Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yuguang Liu | -- | 4825 | 2023-10-16 17:05:07 | | | |
| 2 | Catherine Yang | Meta information modification | 4825 | 2023-10-17 02:38:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Beniwal, S.S.; Lamo, P.; Kaushik, A.; Lorenzo-Villegas, D.L.; Liu, Y.; Mohanasundaram, A. Colorectal Cancer Screening and Diagnostics. Encyclopedia. Available online: https://encyclopedia.pub/entry/50364 (accessed on 03 March 2026).
Beniwal SS, Lamo P, Kaushik A, Lorenzo-Villegas DL, Liu Y, Mohanasundaram A. Colorectal Cancer Screening and Diagnostics. Encyclopedia. Available at: https://encyclopedia.pub/entry/50364. Accessed March 03, 2026.
Beniwal, Shreya Singh, Paula Lamo, Ajeet Kaushik, Dionisio Lorenzo Lorenzo-Villegas, Yuguang Liu, Arunsundar Mohanasundaram. "Colorectal Cancer Screening and Diagnostics" Encyclopedia, https://encyclopedia.pub/entry/50364 (accessed March 03, 2026).
Beniwal, S.S., Lamo, P., Kaushik, A., Lorenzo-Villegas, D.L., Liu, Y., & Mohanasundaram, A. (2023, October 16). Colorectal Cancer Screening and Diagnostics. In Encyclopedia. https://encyclopedia.pub/entry/50364
Beniwal, Shreya Singh, et al. "Colorectal Cancer Screening and Diagnostics." Encyclopedia. Web. 16 October, 2023.
Copy Citation
Colorectal cancer (CRC) is a prevalent and potentially fatal disease categorized based on its high incidences and mortality rates, which raised the need for effective diagnostic strategies for the early detection and management of CRC. While there are several conventional cancer diagnostics available, they have certain limitations that hinder their effectiveness.
colorectal cancer
non-invasive
diagnostic
microfluidics
1. Introduction
Colorectal cancer (CRC) represents a significant global health burden, ranking as the second most prevalent cause of cancer-related mortality and the third most widespread malignant neoplasm. CRC primarily affects the colon and rectum, which are integral components of the gastrointestinal tract responsible for waste processing and elimination [1]. Recent epidemiological data from 2020 to 2021 reported a mortality rate of 9.4%, accompanied by a noteworthy increase in incidents among the elderly [2].
In oncology diagnostics, there has always been a strong emphasis on detecting cancer at an early stage. This principle applies notably to CRC, as the prompt recognition of signs and symptoms associated with CRC during asymptomatic or pre-cancerous stages can profoundly influence treatment modalities (e.g., personalized care) and overall prognosis (e.g., survival rates and enhanced quality of life) [3]. So far, several standard diagnostic modalities have contributed to the early intervention and improved management of CRC and patient outcomes. These modalities encompass screening tests such as fecal occult blood tests (FOBT), fecal immunochemical tests (FIT), and stool DNA tests, as well as standard diagnoses such as colonoscopy, which directly visualizes a biopsy of abnormal tissue or polyps, or computed tomographic colonography (CTC) as a less invasive alternative [4][5]. Furthermore, blood-based biomarkers, such as carcinoembryonic antigen (CEA) and circulating tumor DNA (ctDNA), have also demonstrated promise as non-invasive screening modalities for CRC. Nevertheless, these conventional methodologies are often limited in sensitivity and specificity, and there is a lack of sufficient validation of stool DNA tests. Other practical concerns include the risks associated with the invasive colonoscopy as well as the ionizing radiation exposure inherent in CTC [6].
2. Emerging Paradigms in CRC Diagnostics
CRC diagnostic methods are typically classified into invasive and non-invasive modalities (Figure 1). Invasive tests encompass colonoscopy, flexible sigmoidoscopy, virtual colonoscopy (CT colonography), and double-contrast barium enema [7]. These procedures involve direct access to the colon and rectum to visually examine the colorectal region, identify pathological features, and perform biopsies when required. Conversely, non-invasive tests employ approaches such as fecal occult blood test (FOBT), fecal immunochemical test (FIT), and stool DNA test (MT-sDNA) [8]. These non-invasive techniques analyze stool samples to detect the presence of blood, genetic markers, or DNA alterations associated with CRC. The selection of invasive or non-invasive methods is contingent upon individual risk factors, symptoms, and the recommendations of healthcare providers. Both invasive and non-invasive strategies play pivotal roles in the diagnosis and management of CRC, leading to improved outcomes and tailored treatment modalities. The below discussion gives a brief insight into different invasive and non-invasive methods.
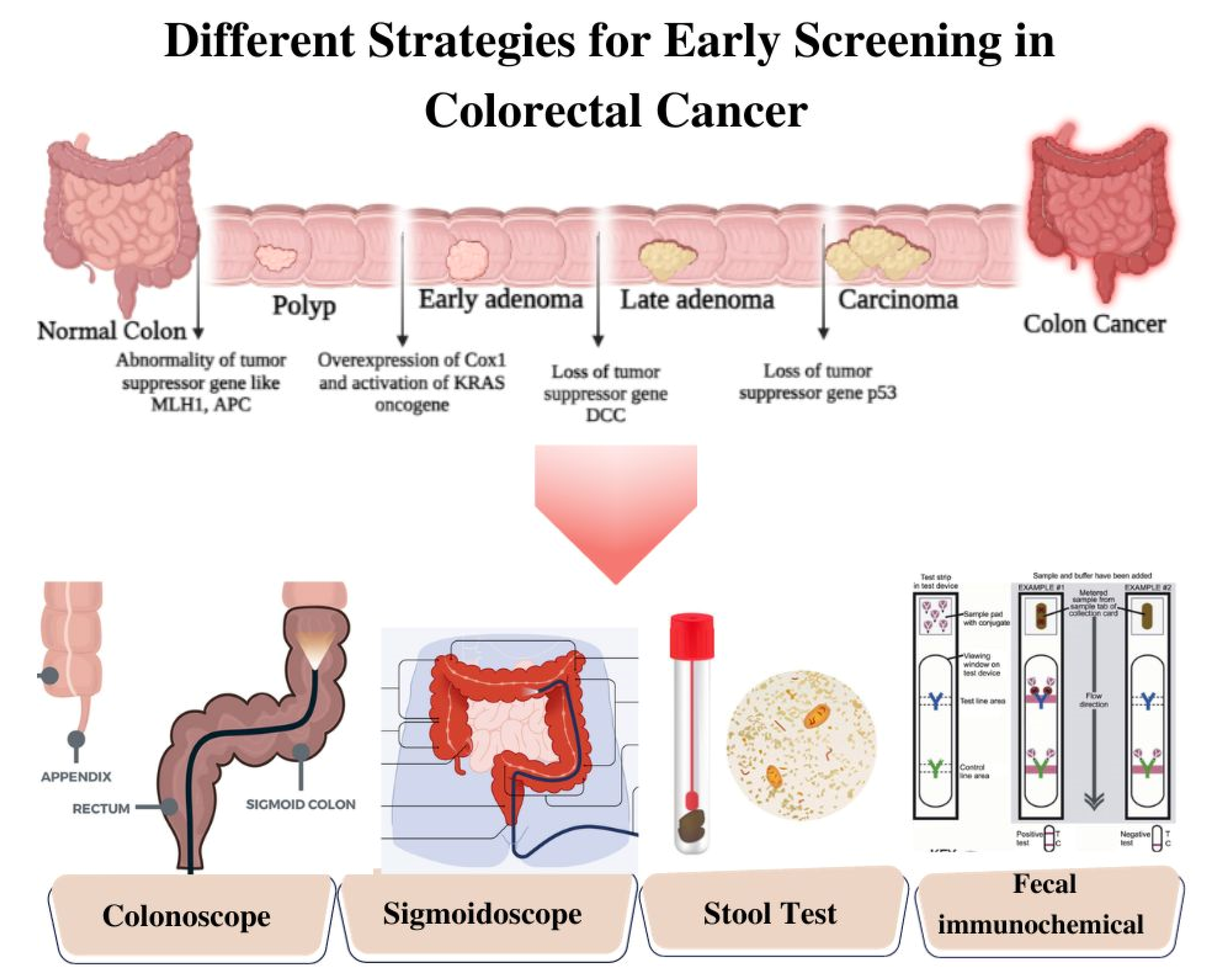
Figure 1. Schematic Illustration of Different Strategies of Screening for Colorectal Cancer.
3. Nanotechnology-Driven Innovations in CRC Diagnosis: Unveiling the Power of Nano-Enabled Tools
Conventional methods demonstrated good sensitivity but could miss some early-stage cases or precancerous lesions. Therefore, the discriminatory power to distinguish individuals with and without CRC is not robust until the onset of primary symptoms, which can delay diagnosis and treatment [9]. It is therefore imperative to increase the sensitivity and specificity of CRC screening methods to detect CRC at precancerous stages, prompting timely and successful interventions [10][11]. Meanwhile, high specificity is equally crucial to minimize false-positive results, as these can lead to unnecessary follow-up procedures that not only increase healthcare costs but also cause undue anxiety and stress for patients. Ensuring high specificity in CRC screening can better ensure that medical resources are allocated to only patients with positive results [6].
In recent years, nanotechnology has emerged as a rapidly evolving field in the advancement of novel diagnostics for CRC. The unique properties and capabilities of nanomaterials offer new avenues for the sensitive and specific detection of CRC biomarkers, enabling early diagnosis and personalized treatment strategies, as represented in Figure 2 [12]. The integration of nanotechnology in CRC diagnostics offers unparalleled precision and efficacy, surpassing conventional techniques. This is attributed to the unique physicochemical properties inherent in nanomaterials, which enable efficient biomarker capture and amplification due to their substantial surface area-to-volume ratio [13][14]. Moreover, nanotechnology-based strategies can enable multiplexed analysis in a single assay. Furthermore, these technologies can be customized to accommodate diverse sample types, including blood, tissue, and stool, extending their applicability in CRC diagnostics [15][16].
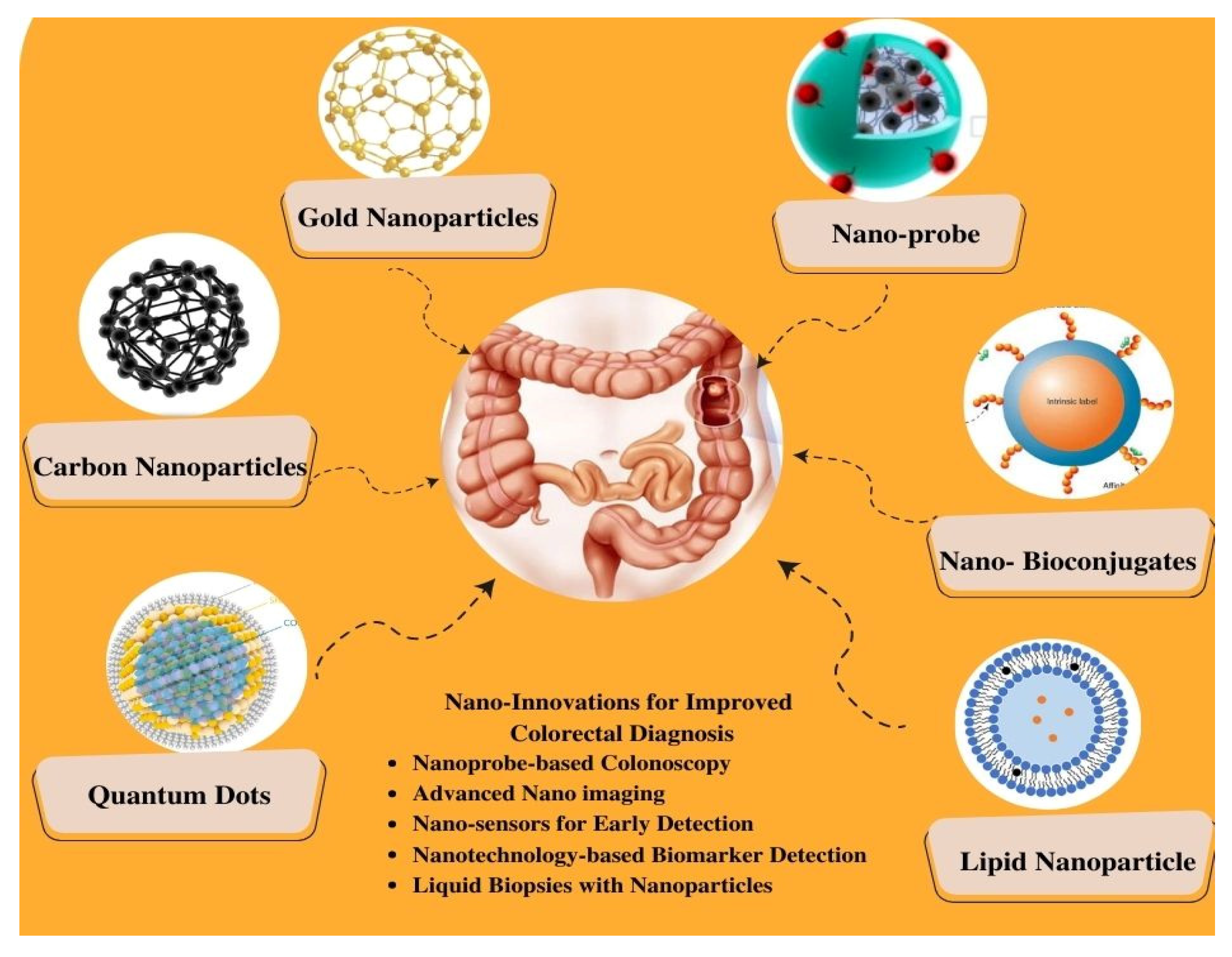
Figure 2. Schematic Representation of Nano-Enhanced Imaging Techniques for Colorectal Cancer Diagnosis.
3.1. Quantum Dots (QDs)
Quantum dots (QDs) are nano-scale fluorescent semiconductor crystals renowned for their unique optical characteristics, especially their exceptional brightness, photo stability, and tunable emission spectra, enabling multiplexed imaging capabilities [17]. QDs can be conjugated with ligands, facilitating their binding to target molecules such as cancer cells or tumor vasculature and thus enabling precise and specific imaging of specific molecular targets [18]. Therefore, QDs present a compelling opportunity for the advancement of sophisticated imaging modalities in CRC research and diagnostics [14].
For instance, Gazouli et al. [19] developed a QD-labeled magnetic immunoassay (QD-MIA) technology that combines QDs and magnetic separation strategies to detect circulating CRC cells. Briefly, this platform introduced a novel, cost-effective methodology for the identification of circulating CTC cells in human biological specimens. This approach involved the utilization of magnetic bead isolation followed by QD fluorescence-based detection, which demonstrated a verified minimum detection limit of 10 DLD-1 CRC cells/mL as quantified through spectrofluorometry. Furthermore, the robustness and reliability of this method were comprehensively assessed through the integration of fluorescence-activated cell sorting analysis and real-time RT-PCR. The findings of the studies underscored the establishment of a straightforward, exceptionally sensitive, and efficient technique with substantial potential for the identification of CTCs in patients with CRC. Notably, the adaptable nature of this method for targeting a wide array of protein markers, whether associated with CTCs or the host organism, implied its versatility and suitability for a diverse range of applications that extended beyond the confines of colorectal cancer detection [19]. Likewise, Carbary-Ganz et al. [20] developed a highly specific and sensitive imaging tool for CRC by exploiting the overexpression of VEGFR2 in the CRC vasculature. In this work, the synthesized QDs were conjugated with VEGFR2-targeting ligands, allowing for the selective binding of the QDs to VEGFR2 in CRC tissues. The study found that the QD655-VEGFR2 agent demonstrated a sensitivity of 85.7% and a specificity of 91.3% in detecting VEGFR2 expression in colorectal cancer. On the other hand, the negative control contrast agent, QD655-IC, had a sensitivity of 5.6% but showed 100% specificity. This suggests the VEGFR2-targeted QDs demonstrated excellent imaging capabilities, providing enhanced contrast and improved visualization of CRC lesions compared to non-targeted QDs or conventional imaging agents [20].
3.2. Carbon-Based Nanoparticles
Carbon-based nanoparticles include carbon nanotubes, graphene, and carbon dots and offer several advantages for CRC diagnosis. Compared with QDs and gold nanoparticles, the biocompatibility of carbon nanoparticles is a notable characteristic that ensures their safe administration in vivo without significant adverse health effects [21]. In addition, these nanoparticles exhibit a large surface area and high electrical conductivity, which makes them ideal for functionalization with specific targeting ligands or biomolecules [22], and they can be integrated with electrical-based sensors such as impedance and electrochemical sensors that are common in point-of-care platforms. For example, Yan et al. [23] assessed the efficacy of carbon nanoparticles in the detection of lymph node metastasis in early-stage (T1–2) CRC. The patient cohort comprised individuals who underwent surgical resection for T1–2 CRC, with carbon nanoparticles administered via submucosal injection around the tumor site. The results revealed that these nanoparticles effectively traced lymphatic drainage and accurately identified sentinel lymph nodes in T1–2 CRC, employing near-infrared fluorescence imaging. Notably, the carbon nanoparticle-guided sentinel lymph node biopsy exhibited a superior detection rate for lymph node metastasis compared to conventional histopathological examination, demonstrating heightened sensitivity of 91.67% and a specificity value of 100% [23]. Similarly, for another group, Zhang et al. [24] demonstrated the efficacy of carbon nanoparticles in identifying sentinel lymph nodes and evaluating lymph node metastasis in elderly patients with CRC who underwent surgical procedures using the same approach. The results showed the successful guidance of carbon nanoparticles in identifying sentinel lymph nodes, enabling accurate evaluation of lymph node metastasis in elderly patients with CRC. These findings indicate the promise of carbon nanoparticles in enhancing lymph node staging precision, aiding treatment decision-making, and reducing the extent of lymphadenectomy in CRC patients [24].
In another example, Cai et al. [25] studied the efficacy of activated carbon nanoparticle suspension and methylene blue for lymph node staining in CRC. In the in vivo experiment, activated carbon nanoparticle suspension was administered through lymphatic vessel injection in mice with CRC, and the successful staining of the regional lymph nodes allowed for their visualization. Additionally, in the in vitro experiment, methylene blue was employed to stain lymph nodes obtained from CRC patients. The comparable staining efficacy between activated carbon nanoparticle suspension and methylene blue indicated their effectiveness in highlighting the lymph nodes, suggesting that activated carbon nanoparticles are a promising alternative to methylene blue for lymph node staining in CRC [25]. In another study by Wang et al. [26] prior to surgery, carbon nanoparticles were injected into the submucosal layer surrounding the tumor site during endoscopic examination. Subsequently, laparoscopic surgery was performed, and the carbon nanoparticles served as a guide for the identification and tracking of lymph nodes. The results demonstrated successful preoperative endoscopic localization of CRC utilizing carbon nanoparticles in all enrolled patients during surgery. Overall, the integration of carbon nanoparticles into laparoscopic procedures provided real-time visualization and improved the precision of lymph node dissection. This innovative approach holds significant potential for optimizing surgical outcomes, reducing complications, and enhancing the overall management of patients with CRC [26].
3.3. Lipid-Based Nanoparticles
Lipid-based nanoparticles, which comprise lipid materials such as phospholipids or cholesterol, offer several advantages for CRC diagnosis [27][28]. As with many other nanoparticles, lipid-based nanoparticles can be functionalized with specific targeting ligands or antibodies that selectively recognize CRC biomarkers or tumor-associated antigens [29]. Moreover, lipid-based nanoparticles can encapsulate imaging agents, such as fluorescent dyes or contrast agents, facilitating enhanced visualization of CRC lesions using various imaging techniques, including fluorescence imaging or MRI. The unique attributes of lipid-based nanoparticles, encompassing their biocompatibility, stability, and capacity to carry imaging payloads, render them well-suited for the precise and non-invasive diagnosis of CRC [30]. For instance, Alrumaihi et al. [31] aimed to enhance the bioavailability of diallyl trisulfide (DATS), a bioactive compound with known anticancer properties, by encapsulating it in polyethylene glycol-coated liposomes (DATSL) and combining it with doxorubicin (DOXO)-encapsulated liposomes (DOXL). DATSL and DOXL exhibited significant sensitivity in inhibiting colon cancer cell proliferation, both individually and in combination, with a synergistic effect observed at specific concentration combinations, lowering the IC50 doses of DATS and DOXO by over 8- and 14-fold, respectively. In an AOM-induced colon cancer mouse model, high-dose DATSL pretreatment followed by DOXL chemotherapy effectively inhibited cancer promotion, with DATSL and DOXL exhibiting approximately 93% and 46% entrapment efficiency, respectively. Additionally, molecular docking analysis identified potential interactions with cancer-related proteins, such as MMP-9, suggesting a mechanism for DATS action. This study presents a promising approach for colorectal cancer prevention and treatment, highlighting the potential of DATSL and DOXL as a novel and efficient therapeutic strategy [31].
Nanotechnology-driven advancements in the diagnosis of CRC come with noteworthy limitations that require careful scientific consideration. Foremost among these constraints is the potential for biocompatibility and toxicity quandaries. Nanoparticles employed in diagnostic applications may engender unforeseen interactions within biological systems, culminating in plausible deleterious repercussions. The imperative of ensuring the safety profile of these nanoparticles for in vivo implementation necessitates comprehensive toxicity assessments and rigorous empirical evaluation. Concomitantly, the scaling-up and mass production of nanoscale diagnostic modalities can be encumbered by formidable cost considerations and intricate technical challenges, potentially hindering their ubiquitous integration into clinical contexts. Furthermore, the long-term stability and durability of nanoscale diagnostic platforms mandate meticulous scrutiny to affirm their sustained reliability. The intricate regulatory approval processes governing the utilization of nanotechnology-based diagnostic tools impose substantial temporal impediments, possibly impeding their expeditious assimilation into customary clinical practice. While nanotechnology offers enticing prospects for advancing CRC diagnosis, these intricacies must be judiciously addressed to unlock its full potential in augmenting early disease detection and clinical management paradigms.
4. Integration of Microfluidics in CRC: Promising Tools for Precise Diagnosis
Microfluidic technologies can precisely manipulate and control fluids at the microscale and provide a foundation for developing lab-on-a-chip devices. These miniaturized systems offer numerous advantages, including reduced sample and reagent consumption, accelerated reaction times, enhanced sensitivity, and portability [32]. The domain of CRC diagnosis has witnessed noteworthy progressions, notably in the realm of microfluidics as a promising and rapidly evolving field. This section aims to elucidate the manifold applications and advantages of microfluidics in CRC diagnostics [33].
The integration of biosensing into microfluidic platforms for the detection of CRC is a notable endeavor. Electrochemical and colorimetric biosensing modalities are particularly pertinent in this context. In the field of CRC, microfluidics has catalyzed profound transformation by significantly innovating enhance detection and diagnosis modalities. The advent of microfluidics has enabled the isolation and analysis of circulating tumor cells (CTCs) and cell-free DNA (cfDNA) from blood specimens, providing a non-invasive avenue for monitoring cancer progression and treatment response [34][35] detection of genetic mutations, tumor markers, and other biomarkers associated with colorectal cancer, thereby enabling early-stage diagnosis and point-of-care testing. The introduction of microfluidic-based liquid biopsies has had a transformative impact on early cancer detection and personalized medicine strategies, as evidenced by a comprehensive analysis of the existing literature [36][37]. As a result, the utilization of miniaturized microfluidic chips has empowered rapid and highly sensitive detection methods, enabling timely interventions and ultimately leading to improved patient outcomes.
Different detection methods used in the study of CRC encompass the identification of CTCs, isolation and examination of tumor exosomes, analysis of DNA biomarkers, and evaluation of microRNAs in microfluidic devices or lab-on-chip systems. These methodologies provide valuable information about the molecular and cellular characteristics of colorectal cancer, enabling early detection and prognosis determination approaches.
4.1. Circulating Tumor Cells Detection
Microfluidic-based methods have gained significant attention in CRC research for the isolation, detection, and analysis of CTCs in patients’ blood samples. By employing immunoaffinity capture, microfluidic platforms selectively capture CT\Cs using specific antibodies immobilized on the device or micro- and nanoscale particles while washing away other blood cells. Various enrichment strategies, such as positive and negative selection, as well as size-based enrichment, are incorporated to enhance CTC enrichment efficiency. Once cells are captured, they can also be subjected to immunofluorescence staining, nucleic acid amplification, and molecular profiling directly on the microfluidic chip, allowing for comprehensive characterization of CTCs.
Microfluidic-based CTC detection offers a number of advantages, such as increased sensitivity and specificity, reduced sample volume requirements, real-time analysis capabilities, and high-throughput screening potential. Various studies have reported the application of microfluidic-based CTC detection in CRC research. For instance, a microfluidic platform can integrate size-based filtration to isolate CTCs from peripheral blood samples from CRC patients, taking advantage of the size differences between CTCs and blood cells [38]. The captured CTCs could then be counted, analyzed, and characterized, providing valuable information for disease monitoring and treatment response assessment. The platform offers potential as a non-invasive method for CTC detection in CRC patients. Likewise, a similar platform has been used by other groups for the enrichment and detection of rare CTCs in the blood stream of cancer patients [39], and the abundance of CTCs provided valuable information about cancer progression and treatment response. However, isolating and analyzing CTCs is still challenging due to their low abundance and heterogeneous nature. To enhance the capture efficiency, a combination of physical and immunomagnetic separation methods can be integrated into the device to enrich CTCs from blood samples. The captured CTCs were then characterized using immunofluorescence staining, allowing for their identification and further analysis. To enhance the accessibility of the platform to minimally trained hands, others designed a microfluidic platform that incorporated an antibody-coated surface for specific CTC capture and integrated automation for enhanced efficiency and reproducibility [40]. The device demonstrated excellent sensitivity and selectivity, enabling the detection and characterization of CTCs from clinical blood samples with high accuracy.
5.2. Microfluidic-Based Isolation and Characterization of Tumor Exosomes
In recent years, substantial advancements have been made in the development of exosome detection chips based on microfluidic platforms. These innovative chips offer rapid, sensitive, and high-throughput detection of exosomes, thereby presenting new avenues for diagnostics, biomarker discovery, and therapeutic applications [41]. Exosome detection chips commonly utilize diverse functional components and techniques to optimize the efficiency and specificity of exosome capture and analysis [42]. A prevalent strategy that is involved in surface functionalization of the microfluidic chip includes antibodies or aptamers that exhibit selective affinity towards exosome surface markers, thereby facilitating their targeted capture and separation from other extracellular vesicles or contaminants. Furthermore, these chips facilitate the integration of downstream analytical techniques into the same device. For instance, the captured exosomes can be subjected to further analysis employing fluorescence microscopy, immunoassays, or nucleic acid amplification techniques. Such integrated platforms offered a streamlined workflow, minimizing sample loss and reducing the overall time required for analysis. These chips hold promise in various applications, such as early cancer detection, monitoring disease progression, and assessing treatment response [43].
A number of studies utilized the use of exosomes for CRC, for instance (Li et al., 2023) demonstrate the construction of a novel exosome detection platform using a three-dimensional (3D) porous microfluidic chip. The platform enabled early diagnosis of CRC by detecting SORL1 protein in exosomes. The 3D porous microfluidic chip offered a significant increase in functionalized surface areas to selectively capture exosomes expressing SORL1, a biomarker associated with CRC. Clinical samples from CRC patients and healthy controls were analyzed, and the captured exosomes were subjected to immunoassays and nucleic acid amplification to detect SORL1. The platform exhibited efficacy of up to 90%, accurately distinguishing CRC patients from healthy individuals based on exosomal SORL1 levels. To enable more streamlined sample processing, others presented an advanced lab-on-a-chip platform that combines pre-concentration and detection of CRC exosomes in a single device [44]. The platform utilizes an anti-CD63 aptamer as a recognition element for specific exosome capture. By integrating microfluidic techniques for efficient pre-concentration and incorporating the aptamer for selective exosome binding, the platform demonstrates detection limit of 1457 particles/mL, thus offering a promising approach for the sensitive and streamlined analysis of colorectal cancer exosomes, enabling improved early detection, prognosis, and personalized treatment strategies.
To further increase the sensitivity of exosome detection, a novel method combines fluorescence signal amplification aided by the DNase I enzyme and graphene oxide-DNA aptamer interactions to capture CRC exosomes [45]. Functionalizing graphene oxide with DNA aptamers targets exosome surface markers with a high sensitivity of 2.1 × 104 particles/μL, thus enabling selective exosome capture. To enhance detection sensitivity, the researchers employ the DNase I enzyme, which selectively degrades free DNA molecules but not those bound to exosomes.
Despite significant advancements in the development of exosome detection chips, several challenges persist in achieving robust and commercially viable platforms. The optimization of capture efficiency, characterized by the ability to capture a high percentage of exosomes from complex biological samples, remains a critical concern. Non-specific binding poses another hurdle, necessitating the minimization of undesired interactions to ensure specific and accurate exosome detection. Standardizing protocols for sample preparation and analysis represents an ongoing research focus to establish consistent and reproducible workflows across different laboratory settings. Addressing these challenges is imperative to drive the further advancement and widespread adoption of exosome detection chips in diverse biomedical research applications and clinical diagnostics.
5.3. Other Cancer-Related Biomarkers Detection
For individuals diagnosed with CRC, the presence and characterization of malignancy-related biomarkers play a pivotal role in understanding the biological activities and pharmacological responses associated with therapeutic interventions. These biomarkers, including free tumor nucleic acids, mRNA expression profiles, proteins, and other substances found in bodily fluids, offer valuable clinical insights to physicians regarding disease status and aid in making informed decisions regarding subsequent treatment strategies [46]. The utilization of microfluidics systems in the measurement of cancer biomarkers has gained significant attention due to their remarkable capabilities in bioanalysis. Microfluidic platforms enable precise detection and analysis of CRC-specific biomarkers, facilitating early diagnosis, treatment monitoring, and personalized therapeutic interventions [37][47].
For instance, efforts have been focused on the monitoring of changes in the mechanobiology of CRC cells using on-chip techniques [48]. The study utilized a microfluidic chip-based platform to analyze mechanical properties and behaviors such as dynamic force deformation, which can serve as a method for detecting mechanical changes linked to the progression of CRC. The research involved subjecting the cells to controlled mechanical forces and monitoring their responses. The researchers were able to identify distinct mechanobiological characteristics associated with different stages of CRC progression. The findings revealed significant changes in the mechanical properties of CRC cells as the disease advanced. The authors observed alterations in cell stiffness, cytoskeletal organization, and migratory behavior, which were correlated with disease stage and aggressiveness. These physical biomarkers hold promise for early detection, prognosis, and monitoring of CRC progression. Another example includes a microfluidic amperometric immunosensor for the determination of claudin7, a biomarker associated with CRC [49]. The immunosensor utilizes porous nanomaterials and was integrated into a microfluidic device, offering the potential for point-of-care applications where it could provide a rapid and reliable diagnosis of CRC in a clinical setting.
The integration of microfluidics technology with CRC represents a significant advancement with substantial potential for enhancing various aspects of CRC research, diagnosis, and treatment. The utilization of microfluidic platforms enables precise manipulation and analysis of biological samples, offering unique advantages such as enhanced sensitivity, high throughput, and improved control over experimental conditions [50]. This technology has demonstrated promising applications in CRC, including the development of advanced diagnostic tools [51]. However, microfluidics in CRC faces certain challenges that need to be addressed for broader adoption and clinical translation. These challenges include standardization of microfluidic protocols, scalability of manufacturing processes, integration with existing clinical workflows, and validation of clinical utility.
6. Exploring the Potential of AI in CRC: Advancements, Challenges, and Future Directions
In the field of CRC pathology, the transformative technology of artificial intelligence (AI) has made significant advancements, shaping current trends in diagnosis. The employment of AI algorithms in the analysis and diagnosis of digital pathology images of colorectal tissue samples represents a noteworthy utilization within the scientific domain [52]. These AI algorithms utilize sophisticated machine-learning techniques to identify and classify malignant cells, offering enhanced diagnostic precision and efficiency. By incorporating AI into the diagnostic process, issues of variability in traditional evaluations can be addressed, leading to more dependable and consistent diagnostic assessments and potentially improving patient outcomes [53].
The integration of microfluidic systems with AI represents a transformative approach to disease diagnosis. The data generated by microfluidic devices, which may include sensor outputs or images, undergoes sophisticated analysis by AI algorithms. These AI algorithms are meticulously trained to process the data, extract relevant features, and make predictions based on patterns and models derived from extensive datasets. What sets AI apart is its capacity for continuous learning and adaptability, leading to heightened diagnostic accuracy and efficiency [33]. The amalgamation of microfluidic systems and AI algorithms holds great promise for enhancing diagnostic capabilities, promising earlier and more precise disease detection, as schematically represented in Figure 3.
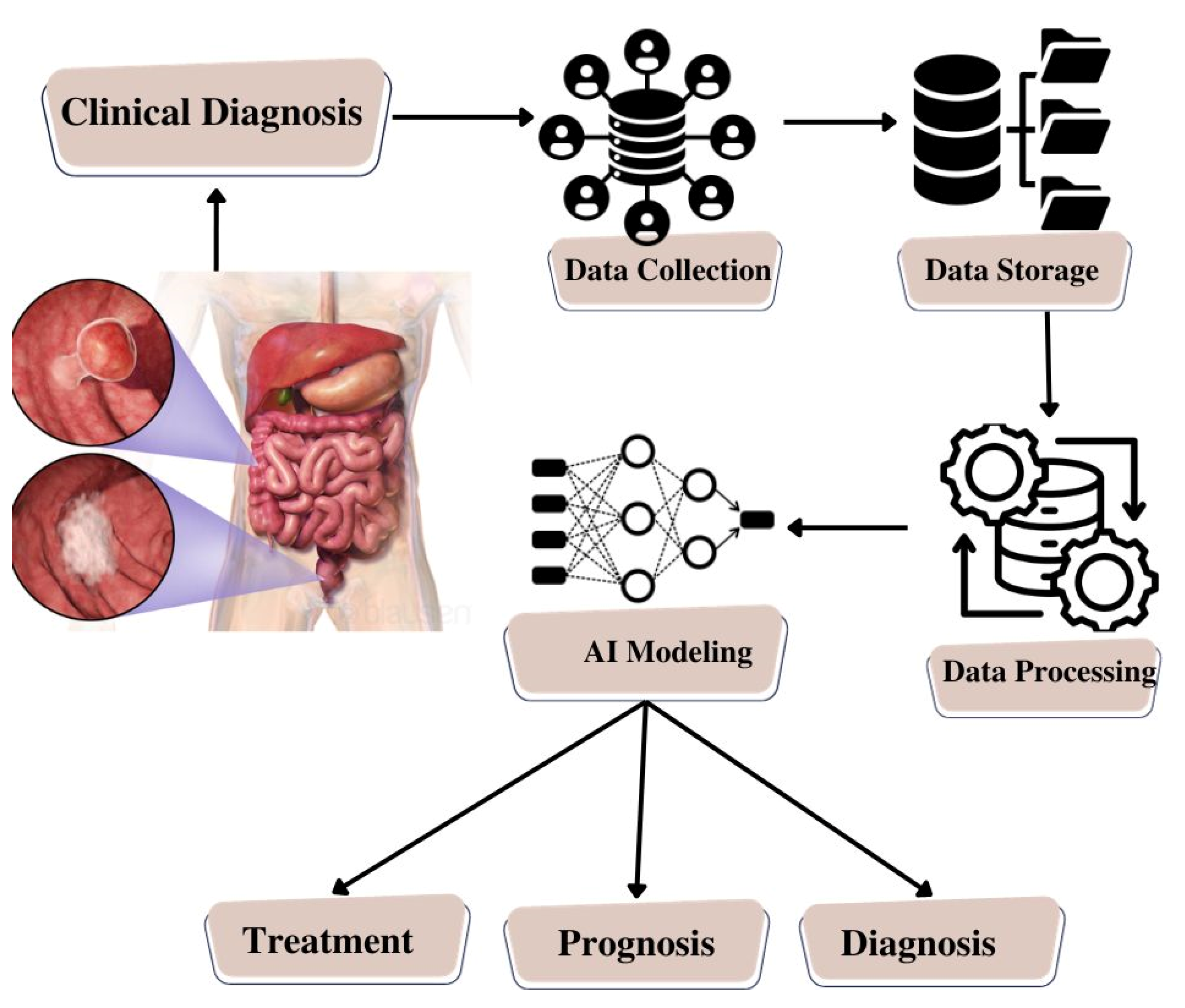
Figure 3. Schematic Illustration of Artificial Intelligence Model Building for Colorectal Cancer.
In concordance with these advancements, a noteworthy trend in this scientific domain pertains to the development of AI-based prognostic and predictive models. These models synthesize diverse datasets encompassing clinical records, genomic information, and histopathological features, with the objective of generating personalized prognostic predictions pertaining to patient outcomes. Through a comprehensive consideration of multiple factors, including genetic profiles, clinical data, and histopathological findings, these AI-driven models offer invaluable insights into the prognosis of individuals afflicted by CRC. Moreover, they exhibit the potential to forecast individual responses to diverse treatment modalities, thereby facilitating the delivery of personalized therapeutic interventions and enhancing the quality of patient care.
Furthermore, AI has evolved into a valuable tool for the identification of novel biomarker targets in CRC. Leveraging vast genomics and transcriptomics datasets, AI algorithms demonstrate proficiency in unveiling intricate patterns and associations that may elude human researchers due to their complexity and scale [33]. This computational approach streamlines the identification of potential biomarkers crucial for early detection and prognostic assessment in CRC. Additionally, AI’s capabilities extend to the exploration of new drug targets and innovative treatment approaches, thus contributing to the ongoing advancement of CRC management strategies.
As a result, the integration of AI with microfluidics and biosensors in the realm of disease diagnosis particularly unveils a multifaceted paradigm with profound scientific implications, such as:
Enhanced Sensing and Detection: Microfluidic platforms endowed with biosensors have the capacity to provide high sensitivity and specificity in the detection of CRC-associated biomarkers. AI algorithms, in tandem, can optimize the analysis of sensor-derived data by discerning subtle patterns or nuanced variations that may hold diagnostic relevance, especially in the early stages of the disease. This amalgamation, therefore, holds the promise of substantially augmenting the precision of CRC detection, enabling the identification of specific biomarkers within bodily fluids or tissue specimens [54][55].
Real-Time Monitoring: Microfluidic systems, when harmonized with biosensors, empower real-time surveillance of dynamic biological processes. The utilization of AI algorithms facilitates the expeditious processing of the continuous influx of data generated by these sensors. This, in turn, furnishes healthcare practitioners with contemporaneous insights into the progression of CRC, the efficacy of therapeutic interventions, or the emergence of potential complications. Such real-time feedback has the potential to be invaluable in facilitating prompt clinical decision-making for individuals afflicted by CRC [56][57].
Point-of-Care Diagnostics: The confluence of microfluidics, biosensors, and AI holds great potential for the creation of point-of-care diagnostic instruments for CRC. These compact, user-friendly devices can swiftly ascertain CRC-specific biomarkers, thereby enabling early diagnosis even in resource-constrained locales or remote geographical areas [58]. AI algorithms play an integral role in the interpretation of sensor-generated data, furnishing rapid diagnostic outcomes to end-users.
Personalized Treatment Strategies: AI-driven models possess the capacity to scrutinize data derived from microfluidic sensors, thereby affording the ability to tailor therapeutic regimens to the unique attributes of individual CRC patients. By considering factors such as genetic profiles, treatment response data, and trends in biomarker levels, AI algorithms can proffer personalized therapeutic recommendations, optimizing the likelihood of favorable treatment outcomes while minimizing the occurrence of adverse side effects [59].
Early Warning Systems: The continuous monitoring capabilities conferred by microfluidic systems and biosensors, when harmonized with AI, have the potential to serve as early warning systems for CRC recurrence or metastasis. AI algorithms can discern subtle alterations in biomarker levels or patterns of disease progression, thereby facilitating timely interventions and subsequently enhancing the long-term prospects for individuals grappling with CRC [60].
Numerous studies have demonstrated the effectiveness of AI in identifying novel biomarkers in CRC [61]. For instance, a multiple diagnosis model for CRC using an artificial neural network has been established [62]. A comprehensive dataset comprising clinical records, histopathological features, and genomic profiles of CRC patients was collected and preprocessed. The dataset was divided into training, validation, and testing subsets. Multiple ANNs with different architectures, such as feed-forward neural networks, convolutional neural networks, and recurrent neural networks, were constructed and trained using the dataset. The study explores the application of AI in endoscopy and laparoscopy, aiming to develop advanced tools that can assist clinicians in identifying and characterizing CRC lesions.
Others explored the application of AI in the intra-operative tissue classification of CRC using indocyanine green (ICG) perfusion [63]. The study focused on utilizing ICG, a fluorescent dye, to assess tissue perfusion during colorectal cancer surgery. By analyzing real-time ICG perfusion images, AI algorithms are trained to distinguish between cancerous and non-cancerous tissues; this showcases the potential of AI algorithms to assist surgeons in real-time tissue classification, enabling them to make informed decisions during surgery.
AI algorithms can also be used to effectively analyze histopathology images and provide reliable CRC diagnoses. The AI models can learn to identify specific features and patterns indicative of CRC, enabling them to distinguish between cancerous and non-cancerous tissues. The study highlights the potential of AI in enhancing CRC diagnosis by providing reliable and objective analysis of histopathology images. By reducing subjectivity and improving efficiency [64].
AI also has the potential to accurately assess the tumor-stroma ratio (TSR) and its association with survival outcomes in CRC [65]. The research utilizes deep learning techniques to train AI models on a large dataset of CRC histopathology images. The AI models learn to identify tumor and stromal regions within the images, enabling them to accurately calculate the TSR. The association between AI-based TSR quantification and survival outcomes in CRC patients can help determine its prognostic value, which indicates that AI can be used as a tool to objectively quantify the TSR and its correlation with survival in CRC.
As an outcome, the investigation of AI in the realm of CRC presents substantial opportunities for advancing diagnostic accuracy, prognostic assessment, and individualized treatment approaches. However, several challenges need to be addressed, including data quality, interpretability, and regulatory considerations. Despite these challenges, with continued research and advancements, artificial intelligence has a promising future in revolutionizing CRC patient care, ultimately leading to improved patient outcomes.
References
- Guren, M.G. The global challenge of colorectal cancer. Lancet Gastroenterol. Hepatol. 2019, 4, 894–895.
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174.
- Klimeck, L.; Heisser, T.; Hoffmeister, M.; Brenner, H. Colorectal cancer: A health and economic problem. Best. Pract. Res. Clin. Gastroenterol. 2023, 101839, in press.
- Chan, S.C.H.; Liang, J.Q. Advances in tests for colorectal cancer screening and diagnosis. Expert. Rev. Mol. Diagn. 2022, 22, 449–460.
- Yao, T.; Sun, Q.; Xiong, K.; Su, Y.; Zhao, Q.; Zhang, C.; Zhang, L.; Li, X.; Fang, H. Optimization of screening strategies for colorectal cancer based on fecal DNA and occult blood testing. Eur. J. Public Health 2023, 33, 336–341.
- Ferrari, A.; Neefs, I.; Hoeck, S.; Peeters, M.; Van Hal, G. Towards Novel Non-Invasive Colorectal Cancer Screening Methods: A Comprehensive Review. Cancers 2021, 13, 1820.
- Gude, S.S.; Veeravalli, R.S.; Vejandla, B.; Gude, S.S.; Venigalla, T.; Chintagumpala, V. Colorectal Cancer Diagnostic Methods: The Present and Future. Cureus 2023, 15, e37622.
- Pickhardt, P.J. Emerging stool-based and blood-based non-invasive DNA tests for colorectal cancer screening: The importance of cancer prevention in addition to cancer detection. Abdom Radiol 2016, 41, 1441–1444.
- Li, D. Recent advances in colorectal cancer screening. Chronic Dis. Transl. Med. 2018, 4, 139–147.
- Allameh, Z.; Davari, M.; Emami, M.H. Sensitivity and Specificity of Colorectal Cancer Mass Screening Methods: A Systematic Review of the Literature. Int. J. Cancer Manag. 2011, 4, 2.
- Trevethan, R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front. Public Health 2017, 5, 307.
- Matos, A.I.; Carreira, B.; Peres, C.; Moura, L.I.F.; Conniot, J.; Fourniols, T.; Scomparin, A.; Martínez-Barriocanal, Á.; Arango, D.; Conde, J.P.; et al. Nanotechnology is an important strategy for combinational innovative chemo-immunotherapies against colorectal cancer. J. Control. Release 2019, 307, 108–138.
- Jindal, S.; Jindal, S. Applications of gold nano particle, quantum dot and magnetic nano particle. AIP Conf. Proc. 2023, 2558, 020037.
- Khan, F.A.; Albalawi, R.; Pottoo, F.H. Trends in targeted delivery of nanomaterials in colon cancer diagnosis and treatment. Med. Res. Rev. 2022, 42, 227–258.
- Ghorbani, F.; Kokhaei, P.; Ghorbani, M.; Eslami, M. Application of different nanoparticles in the diagnosis of colorectal cancer. Gene Rep. 2020, 21, 100896.
- Gogoi, P.; Kaur, G.; Singh, N.K. Nanotechnology for colorectal cancer detection and treatment. World J. Gastroenterol. 2022, 28, 6497–6511.
- Wan, J.; Zhang, X.; Fu, K.; Zhang, X.; Shang, L.; Su, Z. Highly fluorescent carbon dots as novel theranostic agents for biomedical applications. Nanoscale 2021, 13, 17236–17253.
- Raut, N.S.; Umekar, M.J.; Dhoble, S.J. 18—Quantum dots: Novel approach for biological imaging. In Quantum Dots; Thejo Kalyani, N., Dhoble, S.J., Michalska-Domańska, M., Vengadaesvaran, B., Nagabhushana, H., Arof, A.K., Eds.; Woodhead Publishing Series in Electronic and Optical Materials; Woodhead Publishing: Cambridge, UK, 2023; pp. 477–500. ISBN 978-0-323-85278-4.
- Gazouli, M.; Lyberopoulou, A.; Pericleous, P.; Rizos, S.; Aravantinos, G.; Nikiteas, N.; Anagnou, N.P.; Efstathopoulos, E.P. Development of a quantum-dot-labelled magnetic immunoassay method for circulating colorectal cancer cell detection. World J. Gastroenterol. 2012, 18, 4419–4426.
- Carbary-Ganz, J.L.; Barton, J.K.; Utzinger, U. Quantum dots targeted to vascular endothelial growth factor receptor 2 as a contrast agent for the detection of colorectal cancer. J. Biomed. Opt. 2014, 19, 086003.
- Thomas, D.T.; Baby, A.; Raman, V.; Balakrishnan, S.P. Carbon-Based Nanomaterials for Cancer Treatment and Diagnosis: A Review. ChemistrySelect 2022, 7, e202202455.
- Zhang, Y.; Wu, M.; Wu, M.; Zhu, J.; Zhang, X. Multifunctional Carbon-Based Nanomaterials: Applications in Biomolecular Imaging and Therapy. ACS Omega 2018, 3, 9126–9145.
- Yan, J.; Xue, F.; Chen, H.; Wu, X.; Zhang, H.; Chen, G.; Lu, J.; Cai, L.; Xiang, G.; Deng, Z.; et al. A multi-center study of using carbon nanoparticles to track lymph node metastasis in T1-2 colorectal cancer. Surg. Endosc. 2014, 28, 3315–3321.
- Sun, J.; Zhang, J. Assessment of lymph node metastasis in elderly patients with colorectal cancer by sentinel lymph node identification using carbon nanoparticles. J. BUON 2018, 23, 68–72.
- Cai, H.-K.; He, H.-F.; Tian, W.; Zhou, M.-Q.; Hu, Y.; Deng, Y.-C. Colorectal cancer lymph node staining by activated carbon nanoparticles suspension in vivo or methylene blue in vitro. World J. Gastroenterol. 2012, 18, 6148–6154.
- Wang, Q.; Chen, E.; Cai, Y.; Chen, C.; Jin, W.; Zheng, Z.; Jin, Y.; Chen, Y.; Zhang, X.; Li, Q. Preoperative endoscopic localization of colorectal cancer and tracing lymph nodes by using carbon nanoparticles in laparoscopy. World J. Surg. Oncol. 2016, 14, 231.
- Mirahadi, M.; Ghanbarzadeh, S.; Ghorbani, M.; Gholizadeh, A.; Hamishehkar, H. A review on the role of lipid-based nanoparticles in medical diagnosis and imaging. Ther. Deliv. 2018, 9, 557–569.
- Palei, N.N.; Mohanta, B.C.; Sabapathi, M.L.; Das, M.K. Chapter 10—Lipid-based nanoparticles for cancer diagnosis and therapy. In Organic Materials as Smart Nanocarriers for Drug Delivery; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 415–470. ISBN 978-0-12-813663-8.
- Buse, J.; El-Aneed, A. Properties, engineering and applications of lipid-based nanoparticle drug-delivery systems: Current research and advances. Nanomedicine 2010, 5, 1237–1260.
- Sun, J.; Zhang, S.; Jiang, S.; Bai, W.; Liu, F.; Yuan, H.; Ji, J.; Luo, J.; Han, G.; Chen, L.; et al. Gadolinium-Loaded Solid Lipid Nanoparticles as a Tumor-Absorbable Contrast Agent for Early Diagnosis of Colorectal Tumors Using Magnetic Resonance Colonography. J. Biomed. Nanotechnol. 2016, 12, 1709–1723.
- Alrumaihi, F.; Khan, M.A.; Babiker, A.Y.; Alsaweed, M.; Azam, F.; Allemailem, K.S.; Almatroudi, A.A.; Ahamad, S.R.; Alsugoor, M.H.; Alharbi, K.N.; et al. Lipid-Based Nanoparticle Formulation of Diallyl Trisulfide Chemosensitizes the Growth Inhibitory Activity of Doxorubicin in Colorectal Cancer Model: A Novel In Vitro, In Vivo and In Silico Analysis. Molecules 2022, 27, 2192.
- Regmi, S.; Poudel, C.; Adhikari, R.; Luo, K.Q. Applications of Microfluidics and Organ-on-a-Chip in Cancer Research. Biosensors 2022, 12, 459.
- Noor, J.; Chaudhry, A.; Batool, S. Microfluidic Technology, Artificial Intelligence, and Biosensors As Advanced Technologies in Cancer Screening: A Review Article. Cureus 2023, 15, e39634.
- Bargahi, N.; Ghasemali, S.; Jahandar-Lashaki, S.; Nazari, A. Recent advances for cancer detection and treatment by microfluidic technology, review and update. Biol. Proced. Online 2022, 24, 5.
- Kulasinghe, A.; Wu, H.; Punyadeera, C.; Warkiani, M.E. The Use of Microfluidic Technology for Cancer Applications and Liquid Biopsy. Micromachines 2018, 9, 397.
- Gauri, S.; Ahmad, M.R. ctDNA Detection in Microfluidic Platform: A Promising Biomarker for Personalized Cancer Chemotherapy. J. Sens. 2020, 2020, e8353674.
- Pinho, D.; Santos, D.; Vila, A.; Carvalho, S. Establishment of Colorectal Cancer Organoids in Microfluidic-Based System. Micromachines 2021, 12, 497.
- Oh, B.Y.; Kim, J.; Lee, W.Y.; Kim, H.C. A New Size-based Platform for Circulating Tumor Cell Detection in Colorectal Cancer Patients. Clin. Color. Cancer 2017, 16, 214–219.
- Su, W.; Yu, H.; Jiang, L.; Chen, W.; Li, H.; Qin, J. Integrated Microfluidic Device for Enrichment and Identification of Circulating Tumor Cells from the Blood of Patients with Colorectal Cancer. Dis. Markers 2019, 2019, 8945974.
- Gogoi, P.; Sepehri, S.; Zhou, Y.; Gorin, M.A.; Paolillo, C.; Capoluongo, E.; Gleason, K.; Payne, A.; Boniface, B.; Cristofanilli, M.; et al. Development of an Automated and Sensitive Microfluidic Device for Capturing and Characterizing Circulating Tumor Cells (CTCs) from Clinical Blood Samples. PLoS ONE 2016, 11, e0147400.
- Iliescu, F.S.; Vrtačnik, D.; Neuzil, P.; Iliescu, C. Microfluidic Technology for Clinical Applications of Exosomes. Micromachines 2019, 10, 392.
- Chiriacò, M.S.; Bianco, M.; Nigro, A.; Primiceri, E.; Ferrara, F.; Romano, A.; Quattrini, A.; Furlan, R.; Arima, V.; Maruccio, G. Lab-on-Chip for Exosomes and Microvesicles Detection and Characterization. Sensors 2018, 18, 3175.
- Chinnappan, R.; Ramadan, Q.; Zourob, M. An integrated lab-on-a-chip platform for pre-concentration and detection of colorectal cancer exosomes using anti-CD63 aptamer as a recognition element. Biosens. Bioelectron. 2023, 220, 114856.
- Li, P.; Chen, J.; Chen, Y.; Song, S.; Huang, X.; Yang, Y.; Li, Y.; Tong, Y.; Xie, Y.; Li, J.; et al. Construction of Exosome SORL1 Detection Platform Based on 3D Porous Microfluidic Chip and its Application in Early Diagnosis of Colorectal Cancer. Small 2023, 19, 2207381.
- Wang, H.; Chen, H.; Huang, Z.; Li, T.; Deng, A.; Kong, J. DNase I enzyme-aided fluorescence signal amplification based on graphene oxide-DNA aptamer interactions for colorectal cancer exosome detection. Talanta 2018, 184, 219–226.
- Ogunwobi, O.O.; Mahmood, F.; Akingboye, A. Biomarkers in Colorectal Cancer: Current Research and Future Prospects. Int. J. Mol. Sci. 2020, 21, 5311.
- Koncina, E.; Haan, S.; Rauh, S.; Letellier, E. Prognostic and Predictive Molecular Biomarkers for Colorectal Cancer: Updates and Challenges. Cancers 2020, 12, 319.
- Armistead, F.J.; Gala De Pablo, J.; Gadêlha, H.; Peyman, S.A.; Evans, S.D. Physical Biomarkers of Disease Progression: On-Chip Monitoring of Changes in Mechanobiology of Colorectal Cancer Cells. Sci. Rep. 2020, 10, 3254.
- Ortega, F.G.; Gomez, G.E.; Boni, C.; García, I.C.; Navas, C.G.; D’vries, R.F.; Molina Vallejos, M.P.; Serrano, M.J.; Messina, G.A.; Hernández, J.E.; et al. Microfluidic amperometric immunosensor based on porous nanomaterial towards claudin7 determination for colorectal cancer diagnosis. Talanta 2023, 251, 123766.
- Ramos, P.; Carvalho, M.R.; Chen, W.; Yan, L.-P.; Zhang, C.-H.; He, Y.; Reis, R.L.; Oliveira, J.M. Microphysiological systems to study colorectal cancer: State-of-the-art. Biofabrication 2023, 15, 032001.
- Mathur, L.; Ballinger, M.; Utharala, R.; Merten, C.A. Microfluidics as an Enabling Technology for Personalized Cancer Therapy. Small 2020, 16, 1904321.
- Thakur, N.; Yoon, H.; Chong, Y. Current Trends of Artificial Intelligence for Colorectal Cancer Pathology Image Analysis: A Systematic Review. Cancers 2020, 12, 1884.
- Yu, C.; Helwig, E.J. The role of AI technology in prediction, diagnosis and treatment of colorectal cancer. Artif. Intell. Rev. 2022, 55, 323–343.
- Quinchia, J.; Echeverri, D.; Cruz-Pacheco, A.F.; Maldonado, M.E.; Orozco, J. Electrochemical Biosensors for Determination of Colorectal Tumor Biomarkers. Micromachines 2020, 11, 411.
- Özyurt, C.; Uludağ, İ.; İnce, B.; Sezgintürk, M.K. Lab-on-a-chip systems for cancer biomarker diagnosis. J. Pharm. Biomed. Anal. 2023, 226, 115266.
- Tang, Y.; Duan, F.; Zhou, A.; Kanitthamniyom, P.; Luo, S.; Hu, X.; Jiang, X.; Vasoo, S.; Zhang, X.; Zhang, Y. Image-based real-time feedback control of magnetic digital microfluidics by artificial intelligence-empowered rapid object detector for automated in vitro diagnostics. Bioeng. Transl. Med. 2023, 8, e10428.
- Bhuiyan, N.H.; Hong, J.H.; Uddin, M.J.; Shim, J.S. Artificial Intelligence-Controlled Microfluidic Device for Fluid Automation and Bubble Removal of Immunoassay Operated by a Smartphone. Anal. Chem. 2022, 94, 3872–3880.
- Mejía-Salazar, J.R.; Rodrigues Cruz, K.; Materón Vásques, E.M.; Novais de Oliveira, O., Jr. Microfluidic Point-of-Care Devices: New Trends and Future Prospects for eHealth Diagnostics. Sensors 2020, 20, 1951.
- Zare Harofte, S.; Soltani, M.; Siavashy, S.; Raahemifar, K. Recent Advances of Utilizing Artificial Intelligence in Lab on a Chip for Diagnosis and Treatment. Small 2022, 18, 2203169.
- Liu, Y.; Li, S.; Liu, Y. Machine Learning-Driven Multiobjective Optimization: An Opportunity of Microfluidic Platforms Applied in Cancer Research. Cells 2022, 11, 905.
- You, Y.; Lai, X.; Pan, Y.; Zheng, H.; Vera, J.; Liu, S.; Deng, S.; Zhang, L. Artificial intelligence in cancer target identification and drug discovery. Sig. Transduct. Target. Ther. 2022, 7, 156.
- Wang, Q.; Wei, J.; Chen, Z.; Zhang, T.; Zhong, J.; Zhong, B.; Yang, P.; Li, W.; Cao, J. Establishment of multiple diagnosis models for colorectal cancer with artificial neural networks. Oncol. Lett. 2019, 17, 3314–3322.
- Cahill, R.A.; O’Shea, D.F.; Khan, M.F.; Khokhar, H.A.; Epperlein, J.P.; Mac Aonghusa, P.G.; Nair, R.; Zhuk, S.M. Artificial intelligence indocyanine green (ICG) perfusion for colorectal cancer intra-operative tissue classification. Br. J. Surg. 2021, 108, 5–9.
- Wang, K.S.; Yu, G.; Xu, C.; Meng, X.H.; Zhou, J.; Zheng, C.; Deng, Z.; Shang, L.; Liu, R.; Su, S.; et al. Accurate diagnosis of colorectal cancer based on histopathology images using artificial intelligence. BMC Med. 2021, 19, 76.
- Smit, M.A.; Mesker, W.E. The role of artificial intelligence to quantify the tumour-stroma ratio for survival in colorectal cancer. EBioMedicine 2020, 61, 103070.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.7K
Revisions:
2 times
(View History)
Update Date:
17 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





