1. Introduction
In the golden age of antibiotic discovery, the cytoplasmatic membrane was not considered a therapeutic target due to concern that the mammalian cells could be damaged by the antibiotics targeting the bacteria [1]. This view, however, is changing. Lipophosphonoxins (LPPOs) belong to a small molecule antibacterial membrane targeting peptidomimetics. Hence they target the cytoplasmic membrane. This new and very promising class of antibiotics was introduced for the first time in 2011 by Rejman et al. in the Journal of Medicinal Chemistry [2]. At that time, the LPPO group that was investigated was called the first-generation today, as today there is already a second and a third generation of LPPOs, which seems to be even more promising for clinical use than the first one. The third generation is also termed LEGO-LPPOs [3].
While first-generation LPPOs demonstrated selectivity against prokaryotic cells, showing no or limited genotoxicity against human cell lines at minimal bactericidal concentration, having a mode of action that kills the prokaryotic cells by pore formation in their cytoplasmic membrane, and having a low propensity for resistance development, first-generation LPPOs are active only against Gram-positive pathogens and are unsuitable for systemic treatment. To overcome these limiting factors of first-generation, second-generation was synthesized and tested.
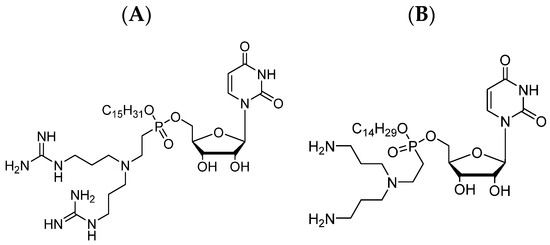
As mentioned earlier, the molecule of LPPO comprises four different modules that can be modified, and by these modifications, different LPPO agents can be created. The lack of susceptibility of Gram-negative bacteria to first-generation LPPOs is probably caused by the limited LPPO–membrane interaction due to a weak positive charge of the first-generation. As the positive charge in the first-generation structure is located on the imino-sugar module, the structural modification for the second generation was created here. Such a re-design of the imino-sugar module increased the number of positive charges and thus increased the affinity to the cytoplasmic membrane of the Gram-negative bacteria (see Figure 1).
Figure 1. Example of the second-generation LPPOs: (A) Compound DR-6180; (B) Compound DR-6155.
The second-generation LPPOs demonstrated in vitro an increased efficacy against Gram-positive pathogens vs. first-generation (MIC < 1–6 mg/L) as well as antibacterial activity against Gram-negative pathogens, including clinically relevant strains of
Escherichia coli,
Pseudomonas aeruginosa, and
Salmonella Enteritidis
[4]. Both generations of LPPOs share the same mode of action—the creation of pores in the cytoplasmic membrane, resulting in the efflux of the bacterial cytosol and cell disintegration. As in the first-generation, second-generation showed no effect on the eukaryotic cells at their bactericidal concentrations, no in vivo inhibition of biosynthesis of the cell macromolecules (DNA, RNA, protein, peptidoglycan, and membrane lipids), excellent thermostability and stability in low pH, and inability to pass through the Caco-2 monolayer.
Of all synthesized second-generation LPPOs, DR-6155 and DR-6180 were selected for further investigation. Because of the inability of LPPOs to cross the eukaryotic cytoplasmic membrane and recommended local gastrointestinal and topical/skin application, LPPOs’ in vivo toxicity was investigated accordingly. The Maximum Tolerated Dose (MTD) was investigated in mice using an orally administered dose of 2000 mg/kg of body weight. No death, body weight loss, or gross pathology changes were observed with either compound during a period of two weeks
[4]. Skin irritation test was performed in rabbits and lasted for at least one week. All tested animals were without any morphological, physiological, or behavioral abnormalities
[4]. All these tests suggest a favorable safety profile of second-generation LPPOs in mammals.
One of the biggest assets of the first generation was a low propensity for resistance development, and thus resistance development was also investigated in the second generation. Twenty one-day passages with subinhibitory concentrations were carried out in vitro with
Pseudomonas aeruginosa and ciprofloxacin as a control. The MIC remained relatively unchanged for LPPO and significantly increased for ciprofloxacin (from 0.25 to 4 mg/L). No
Pseudomonas aeruginosa cells resistant to second-generation LPPOs were cultivated, but cells resistant to ciprofloxacin emerged
[4]. The low propensity for pathogen resistance was shown also in Gram-negative bacteria.
Second-generation LPPOs keep all the beneficial properties of first-generation and, in addition, are effective against a broad spectrum of bacteria, including bacterial strains that are difficult to kill—Gram-positive as well as Gram-negative ones. Therefore, the logical step was to investigate further only the second generation. One of the few communicated disadvantages so far has been the unsuitability of LPPOs for systemic treatment, not only because of their inability to be absorbed from the intestine (that can be overcome by intravenous administration) but because of the interaction with the serum albumins that abolish the LPPOs’ antibacterial activity
[3]. Hence, the second generation was investigated in the non-systemic applications—skin infections, as antimicrobial additives in bone cement, and in tooth root canal infection treatment.
2. Second-Generation LPPOs as Antimicrobial Additives in Bone Cement
Infections are serious complications associated with 1–2% of primary joint replacements and may result in the development of bacterial biofilms on the implant surface that can result in implant removal
[5]. About two-thirds of orthopedic implant infections are caused by staphylococci, and the second most common pathogens are streptococci
[6]. To prevent the formation of bacterial biofilms, gentamicin is the most commonly used antibiotic mixed with poly(methyl methacrylate) (PMMA) bone cement. However, some bacterial strains are already resistant to gentamicin, and therefore, there is a need to find more potent antibiotics that can be mixed with bone cement without compromising the cement’s mechanical properties that would be released from the cement in sufficient amounts to prevent biofilm formation. Second-generation LPPOs, DR-6155 and DR-6180, were evaluated as additives to surgical bone cement in a series of in vitro studies.
During the polymerization process, exothermic reaction increases the temperature of the PMMA bone cement up to 90 °C
[7]; hence LPPOs’ thermostability at high temperatures was evaluated. Both tested LPPOs were dissolved in 80 °C water for up to 8 h, although the polymerization takes only about 10 min. Then, LPPOs were tested with liquid chromatography–mass spectrometry, which showed no degradation and proved excellent thermostability of LPPOs. This is essential for withstanding high temperatures associated with surgical bone cement polymerization
[8].
Another experiment tested whether adding LPPO into bone cement changes the characteristics of the material, such as strength and elongation, at break. The experiment demonstrated that composite cements containing up to 0.2 g of LPPO/10 g of cement did not negatively affect the polymerization process, and cement containing LPPOs had similar qualities as compared to one without LPPOs.
Investigators also tested whether LPPOs are released from the polymerized cement in an active form and in a sufficient amount. LPPO amounts ranging from 0.05 g to 0.2 g per 10 g of the PMMA bone cement were investigated, and the 0.18 g/10 g combination was selected as the one with the optimal LPPO release and still not compromising the mechanical properties of the bone cement
[8]. LPPO is released in two phases. First comes an initial spike that lasts for a few hours and is followed by a gradual lower release in subsequent days. This biphasic release is optimal for local infection treatment when elevated initial concentration kills present bacteria, and subsequently released lower dose still suffices to prevent new bacterial infection from occurring
[8].
The final test was designed to answer the question of whether LPPOs released from the bone cement can prevent bacterial biofilm formation. Bacterial strains of
Enterococcus faecalis,
Staphylococcus aureus,
ica operon-positive
Staphylococcus epidermidis (
ica operon, a gene cluster encoding the production of intercellular adhesin, a polysaccharide that mediates the intercellular adherence of bacteria and biofilm accumulation),
Escherichia coli, and
Pseudomonas aeruginosa were investigated. Gentamicin (0.9 g per 40 g of PMMA bone cement) was used as a control. DR-6155 demonstrated complete inhibition of the biofilm formation for all tested bacterial strains, and DR-6180 showed complete biofilm inhibition for all strains except for
Pseudomonas aeruginosa. Gentamicin prevented biofilm formation for all strains, with the exception of gentamicin-resistant
Staphylococcus epidermidis [8].
3. Nanofiber Dressing Loaded with Second-Generation LPPO in Treatment of Wound Infection Induced by Staphylococcus aureus
Systemic antibiotic therapy is the standard of care for the treatment of wound infections, especially burns, but it may be associated with systemic toxicity and/or with the emergence of resistant bacterial pathogens. Topical antibiotic therapy rarely causes systemic toxicity; however, it may have cytotoxic effects on keratinocytes and fibroblasts and thus delay wound healing
[9]. Conventional wound dressings such as gauze do not prevent infection and drying of the wound beds. In addition, it needs to be replaced regularly, and this process often damages the healing tissue. From this perspective, the ideal wound dressing would be biodegradable, would support gas exchange, and would have antibacterial activity at the same time.
One of the most promising wound dressing systems is a polycaprolactone (PCL)-based nanofibrous scaffold (NANO) produced by electrospinning. NANO mimics, with its network of cross-linked smooth fibers, the structure of the native extracellular matrix (diameter of fibers around 1 μm and pores of several micrometers). It delivers oxygen, extracts wound exudate, and is biocompatible and biodegradable. It degrades into naturally occurring metabolite 6-hydroxyhexanoic acid and so reduces the rate of dressing replacements
[10]. PCL scaffold is approved by the FDA for biomedical applications.
To enhance the NANO wound dressing properties and for the purpose of this experiment, NANO was loaded with second-generation LPPO DR-6180, and investigators tested whether LPPO is released from NANO and if so, whether it has antimicrobial activity against Staphylococcus aureus, whether it affects proliferation, differentiation, and migration of fibroblasts and keratinocytes, and whether LPPO is absorbed and has any systemic effect. NANO with loaded LPPO of concentrations 0, 2, 5, and 10% was used. NANO loaded with LPPO did not significantly affect the fiber size.
For the proper antibiotic function of the NANO–LPPO, it is crucial that LPPO is released in a sufficient amount. LPPO release was tested in a phosphate-buffered saline (PBS) environment and showed a dose-dependent pattern. LPPO from the NANO–LPPO10% was released rapidly in the first 24 h, followed by continuous slower release over the next six days. However, there was no detectable release from NANO–LPPO2% observed during the seven-day incubation period in the PBS. It suggests that the release of LPPO by simple diffusion occurs only at higher LPPO concentrations. Because lipases are ubiquitous enzymes produced by bacteria, in the next step, LPPO release from the NANO was tested in the presence of a lipase. In this environment, the release was observed even from NANO–LPPO2% (75% of LPPO released during the first 24 h, followed by slower release afterward). All the LPPO from NANO–LPPO10% was released, and the NANO was degraded during the first 24 h (50% during the first 8 h). The degradation of the nanofibers by lipase started as surface modifications and continued from day two in significant restructuring of the entire fiber, and the degradation was more pronounced with the higher LPPO concentration load. Interestingly, and this phenomenon was not described before, the degradation of the nanomaterial is accelerated not only by the presence of LPPO but also by the microbial lytic enzymes. It means that more bacteria produce more enzymes (lipases), and this causes bigger and faster NANO degradation with more LPPO released. Overall, LPPO is released from the NANO by two main mechanisms: simple diffusion and the enzymatically catalyzed degradation of NANO
[11]. LPPO biphasic release, described as an initial spike within a few hours followed by a gradual lower release in subsequent days, is optimal for local infection treatment when elevated initial concentration kills present bacteria and subsequently released lower dose still suffices to prevent bacterial inhibition.
Wettability is defined as the ability of a liquid to spread over a surface, and the wettability of the dressing is an important characteristic that provides information about the ability of the dressing to absorb the fluids. The Washburn adsorption test showed that the presence of LPPO in the nanomaterial increased its wettability as compared to NANO without an LPPO load. LPPO-loaded NANO increases the ability to absorb the fluids and thus control the moisture in the wound
[11].
Fibroblasts and keratinocytes are two of the major cell types responsible for the wound regeneration process, and their impairment by antibiotics may have a negative effect on wound healing. Therefore, it was investigated in vitro whether LPPO at bactericidal concentration has any effect on human dermal fibroblasts (HDFs) and immortalized human keratinocyte cells (HaCaT), and whether LPPO interferes with TGF-1 signaling, which is important for proper wound closure. It was demonstrated that LPPO in the concentration range from 0.1 to 25 mg/L does not impair the function of fibroblasts and keratinocytes. However, higher LPPO concentrations of 50 and 100 mg/L were found to be toxic to them. The keratinocyte migration, which is crucial for proper wound re-epithelization, is accelerated in the presence of pro-fibrotic cytokine (TGF-1β). In vitro studies showed that LPPO did not interfere with TGF-β1 signaling and thus did not alter the ability of keratinocytes to migrate
[11].
The positive impact of nanomaterials on wound healing has been published before. It was proved that nanofibrous scaffolds hindered bacterial growth and thus promoted wound repair
[12]. The effectiveness of NANO-LPPO dressing was investigated in non-infected as well as
Staphylococcus aureus-induced wound infection in mice as
Staphylococcus aureus impairs the wound healing process. In non-infected mice wounds, the NANO-LPPO dressing did not impair wound healing at any of the tested LPPO concentrations and even slightly improved granulation tissue formation. In
Staphylococcus aureus-infected mice wounds, increased re-epithelization, granulation tissue formation, and newly formed collagen in the granulation tissue were described with NANO-LPPO5% and NANO-LPPO10% dressing, and no differences were observed as compared to non-infected control. These histologically infected wound healing properties of 5% and 10% NANO-LPPO concentrations were almost not observed with NANO-LPPO2%, where the histological wound picture looked similar as compared to infected wounds with NANO dressing without LPPO (see
Figure 2)
[11].
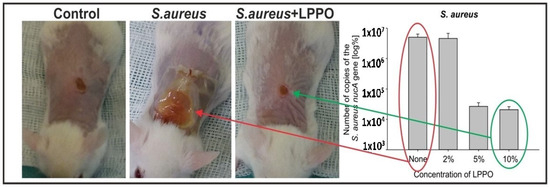
Figure 2. Wound healing in BALB/c mice infected with Staphylococcus aureus promoted by NANO–LPPO. The right panel represents qPCR quantification of Staphylococcus aureus (Control, NANO–LPPO2%, NANO–LPPO5%, NANO–LPPO10%).
The higher positive impact of NANO-LPPO5% and 10% on infected wound healing was translated into an inspection of bacteria from wound swabs grown on agar media and
Staphylococcus aureus qPCR-based quantification in wounds. On agar media,
Staphylococcus aureus was detected in all samples without LPPO and with NANO-LPPO2% dressing; however, only in one sample with LPPO5%. In qPCR-based quantitation that also detects DNA released from dead bacteria, the bacterial load from wounds and surrounding skin treated with NANO–LPPO5% and 10% was significantly lower versus that from wounds treated with NANO2% and NANO only (in the range of 10
4 vs. 10
6, respectively)
[11].
The report of Do Pham et al.
[11] was the first one that described, at least partially, LPPO absorption when used locally and subsequent systemic distribution in the blood and liver of a mammal (mice in this case). In NANO–LPPO2%, the concentration of LPPO detected in mice plasma was 1.62 ng/mL in the non-infected mice and 3.39 ng/mL in the infected mice. In NANO–LPPO5%, the plasma concentration was 3.96 ng/mL and 1.58 ng/mL, respectively. In NANO–LPPO10%, the plasma concentration was 9.56 ng/mL and 8.49 ng/mL, respectively. In NANO–LPPO2%, the concentration of LPPO detected in the liver was 113.8 ng/mL in the non-infected mice and 72.23 ng/mL in the infected mice. In NANO–LPPO5%, the liver concentration was 222.5 ng/mL and 127.9 ng/mL, respectively. In NANO-LPPO10%, the liver concentration was 659.2 ng/mL and 161.7 ng/mL, respectively. There were three mice in each group. Overall, the concentration of LPPO detected in blood and liver correlated with its amounts in the NANO–LPPO dressing, and the systemic exposure was negligible from the cytotoxicity point of view
[11].