
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Teddie Onkabetse Rahube | -- | 3619 | 2023-10-16 12:40:30 | | | |
| 2 | Peter Tang | + 1 word(s) | 3620 | 2023-10-17 03:03:51 | | |
Video Upload Options
Antimicrobial resistance is considered a “One-Health” problem, impacting humans, animals, and the environment. The problem of the rapid development and spread of bacteria resistant to multiple antibiotics is a rising global health threat affecting both rich and poor nations. Low- and middle-income countries are at highest risk, in part due to the lack of innovative research on the surveillance and discovery of novel therapeutic options. Fast and effective drug discovery is crucial towards combatting antimicrobial resistance and reducing the burden of infectious diseases. African medicinal plants have been used for millennia in folk medicine to cure many diseases and ailments. Over 10% of the Southern African vegetation is applied in traditional medicine, with over 15 species being partially or fully commercialized. These include the genera Euclea, Ficus, Aloe, Lippia. And Artemisia, amongst many others. Bioactive compounds from indigenous medicinal plants, alone or in combination with existing antimicrobials, offer promising solutions towards overcoming multi-drug resistance. Secondary metabolites have different mechanisms and modes of action against bacteria, such as the inhibition and disruption of cell wall synthesis; inhibition of DNA replication and ATP synthesis; inhibition of quorum sensing; inhibition of AHL or oligopeptide signal generation, broadcasting, and reception; inhibition of the formation of biofilm; disruption of pathogenicity activities; and generation of reactive oxygen species.
1. Introduction
2. Clinically Important Multi-Drug-Resistant Bacteria and Modes of Antibiotic Resistance
3. Diversity and Distribution of African Medicinal Plants with Potential Antimicrobial Properties
4. Plant Secondary Metabolites with Antimicrobial Potential
4.1. Alkaloids
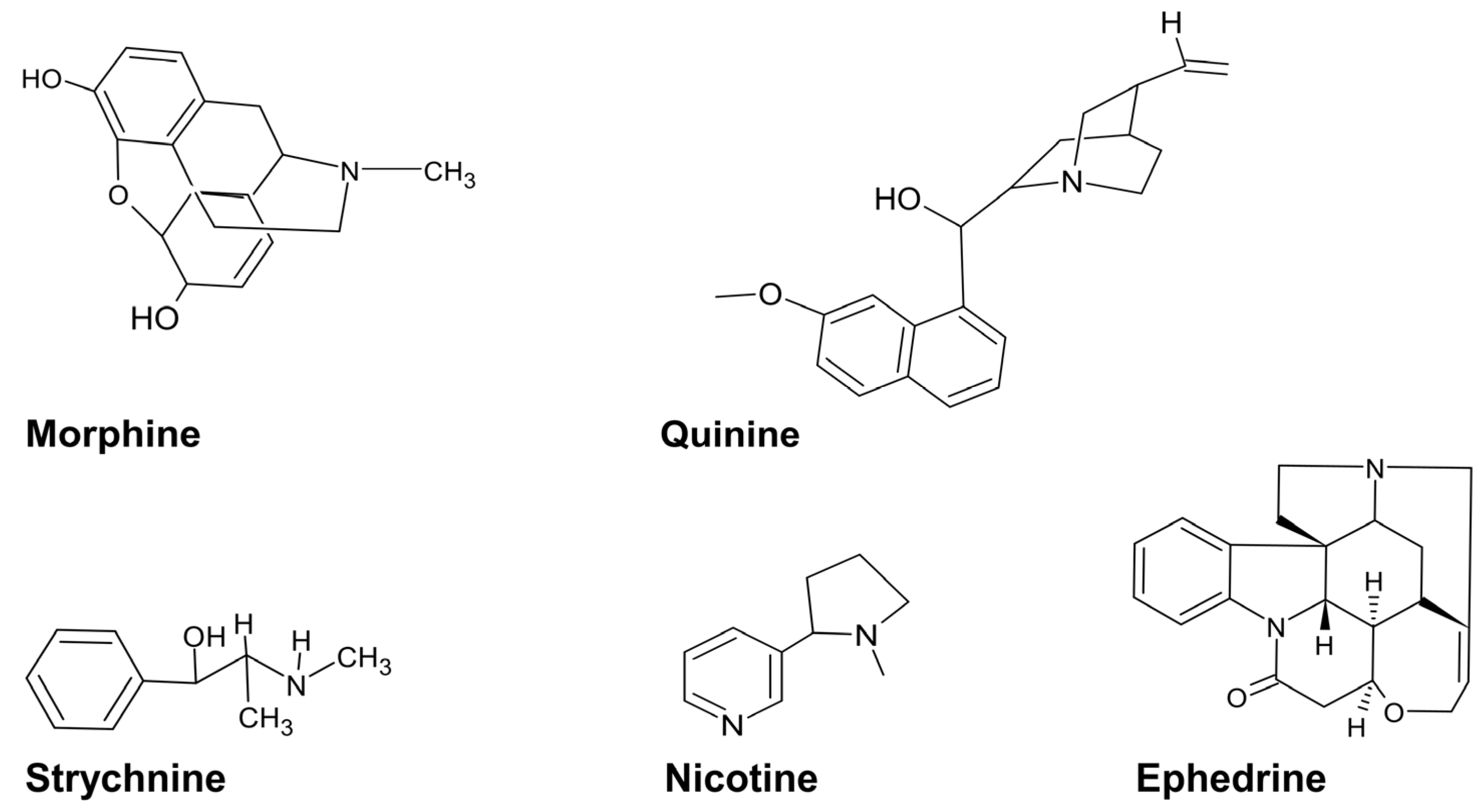
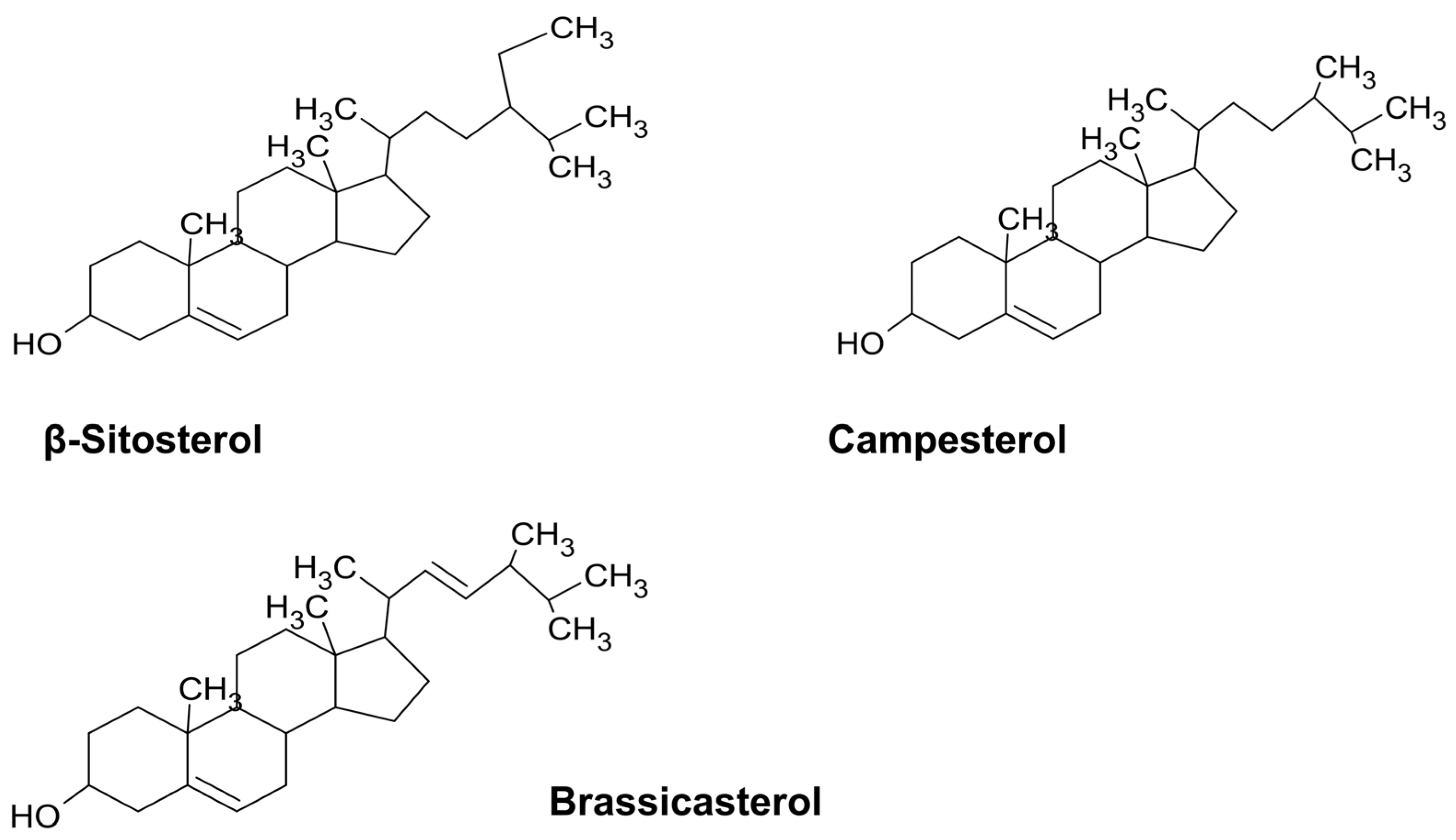
4.2. Polyphenols
4.3. Terpenes
5. Therapeutic Potential of Traditional Medicinal Plants against MDR Bacteria
References
- Littmann, J.; Buyx, A.; Cars, O. Antibiotic resistance: An ethical challenge. Int. J. Antimicrob. Agents 2015, 46, 359–361.
- Kongkham, B.; Prabakaran, D.; Puttaswamy, H. Opportunities and challenges in managing antibiotic resistance in bacteria using plant secondary metabolites. Fitoterapia 2020, 147, 104762.
- Ibrahim, N.; Kebede, A. In vitro antibacterial activities of methanol and aqueous leave extracts of selected medicinal plants against human pathogenic bacteria. Saudi J. Biol. Sci. 2020, 27, 2261–2268.
- Demgne, O.M.; Tchinda, C.F.; Mbaveng, A.T.; Beng, V.P.; Kuete, V. Antibacterial and antibiotic-potentiating activities of nine Cameroonian medicinal plants against multidrug-resistant bacteria expressing active efflux pumps. Investig. Med. Chem. Pharmacol. 2022, 5, 58.
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the antibacterial mechanism of plant-derived compounds against multidrug-resistant bacteria (MDR). Evid.-Based Complement. Altern. Med. 2021, 2021, 3663315.
- Van Duin, D.; Paterson, D.L. Multidrug-resistant bacteria in the community: Trends and lessons learned. Infect. Dis. Clin. 2016, 30, 377–390.
- Bhatia, P.; Sharma, A.; George, A.J.; Anvitha, D.; Kumar, P.; Dwivedi, V.P.; Chandra, N.S. Antibacterial activity of medicinal plants against ESKAPE: An update. Heliyon 2021, 7, e06310.
- Blake, K.S.; Choi, J.; Dantas, G. Approaches for characterizing and tracking hospital-associated multidrug-resistant bacteria. Cell Mol. Life Sci. 2021, 78, 2585–2606.
- Kebede, T.; Gadisa, E.; Tufa, A. Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: A possible alternative in the treatment of multidrug-resistant microbes. PLoS ONE 2021, 16, e0249253.
- Raskin, I.; Ribnicky, D.M.; Komarnytsky, S.; Ilic, N.; Poulev, A.; Borisjuk, N.; Brinker, A.; Moreno, D.A.; Ripoll, C.; Yakoby, N.; et al. Plants and human health in the twenty-first century. Trends Biotechnol. 2002, 20, 522–531.
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The role and place of medicinal plants in the strategies for disease prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210–229.
- Soulaimani, B.; El Hidar, N.; El Fakir, S.B.; Mezrioui, N.; Hassani, L.; Abbad, A. Combined antibacterial activity of essential oils extracted from Lavandula maroccana (Murb.), Thymus pallidus Batt. and Rosmarinus officinalis L. against antibiotic-resistant Gram-negative bacteria. Eur. J. Integr. Med. 2021, 43, 101312.
- Song, M.; Liu, Y.; Li, T.; Liu, X.; Hao, Z.; Ding, S.; Panichayupakaranant, P.; Zhu, K.; Shen, J. Plant natural flavonoids against multidrug resistant pathogens. Adv. Sci. 2021, 8, 2100749.
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. EHP 2001, 109, 69–75.
- Rout, S.; Choudary, K.; Kar, D.; Das, L.; Jain, A. Plants in traditional medicinal system-future source of new drugs. Int. J. Pharm.Sci. 2009, 1, 1–23. Available online: https://www.researchgate.net/publication/26626847_Plants_in_traditional_medicinal_system-Future_source_on_new_drugs (accessed on 14 July 2023).
- Terreni, M.; Taccani, M.; Pregnolato, M. New antibiotics for multidrug-resistant bacterial strains: Latest research developments and future perspectives. Molecules 2021, 26, 2671.
- Ballot, D.E.; Bandini, R.; Nana, T.; Bosman, N.; Thomas, T.; Davies, V.A.; Cooper, P.A.; Mer, M.; Lipman, J. A review of-multidrug-resistant Enterobacteriaceae in a neonatal unit in Johannesburg, South Africa. BMC Pediatr. 2019, 19, 320.
- Adesanya, O.A.; Igwe, H.A. Carbapenem-resistant Enterobacteriaceae (CRE) and Gram-negative bacterial infections in south-west Nigeria: A retrospective epidemiological surveillance study. AIMS Pub. Health 2020, 7, 804.
- Orababa, O.Q.; Arowolo, M.T.; Olaitan, M.O.; Osibeluwo, B.V.; Essiet, U.U.; Batholomew, O.H.; Ogunrinde, O.G.; Lagoke, O.A.; Soriwe, J.D.; Ishola, O.D.; et al. Prevalence Of carbapenem resistance in Acinetobacter baumanii and Pseudomonas aeruginosa in sub-Saharan Africa: A systematic review and meta-analysis. medRxiv 2022, 11, 22282516.
- Tolba, S.; El Shatoury, E.H.; Abo AlNasr, N.M. Prevalence of carbapenem resistant acinetobacter baumannii (CRAB) in some Egyptian hospitals: Evaluation of the use of blaOXA-51-like gene as species specific marker for CRAB. Egypt. J. Bot. 2019, 59, 723–733.
- Hosu, M.C.; Vasaikar, S.D.; Okuthe, G.E.; Apalata, T. Detection of extended spectrum beta-lactamase genes in Pseudomonas aeruginosa isolated from patients in rural Eastern Cape Province, South Africa. Sci. Rep. 2021, 11, 7110.
- Kindu, M.; Derseh, L.; Gelaw, B.; Moges, F. Carbapenemase-producing non-glucose-fermenting Gram-negative Bacilli in Africa, Pseudomonas aeruginosa and Acinetobacter baumannii: A systematic review and meta-analysis. Int. J. Microbiol. 2020, 2020, 9461901.
- Al-Orphaly, M.; Hadi, H.A.; Eltayeb, F.K.; Al-Hail, H.; Samuel, B.G.; Sultan, A.A.; Skariah, S. Epidemiology of multidrug-resistant Pseudomonas aeruginosa in the Middle East and North Africa Region. Msphere 2021, 6, e00202–e00221.
- Shrivastava, S.R.; Shrivastava, P.S.; Ramasamy, J. World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. J. Med. Soc. 2018, 32, 76–77.
- Melese, A.; Genet, C.; Andualem, T. Prevalence of Vancomycin resistant enterococci (VRE) in Ethiopia: A systematic review and meta-analysis. BMC Infect. Dis. 2020, 20, 124.
- Orababa, O.Q.; Soriwei, J.D.; Akinsuyi, S.O.; Essiet, U.U.; Solesi, O.M. A systematic review and meta-analysis on the prevalence of vancomycin-resistant enterococci (VRE) among Nigerians. Porto. Biomed. J. 2021, 6, e125.
- Mkhize, S.; Amoako, D.G.; Shobo, C.O.; Zishiri, O.T.; Bester, L.A. Genotypic and Phenotypic Characterizations of Methicillin-Resistant Staphylococcus aureus (MRSA) on Frequently Touched Sites from Public Hospitals in South Africa. Int. J. Microbiol. 2021, 2021, 6011045.
- Omoshaba, E.; Ojo, O.; Oyekunle, M.; Sonibare, A.; Adebayo, A. Methicillin-resistant Staphylococcus aureus (MRSA) isolated from raw milk and nasal swabs of small ruminants in Abeokuta, Nigeria. Trop. Anim. Health. Prod. 2020, 52, 2599–2608.
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661.
- Goda, K.; Kenzaka, T.; Hoshijima, M.; Yachie, A.; Akita, H. Toxic shock syndrome with a cytokine storm caused by Staphylococcus simulans: A case report. BMC Infect. Dis. 2021, 21, 19.
- Taylor, T.A.; Unakal, C.G. Staphylococcus aureus Infection; StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2022; Available online: https://www.ncbi.nlm.nih.gov/books/NBK441868/ (accessed on 14 July 2023).
- Adeiza, S.S.; Onaolapo, J.A.; Olayinka, B.O. Prevalence, risk-factors, and antimicrobial susceptibility profile of methicillin-resistant Staphylococcus aureus (MRSA) obtained from nares of patients and staff of Sokoto state-owned hospitals in Nigeria. GMS Hyg. Infect. Control. 2020, 15, Doc25.
- Parmanik, A.; Das, S.; Kar, B.; Bose, A.; Dwivedi, G.R.; Pandey, M.M. Current treatment strategies against multidrug-resistant bacteria: A review. Curr. Microbiol. 2022, 79, 388.
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109.
- Bromley, A.; Cock, I. Antibacterial Activity of Harpagophytum procumbens (Burch.) DC. ex Meisn. Root Extracts against Gastrointestinal Pathogens and Bacterial Triggers of Autoimmune Diseases. Pharmacogn. Commn. 2022, 12, 14–22.
- Endris, A.; Asfaw, N.; Bisrat, D. Chemical composition, antimicrobial and antioxidant activities of the essential oil of Lippia javanica leaves from Ethiopia. J. Essenti. Oil. Res. 2016, 28, 221–226.
- Hikaambo, C.N.; Chisanga, T.; Kampamba, M.; Akapelwa, T.M.; Chimombe, T.; Chulu, M.; Nanyangwe, N.; Kabuka, R.; Mudenda, S. Antibacterial Activity of Cassia abbreviata Oliv Bark Extract against Escherichia coli and Staphylococcus aureus. J. Pharma. Res. Sci. Technol. 2022, 6, 76–83.
- Kambiz, L.; Afolayan, A. Extracts from Aloe ferox and Withania somnifera inhibit Candida albicans and Neisseria gonorrhoea. Afr. J. Biotechnol. 2008, 7, 12–15.
- Siddiqui, N.A.; Al-Yousef, H.M.; Alhowiriny, T.A.; Alam, P.; Hassan, W.; Amina, M.; Hussain, A.; Abdelaziz, S.; Abdallah, R.H. Concurrent analysis of bioactive triterpenes oleanolic acid and β-amyrin in antioxidant active fractions of Hibiscus calyphyllus, Hibiscus deflersii and Hibiscus micranthus grown in Saudi Arabia by applying validated HPTLC method. Saudi. Pharm. J. 2018, 26, 266–273.
- Mongalo, N.; Mafoko, B. Cassia abbreviata Oliv. A review of its ethnomedicinal uses, toxicology, phytochemistry, possible propagation techniques and Pharmacology. Afr. J. Pharm. Pharmacol. 2013, 7, 2901–2906.
- Danley, K. Letters of the bush: A case study of traditional Setswana herbal medicine. ISP Collect. 2006, 270, 1–30. Available online: https://digitalcollections.sit.edu/isp_collection/270 (accessed on 14 July 2023).
- du Toit, A.; van der Kooy, F. Artemisia afra, a controversial herbal remedy or a treasure trove of new drugs? J. Ethnoph. 2019, 244, 112127.
- Kane, N.; Kyama, M.; Nganga, J.; Hassanali, A.; Diallo, M.; Kimani, F. Comparison of phytochemical profiles and antimalarial activities of Artemisia afra plant collected from five countries in Africa. Afr. J. Bot. 2019, 125, 126–133.
- Liu, C.; Huan, H.; Zhou, Q.; Liu, B.; Wang, Y.; Li, P.; Liao, K.; Su, W. Antibacterial and antibiotic synergistic activities of the extract from Pithecellobium clypearia against clinically important multidrug-resistant gram-negative bacteria. Eur. J. Integr. Med. 2019, 32, 100999.
- Asowata-Ayodele, A.M.; Otunola, G.A.; Afolayan, A.J. Assessment of the polyphenolic content, free radical scavenging, anti-inflammatory, and antimicrobial activities of acetone and aqueous extracts of Lippia javanica (Burm. F.) spreng. Pharmacogn. Mag. 2016, 12, S353.
- Motlhanka, D.M.; Makhabu, S.W. Medicinal and edible wild fruit plants of Botswana as emerging new crop opportunities. J. Med. Plants Res. 2011, 5, 1836–1842.
- Mncwangi, N.; Chen, W.; Vermaak, I.; Viljoen, A.M.; Gericke, N. Devil’s Claw—A review of the ethnobotany, phytochemistry and biological activity of Harpagophytum procumbens. J. Ethnopharnacol. 2012, 143, 755–771.
- Chen, W.; Van Wyk, B.E.; Vermaak, I.; Viljoen, A.M. Cape aloes—A review of the phytochemistry, pharmacology and commercialisation of Aloe ferox. Phytochem. Lett. 2012, 5, 1–12.
- Nalimu, F.; Oloro, J.; Kahwa, I.; Ogwang, P.E. Review on the phytochemistry and toxicological profiles of Aloe vera and Aloe ferox. Futur. J. Pharm. Sci. 2021, 7, 145.
- Maroyi, A. Boscia albitrunca: Review of its botany, medicinal uses, phytochemistry, and biological activities. Asian. Pac. J. Trop. Med. 2019, 12, 51–56.
- Madzibane, J.; Potgieter, M. Uses of Colophospermum mopane (Leguminosae: Caesalpinioideae) by the Vhavenda. S. Afr. J. Bot. 1999, 65, 440–444.
- Bakrim, W.B.; Nurcahyanti, A.D.R.; Dmirieh, M.; Mahdi, I.; Elgamal, A.M.; El Raey, M.A.; Wink, M.; Sobeth, M. Phytochemical Profiling of the Leaf Extract of Ximenia Americana Var. Caffra and Its Antioxidant, Antibacterial, and Antiaging Activities In Vitro and in Caenorhabditis Elegans: A Cosmeceutical and Dermatological Approach. Oxid. Med. Cell Longev. 2022, 2022, 3486257.
- Majinda, R.R.; Motswaledi, M.S. Antibiotic activity of selected Botswana medicinal plants. Botsw. Notes Rec. 1998, 30, 157–162.
- Maroyi, A. Ximenia caffra Sond.(Ximeniaceae) in sub-Saharan Africa: A synthesis and review of its medicinal potential. J. Ethnopharmacol. 2016, 184, 81–100.
- Steenkamp, V.; Fernandes, A.C.; Jansen van Rensburg, C.E. Antibacterial activity of Venda medicinal plants. Fitoterapia 2007, 78, 561–564.
- Banso, A.; Adeyemo, S. Evaluation of antibacterial properties of tannins isolated from Dichrostachys cinerea. Afr. J. Biotechnol. 2007, 6, 15.
- Neondo, J.O.; Mbithe, C.M.; Njenga, P.K.; Muthuri, C.W. Phytochemical characterization, antibacterial screening and toxicity evaluation of Dichrostachys cinerea. Int. J. Med. Plant. Res. 2012, 1, 32–37.
- Gebrehiwot, S.; Chaithanya, K.K. Traditional uses, phytochemistry, and pharmacological properties of Capparis tomentosa Lam.: A review. Drug Invent. 2020, 13, 1006–10011.
- Steenkamp, V.; Mathivha, E.; Gouws, M.; Van Rensburg, C. Studies on antibacterial, antioxidant and fibroblast growth stimulation of wound healing remedies from South Africa. J. Ethnopharmacol. 2004, 95, 353–357.
- Ahmed, A.S.; McGaw, L.J.; Moodley, N.; Naidoo, V.; Eloff, J.N. Cytotoxic, antimicrobial, antioxidant, antilipoxygenase activities and phenolic composition of Ozoroa and Searsia species (Anacardiaceae) used in South African traditional medicine for treating diarrhoea. S. Afr. J. Bot. 2014, 95, 9–18.
- Maroyi, A. Euclea undulata Thunb.: Review of its botany, ethnomedicinal uses, phytochemistry and biological activities. Asian Pac. J. Trop. Med. 2017, 10, 1030–1036.
- Mbanga, J.; Ncube, M.; Magumura, A. Antimicrobial activity of Euclea undulata, Euclea divinorum and Diospyros lycioides extracts on multi-drug resistant Streptococcus mutans. J. Med. Plant. Res. 2013, 7, 2741–2746.
- Mongalo, N.; McGaw, L.; Segapelo, T.; Finnie, J.; Van Staden, J. Ethnobotany, phytochemistry, toxicology and pharmacological properties of Terminalia sericea Burch. ex DC. (Combretaceae)—A review. J. Ethnopharmacol 2016, 194, 789–802.
- Akindele, A.J.; Wani, Z.A.; Sharma, S.; Mahajan, G.; Satti, N.K.; Adeyemi, O.O.; Mondhe, D.M.; Saxena, A.K. In vitro and in vivo anticancer activity of root extracts of Sansevieria liberica Gerome and Labroy (Agavaceae). Evid.-Based Complement. Altern. Med. 2015, 2015, 560404.
- Moshi, M.; Mbwambo, Z. Some pharmacological properties of extracts of Terminalia sericea roots. J. Ethnopharmacol. 2005, 97, 43–47.
- Tkachenko, H.; Buyun, L.; Osadowski, Z.; Maryniuk, M. The Antibacterial Activity of Certain Sansevieria Thunb. species against Escherichia coli. Agrobiodivers. Improv. Nutr. Health Life Qual. 2017, 446–453.
- Hübsch, Z.; Van Zyl, R.; Cock, I.; Van Vuuren, S. Interactive antimicrobial and toxicity profiles of conventional antimicrobials with Southern African medicinal plants. S. Afr. J. Bot. 2014, 93, 185–197.
- Viljoen, A.M.; Subramoney, S.; van Vuuren, S.F.; Başer, K.; Demirci, B. The composition, geographical variation and antimicrobial activity of Lippia javanica (Verbenaceae) leaf essential oils. J. Ethnopharmacol. 2005, 96, 271–277.
- Ding, J.; Wang, L.; He, C.; Zhao, J.; Si, L.; Huang, H. Artemisia scoparia: Traditional uses, active constituents and pharmacological effects. J. Ethnopharmacol. 2021, 273, 113960.
- Hussain, M.S.; Fareed, S.; Ansari, S.; Rahman, M.A.; Ahmad, I.Z.; Saeed, M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied. Sci. 2012, 4, 10.
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research progress on antibacterial activities and mechanisms of natural alkaloids: A review. Antibiotics 2021, 10, 318.
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019, 10, 911.
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food. Biochem. 2022, 46, e14264.
- Ali, A.; Parisi, A.; Normanno, G. Polyphenols as emerging antimicrobial agents. In Emerging Modalities in Mitigation of Antimicrobial Resistance; Akhtar, N., Singh, K.S., Prerna Goyal, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 219–259.
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Encinar, J.A.; Rodríguez-Díaz, J.C.; Micol, V. Antimicrobial capacity of plant polyphenols against gram-positive bacteria: A comprehensive review. Curr. Med. Chem. 2020, 27, 2576–2606.
- Boncan, D.A.T.; Tsang, S.S.; Li, C.; Lee, I.H.; Lam, H.M.; Chan, T.F.; Hui, J.H.L. Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 2020, 21, 7382.
- Abdallah, I.I.; Quax, W.J. A Glimpse into the Biosynthesis of Terpenoids. KnE Life Sci. 2017, 3, 81–98.
- Gach, K.; Długosz, A.; Janecka, A. The role of oxidative stress in anticancer activity of sesquiterpene lactones. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015, 388, 477–486.
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555.
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471.
- Erhabor, C.; Erhabor, J.; McGaw, L. The potential of South African medicinal plants against microbial biofilm and quorum sensing of foodborne pathogens: A review. S. Afr. J. Bot. 2019, 126, 214–231.
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1.
- Alamgir, A. Pharmacognostical Botany: Classification of medicinal and aromatic plants (maps), botanical taxonomy, morphology, and anatomy of drug plants. In Therapeutic Use of Medicinal Plants and Their Extracts: Volume 1: Pharmacogn; Springer: Cham, Switzerland, 2017; pp. 177–293.
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176.
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258.
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567.
- Vadhana, P.; Singh, B.R.; Bharadwaj, M.; Singh, S.V. Emergence of herbal antimicrobial drug resistance in clinical bacterial isolates. Pharm. Anal. Acta 2015, 6, 10.
- Chabán, M.F.; Karagianni, C.; Joray, M.B.; Toumpa, D.; Sola, C.; Crespo, M.I.; Palacios, S.M.; Athanassopoulos, C.M.; Carpinella, M.C. Antibacterial effects of extracts obtained from plants of Argentina: Bioguided isolation of compounds from the anti-infectious medicinal plant Lepechinia meyenii. J. Ethnopharmacol. 2019, 239, 111930.
- Ganesan, K.; Xu, B. A critical review on polyphenols and health benefits of black soybeans. Nutrients 2017, 9, 455.
- Ganesan, K.; Xu, B. A critical review on phytochemical profile and health promoting effects of mung bean (Vigna radiata). Food. Sci. Hum. Wellness 2018, 7, 11–33.
- Ganesh, P.S.; Rai, V.R. Attenuation of quorum-sensing-dependent virulence factors and biofilm formation by medicinal plants against antibiotic resistant Pseudomonas aeruginosa. J. Tradit. Complement. Med. 2017, 8, 170–177.
- Gledhill, J.R.; Montgomery, M.G.; Leslie, A.G.; Walker, J.E. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc. Natl. Acad. Sci. USA 2007, 104, 13632–13637.
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272.
- Gregoire, S.; Singh, A.; Vorsa, N.; Koo, H. Influence of cranberry phenolics on glucan synthesis by glucosyltransferases and Streptococcus mutans acidogenicity. J. Appl. Micribiol. 2007, 103, 1960–1968.




