptosis [47]. Nrf2 is an important regulatory factor in the maintenance of intracellular REDOX homeostasis. Through the p62-Keap1-Nrf2 pathway, the expression of multiple genes involved in iron and ROS metabolism (NQO1, HO1, and FTH1) is upregulated, and cell ferroptosis is inhibited [48]. GPX4 reduces cytotoxic lipid peroxides (L-OOH) to the corresponding alcohols (L-OH), and inhibition of GPX4 activity leads to the accumulation of lipid peroxides in cell membranes. For example, RSL3, as an inducer of ferroptosis, can directly act on GPX4 and inhibit its activity, thus reducing the antioxidant capacity of cells and accumulating ROS, resulting in ferroptosis [19]. However, this research is now controversial, with newer studies finding that RSL3 and ML162 do not have the ability to inhibit the enzyme activity of the recombinant selenium protein GPX4. Surprisingly, another selenium protein, TXNRD1, can effectively inhibit GPX4 activity [49].
3. The Function of Ferroptosis in Head and Neck Squamous Cell Carcinoma (HNSCC)
3.1. Ferroptosis and Tumor Cell Death
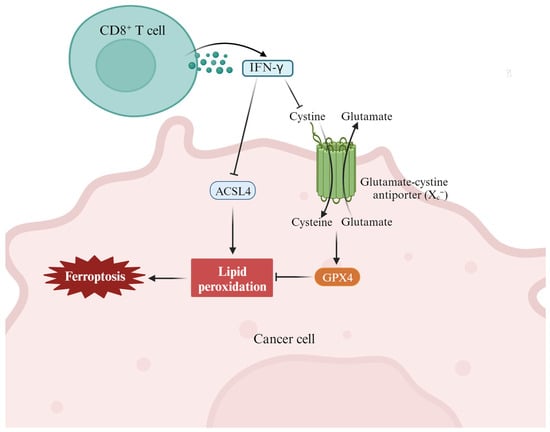
Interferon γ (IFN-γ) has been implicated in T helper type 1 (Th1) cell development through its ability to optimize interleukin 12 (IL-12) production from macrophages and IL-12 receptor expression on activated T cells. As a new mode of action for cytotoxic T-cell-mediated tumor killing, T-cell-derived IFN-γ stimulates ACSL4 and alters tumor cell lipid patterns by binding to arachidonic acid to induce ferroptosis in immunogenic tumor cells [50]. In a clinical context, the presence of tumor ACSL4 is associated with T cell markers and improved survival in cancer patients treated with immune checkpoint blockade (ICB) therapy. Therefore, the combination of IFN-γ signaling and specific fatty acids, such as arachidonic acid, represents an inherent mechanism for promoting ferroptosis within tumors and underscores the role of cytotoxic T cells in this process. Studies have revealed that erlotinib can promote the production of ROS in HNSCC cells and induce ferroptosis by inhibiting EGFR, thus killing tumor cells [51]. Dihydroartemisinin (DHA), a semi-synthetic derivative of artemisinin, has high antitumor biological activity. DHA was used to treat five HNSCC cell lines and two non-oncogenic normal epithelial cell lines. The results showed that DHA induces cell cycle arrest through Forkhead box protein M1 (FOXM1) and induces ferroptosis and apoptosis in HNSCC. Therefore, DHA may be promising for the treatment of HNSCC [36].
3.2. Ferroptosis and Tumor Metastasis
Tumor metastasis is the main factor in malignant tumor treatment failure. The importance of ferroptosis in tumor metastasis has attracted increasing attention. It was found that the ferroptosis driver SOCS1 and inhibitor FTH1 are closely related to the degree of macrophage infiltration in HNSCC, and both can be used as prognostic indicators for HNSCC, suggesting that ferroptosis plays an important role in the infiltration process of HNSCC [34]. The study showed that low expression of the DNA-damage-inducible transcript 4 (DDIT4) could significantly inhibit the invasion and migration of HNSCC cells, but overexpression of DDIT4 was negatively correlated with cell infiltration. It was suggested that DDIT4 plays a key role in HNSCC metastasis and may be a potential target for HNSCC treatment [52].
3.3. Ferroptosis and Antitumor Immunity
Immunotherapy is one of the most promising antitumor therapies and is achieved by activating the immune system to enhance its inherent cancer treatment ability [53]. In recent years, ferroptosis has been found to be closely related to tumor immunotherapy (Figure 3) [54][55][56]. With the development of immunology research, the combination of immunotherapy and ferroptosis will provide a new therapeutic strategy for HNSCC patients. Yang et al. found that ferroptotic stress can induce PD-L1 expression in HNSCC by regulating the NF-KB signaling pathway and calcium influx through ROS [28]. This study reveals a subgroup of ferroptotic HNSCC with immune-active signatures and indicates the potential for ferroptosis inducers to increase the HNSCC efficacy of immune checkpoint inhibitors. Zhang et al. showed that PKCβII senses initial lipid peroxides and activates ferroptosis-related lipid peroxidation through phosphorylation of ACSL4 [57].

Figure 3. The cross-talk between the immune system (such as CD8+ T cells and IFN-γ) and lipid metabolism (such as different fatty acids) in the context of tumor ferroptosis in the tumor microenvironment. In this case, T-cell-derived IFN-γ stimulates ACSL4 and alters tumor cell lipid patterns by binding to arachidonic acid to induce ferroptosis in immunogenic tumor cells [50].
3.4. Ferroptosis and Drug Resistance
Traditional cytotoxic drugs and targeted drugs generally slow or stop tumor growth by inducing cancer cell death, leaving normal cells unaffected. However, the emergence of drug resistance during chemotherapy or targeted therapy remains a largely insurmountable challenge [58]. Studies have shown that ferroptosis is associated with drug resistance in cancer treatments, and inducing ferroptosis has been shown to reverse drug resistance [59][60]. Now, increasing preclinical evidence suggests that inducing ferroptosis may be an effective treatment strategy to prevent the acquired resistance to certain tumor therapies, such as gefitinib, lapatinib, erlotinib, trametinib, sorafenib, and vemurafenib [61][62]. Often some resistant cancer cells exhibit epithelial-mesenchymal transition, making them more sensitive to ferroptosis. Therefore, enhancing the sensitivity of drug-resistant cells to drugs by regulating ferroptosis is of great significance for improving the effectiveness of chemotherapy or targeted drugs in tumor therapy. Epithelial membrane protein (EMP1) overexpression has been reported to enhance RSL3-induced iron sagging in HNSCC cells by promoting gefitinib resistance through targeting the MAPK pathway [29].
4. Targeting Ferroptosis in the Prevention and Intervention of HNSCC
4.1. Ferroptosis and Cancer Diagnosis and Prognosis
Early diagnosis and prognostic monitoring hold immense importance in cancer treatment and prognosis. Evaluation of the correlation between ferroptosis-related genes (FRGs) and the prognosis of HNSCC will provide an important reference for our ability to predict recurrence of HNSCC patients. Therefore, researchers conducted a series of studies on the influence of FRGs on HNSCC prognosis through different research methods. Bioinformatics methods were used to study the correlation between FRGs and HNSCC prognosis, and it was found that ferroptosis-related lncRNA has predictive value in the prognosis evaluation of HNSCC [63]. Sun’s group analyzed the association of 11 FRGs with prognosis through the TCGA database, constructed a prognostic risk model, and further selected receiver operating characteristic (ROC), nomogram analysis, Gene Expression Omnibus, univariate cox regression analysis, and Kaplan–Meier curves. Finally, five ferroptosis-related molecular markers were selected that may affect the treatment targets of ferroptosis, providing new directions for the treatment of HNSCC [64].
4.2. Ferroptosis and HNSCC Therapeutic Strategy
Ferroptosis has garnered growing interest as a potential therapeutic approach for malignant tumors. With the rapid development of nanotechnology and biomaterial technology, multifunctional nano-mediated ferroptosis combination therapy has shown great promise for clinical application in tumor diagnosis and treatment [65][66][67]. Recently, research has shown that ferroptosis shows great potential in overcoming multi-drug resistance in cancer therapy [68]. DHA and sodium nitroprusside (SNP) are among the ferroptosis inducers. By loading SNP and DHA with surface-immobilized FA onto imidazoline-zeolite frame (Zif-8) nanoparticles, scholars synthesized DHA/SNP@Zif-8-FA nanocomposite drugs that target Fr-overexpressing HNSCC cells via ferroptosis.
4.3. Ferroptosis Resistance in HNSCC
Malignant tumors pose a serious threat to human health. Despite various clinical antitumor treatments, patients still have poor prognosis, frequent relapse, and metastasis, which eventually lead to death. How to overcome tumor treatment resistance remains an urgent clinical problem. Recent studies have shown that the ferroptosis pathway is closely related to tumor therapeutic efficacy. Although studies on ferroptosis have shown that it provides a new strategy for tumor therapy, cancer cells can acquire resistance to ferroptosis through upregulation of antiferroptosis proteins or downregulation of pro-ferroptosis proteins, thereby affecting the positive role of ferroptosis in tumor therapy [69]. This study focused on the regulatory role of interleukin-6 (IL-6) in the resistance to ferroptosis in HNSCC. It was found that the expression level of IL-6 gradually increased during the development of HNSCC, and the upregulation of xCT expression was associated with a poor prognosis of HNSCC. IL-6 not only activates xCT expression through JAK2/STAT3 signaling pathway transcription, but also reverses ferroptosis and growth inhibition induced by xCT knockout or ferroptosis inducer erastin. Therefore, the induction of ferroptosis resistance by IL-6 plays a significant role in the development of HNSCC [30].
References
- Torti, S.V.; Torti, F.M. Iron: The cancer connection. Mol. Asp. Med. 2020, 75, 100860.
- Lelievre, P.; Sancey, L.; Coll, J.L.; Deniaud, A.; Busser, B. Iron Dysregulation in Human Cancer: Altered Metabolism, Biomarkers for Diagnosis, Prognosis, Monitoring and Rationale for Therapy. Cancers 2020, 12, 3524.
- You, C.; Gao, Z.; Wu, H.; Sun, K.; Ning, L.; Lin, F.; Sun, B.; Wang, F. Reactive oxygen species mediated theranostics using a Fenton reaction activable lipo-polymersome. J. Mater. Chem. B 2019, 7, 314–323.
- Tang, D.; Kroemer, G. Ferroptosis. Curr. Biol. 2020, 30, R1292–R1297.
- Crielaard, B.J.; Lammers, T.; Rivella, S. Targeting iron metabolism in drug discovery and delivery. Nat. Rev. Drug Discov. 2017, 16, 400–423.
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849.
- Lin, Z.; Yang, X.; Guan, L.; Qin, L.; Ding, J.; Zhou, L. The link between ferroptosis and airway inflammatory diseases: A novel target for treatment. Front. Mol. Biosci. 2022, 9, 985571.
- Wang, K.; Chen, X.Z.; Wang, Y.H.; Cheng, X.L.; Zhao, Y.; Zhou, L.Y.; Wang, K. Emerging roles of ferroptosis in cardiovascular diseases. Cell Death. Discov. 2022, 8, 394.
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843.
- De Bortoli, M.; Taverna, E.; Maffioli, E.; Casalini, P.; Crisafi, F.; Kumar, V.; Caccia, C.; Polli, D.; Tedeschi, G.; Bongarzone, I. Lipid accumulation in human breast cancer cells injured by iron depletors. J. Exp. Clin. Cancer. Res. 2018, 37, 75.
- Hino, K.; Yanatori, I.; Hara, Y.; Nishina, S. Iron and liver cancer: An inseparable connection. FEBS J. 2022, 289, 7810–7829.
- Wang, D.; Tang, L.; Zhang, Y.; Ge, G.; Jiang, X.; Mo, Y.; Wu, P.; Deng, X.; Li, L.; Zuo, S.; et al. Regulatory pathways and drugs associated with ferroptosis in tumors. Cell Death. Dis. 2022, 13, 544.
- Rishi, G.; Huang, G.; Subramaniam, V.N. Cancer: The role of iron and ferroptosis. Int. J. Biochem. Cell Biol. 2021, 141, 106094.
- Edinger, A.L.; Thompson, C.B. Death by design: Apoptosis, necrosis and autophagy. Curr. Opin. Cell Biol. 2004, 16, 663–669.
- Ray, L.B. How RAS mutations really work. Science 2020, 370, 927–928.
- Prior, I.A.; Hood, F.E.; Hartley, J.L. The Frequency of Ras Mutations in Cancer. Cancer Res. 2020, 80, 2969–2974.
- Murugan, A.K.; Grieco, M.; Tsuchida, N. RAS mutations in human cancers: Roles in precision medicine. Semin. Cancer Biol. 2019, 59, 23–35.
- Wang, J.; Millstein, J.; Arai, H.; Battaglin, F.; Kawanishi, N.; Jayachandran, P.; Soni, S.; Wu, Z.; Mancao, C.; Cremolini, C.; et al. The role of genetic variants involved with ferroptosis regulator genes in predicting outcomes in patients (pts) with RAS-mutant metastatic colorectal cancer (mCRC): Data from MAVERICC and TRIBE trials. J. Clin. Oncol. 2022, 40, 197.
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072.
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death. Differ. 2018, 25, 486–541.
- Loveless, R.; Bloomquist, R.; Teng, Y. Pyroptosis at the forefront of anticancer immunity. J. Exp. Clin. Cancer Res. 2021, 40, 264.
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176.
- Hadian, K.; Stockwell, B.R. SnapShot: Ferroptosis. Cell 2020, 181, 1188–1188.e1.
- Yan, H.F.; Zou, T.; Tuo, Q.Z.; Xu, S.; Li, H.; Belaidi, A.A.; Lei, P. Ferroptosis: Mechanisms and links with diseases. Signal Transduct. Target. Ther. 2021, 6, 49.
- Qu, M.; Zhang, H.; Chen, Z.; Sun, X.; Zhu, S.; Nan, K.; Chen, W.; Miao, C. The Role of Ferroptosis in Acute Respiratory Distress Syndrome. Front. Med. 2021, 8, 651552.
- Cao, J.Y.; Dixon, S.J. Mechanisms of ferroptosis. Cell Mol. Life Sci. 2016, 73, 2195–2209.
- Lee, J.; You, J.H.; Kim, M.S.; Roh, J.L. Epigenetic reprogramming of epithelial-mesenchymal transition promotes ferroptosis of head and neck cancer. Redox Biol. 2020, 37, 101697.
- Chung, C.H.; Lin, C.Y.; Chen, C.Y.; Hsueh, C.W.; Chang, Y.W.; Wang, C.C.; Chu, P.Y.; Tai, S.K.; Yang, M.H. Ferroptosis Signature Shapes the Immune Profiles to Enhance the Response to Immune Checkpoint Inhibitors in Head and Neck Cancer. Adv. Sci. 2023, 10, e2204514.
- Wang, Y.; Zhang, L.; Yao, C.; Ma, Y.; Liu, Y. Epithelial Membrane Protein 1 Promotes Sensitivity to RSL3-Induced Ferroptosis and Intensifies Gefitinib Resistance in Head and Neck Cancer. Oxid. Med. Cell. Longev. 2022, 2022, 4750671.
- Li, M.; Jin, S.; Zhang, Z.; Ma, H.; Yang, X. Interleukin-6 facilitates tumor progression by inducing ferroptosis resistance in head and neck squamous cell carcinoma. Cancer Lett. 2022, 527, 28–40.
- Jehl, A.; Conrad, O.; Burgy, M.; Foppolo, S.; Vauchelles, R.; Ronzani, C.; Etienne-Selloum, N.; Chenard, M.P.; Danic, A.; Dourlhes, T.; et al. Blocking EREG/GPX4 Sensitizes Head and Neck Cancer to Cetuximab through Ferroptosis Induction. Cells 2023, 12, 733.
- Lee, J.; You, J.H.; Shin, D.; Roh, J.L. Inhibition of Glutaredoxin 5 predisposes Cisplatin-resistant Head and Neck Cancer Cells to Ferroptosis. Theranostics 2020, 10, 7775–7786.
- Liu, S.; Yan, S.; Zhu, J.; Lu, R.; Kang, C.; Tang, K.; Zeng, J.; Ding, M.; Guo, Z.; Lai, X.; et al. Combination RSL3 Treatment Sensitizes Ferroptosis- and EGFR-Inhibition-Resistant HNSCCs to Cetuximab. Int. J. Mol. Sci. 2022, 23, 9014.
- Hu, Z.W.; Wen, Y.H.; Ma, R.Q.; Chen, L.; Zeng, X.L.; Wen, W.P.; Sun, W. Ferroptosis Driver SOCS1 and Suppressor FTH1 Independently Correlate With M1 and M2 Macrophage Infiltration in Head and Neck Squamous Cell Carcinoma. Front. Cell Dev. Biol. 2021, 9, 727762.
- Roh, J.L.; Kim, E.H.; Jang, H.; Shin, D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017, 11, 254–262.
- Lin, R.; Zhang, Z.; Chen, L.; Zhou, Y.; Zou, P.; Feng, C.; Wang, L.; Liang, G. Dihydroartemisinin (DHA) induces ferroptosis and causes cell cycle arrest in head and neck carcinoma cells. Cancer Lett. 2016, 381, 165–175.
- Li, J.; Xiao, W.; Wei, W.; Wu, M.; Xiong, K.; Lyu, J.; Li, Y. HSPA5, as a ferroptosis regulator, may serve as a potential therapeutic for head and neck squamous cell carcinoma. Mol. Immunol. 2023, 158, 79–90.
- Shin, D.; Kim, E.H.; Lee, J.; Roh, J.L. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic. Biol. Med. 2018, 129, 454–462.
- You, J.H.; Lee, J.; Roh, J.L. Mitochondrial pyruvate carrier 1 regulates ferroptosis in drug-tolerant persister head and neck cancer cells via epithelial-mesenchymal transition. Cancer Lett. 2021, 507, 40–54.
- Shin, D.; Lee, J.; You, J.H.; Kim, D.; Roh, J.L. Dihydrolipoamide dehydrogenase regulates cystine deprivation-induced ferroptosis in head and neck cancer. Redox Biol. 2020, 30, 101418.
- Liu, F.; Tang, L.; Li, Q.; Chen, L.; Pan, Y.; Yin, Z.; He, J.; Tian, J. Single-cell transcriptomics uncover the key ferroptosis regulators contribute to cancer progression in head and neck squamous cell carcinoma. Front. Mol. Biosci. 2022, 9, 962742.
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296.
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698.
- Wang, Y.; Wei, Z.; Pan, K.; Li, J.; Chen, Q. The function and mechanism of ferroptosis in cancer. Apoptosis 2020, 25, 786–798.
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975.
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98.
- Xie, Y.; Zhu, S.; Song, X.; Sun, X.; Fan, Y.; Liu, J.; Zhong, M.; Yuan, H.; Zhang, L.; Billiar, T.R.; et al. The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep. 2017, 20, 1692–1704.
- Li, X.; Chen, J.; Yuan, S.; Zhuang, X.; Qiao, T. Activation of the P62-Keap1-NRF2 Pathway Protects against Ferroptosis in Radiation-Induced Lung Injury. Oxid. Med. Cell. Longev. 2022, 2022, 8973509.
- Cheff, D.M.; Huang, C.; Scholzen, K.C.; Gencheva, R.; Ronzetti, M.H.; Cheng, Q.; Hall, M.D.; Arner, E.S.J. The ferroptosis inducing compounds RSL3 and ML162 are not direct inhibitors of GPX4 but of TXNRD1. Redox Biol. 2023, 62, 102703.
- Liao, P.; Wang, W.; Wang, W.; Kryczek, I.; Li, X.; Bian, Y.; Sell, A.; Wei, S.; Grove, S.; Johnson, J.K.; et al. CD8(+) T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 2022, 40, 365–378.e366.
- Orcutt, K.P.; Parsons, A.D.; Sibenaller, Z.A.; Scarbrough, P.M.; Zhu, Y.; Sobhakumari, A.; Wilke, W.W.; Kalen, A.L.; Goswami, P.; Miller, F.J., Jr.; et al. Erlotinib-mediated inhibition of EGFR signaling induces metabolic oxidative stress through NOX4. Cancer Res. 2011, 71, 3932–3940.
- Zhang, Z.; Zhu, H.; Zhao, C.; Liu, D.; Luo, J.; Ying, Y.; Zhong, Y. DDIT4 promotes malignancy of head and neck squamous cell carcinoma. Mol. Carcinog. 2023, 62, 332–347.
- Duan, X.; Chan, C.; Lin, W. Nanoparticle-Mediated Immunogenic Cell Death Enables and Potentiates Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2019, 58, 670–680.
- Zhang, F.; Li, F.; Lu, G.H.; Nie, W.; Zhang, L.; Lv, Y.; Bao, W.; Gao, X.; Wei, W.; Pu, K.; et al. Engineering Magnetosomes for Ferroptosis/Immunomodulation Synergism in Cancer. ACS Nano. 2019, 13, 5662–5673.
- Wang, W.; Green, M.; Choi, J.E.; Gijon, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019, 569, 270–274.
- Lang, X.; Green, M.D.; Wang, W.; Yu, J.; Choi, J.E.; Jiang, L.; Liao, P.; Zhou, J.; Zhang, Q.; Dow, A.; et al. Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov. 2019, 9, 1673–1685.
- Zhang, H.L.; Hu, B.X.; Li, Z.L.; Du, T.; Shan, J.L.; Ye, Z.P.; Peng, X.D.; Li, X.; Huang, Y.; Zhu, X.Y.; et al. PKCbetaII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat. Cell. Biol. 2022, 24, 88–98.
- Nie, Z.; Chen, M.; Gao, Y.; Huang, D.; Cao, H.; Peng, Y.; Guo, N.; Wang, F.; Zhang, S. Ferroptosis and Tumor Drug Resistance: Current Status and Major Challenges. Front. Pharmacol. 2022, 13, 879317.
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in cancer therapy: A novel approach to reversing drug resistance. Mol. Cancer 2022, 21, 47.
- Friedmann Angeli, J.P.; Krysko, D.V.; Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 2019, 19, 405–414.
- Song, X.; Wang, X.; Liu, Z.; Yu, Z. Role of GPX4-Mediated Ferroptosis in the Sensitivity of Triple Negative Breast Cancer Cells to Gefitinib. Front. Oncol. 2020, 10, 597434.
- Huang, W.; Chen, K.; Lu, Y.; Zhang, D.; Cheng, Y.; Li, L.; Huang, W.; He, G.; Liao, H.; Cai, L.; et al. ABCC5 facilitates the acquired resistance of sorafenib through the inhibition of SLC7A11-induced ferroptosis in hepatocellular carcinoma. Neoplasia 2021, 23, 1227–1239.
- Tang, Y.; Li, C.; Zhang, Y.J.; Wu, Z.H. Ferroptosis-Related Long Non-Coding RNA signature predicts the prognosis of Head and neck squamous cell carcinoma. Int. J. Biol. Sci. 2021, 17, 702–711.
- Zhao, Y.Y.; Lian, J.X.; Lan, Z.; Zou, K.L.; Wang, W.M.; Yu, G.T. Ferroptosis promotes anti-tumor immune response by inducing immunogenic exposure in HNSCC. Oral Dis. 2023, 29, 933–941.
- Luo, L.; Wang, H.; Tian, W.; Li, X.; Zhu, Z.; Huang, R.; Luo, H. Targeting ferroptosis-based cancer therapy using nanomaterials: Strategies and applications. Theranostics 2021, 11, 9937–9952.
- Wang, Y.; Sun, T.; Jiang, C. Nanodrug delivery systems for ferroptosis-based cancer therapy. J. Control. Release. 2022, 344, 289–301.
- Guan, Q.; Guo, R.; Huang, S.; Zhang, F.; Liu, J.; Wang, Z.; Yang, X.; Shuai, X.; Cao, Z. Mesoporous polydopamine carrying sorafenib and SPIO nanoparticles for MRI-guided ferroptosis cancer therapy. J. Control. Release. 2020, 320, 392–403.
- Peng, H.; Zhang, X.; Yang, P.; Zhao, J.; Zhang, W.; Feng, N.; Yang, W.; Tang, J. Defect self-assembly of metal-organic framework triggers ferroptosis to overcome resistance. Bioact. Mater. 2023, 19, 1–11.
- Brown, C.W.; Chhoy, P.; Mukhopadhyay, D.; Karner, E.R.; Mercurio, A.M. Targeting prominin2 transcription to overcome ferroptosis resistance in cancer. EMBO Mol. Med. 2021, 13, e13792.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
726
Revisions:
2 times
(View History)
Update Date:
20 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




