| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michał Filipiak | + 3386 word(s) | 3386 | 2020-04-03 14:21:01 | | | |
| 2 | Michał Filipiak | + 7 word(s) | 3393 | 2020-04-03 16:32:26 | | | | |
| 3 | Vicky Zhou | -22 word(s) | 3371 | 2020-10-30 10:13:48 | | | | |
| 4 | Catherine Yang | Meta information modification | 3371 | 2020-11-05 11:32:50 | | |
Video Upload Options
For all bee species, the pollen quality determines the overall quality of the larval food, influences the development of individuals and shapes their populations. However, not all plants produce pollen that fully satisfies the nutritional requirements of bees. Lack of understanding of the nutritional requirements of wild bees may lead to unintended negative effects of conservation efforts. Ecological stoichiometry provides an approach to better understand the nutritional constraints on growing and developing organisms and their colonies and populations. It makes reference to elements that, if scarce in the environment, prevent the construction of biologically important organic molecules. The least understood aspect of the nutritional requirements of bees concerns stoichiometric balancing and the need for adequate ratios of nutritional elements in consumed food. This text provides theoretical foundation for the project aiming at determining the likely limitations imposed on wild bees by the lack of nutritionally balanced pollen.
The following hypotheses may be tested:
1. Pollen stoichiometry vary among plant species and populations but will differ more widely among species than within different populations of the same species.
2. The stoichiometry of bees will vary substantially among bee species and between sexes within a species, which suggests the existence of different nutritional demands. Therefore, it is expected that the stoichiometric mismatches experienced by bees will vary in a species-specific and sex-specific manner.
3. For a given bee species, specific pollen species allow the overcoming of stoichiometric mismatches and will balance the diet. Accordingly, it is expected expect that flora diversity and, thus, pollen diversity matches the stoichiometric niches of bees.
I predict that the occurrence of specific key host plant species that produce stoichiometrically desirable pollen allows bees to stoichiometrically balance their diets. The project may ask if and how floral diversity, particularly the accessibility of nutritionally desirable key species, may influence bee populations. The obtained data will allow the parameterization of a conceptual model of nutritional limitations (to be developed in the future), which will enable to predict how floral community influences wild bee populations via supply and demand of nutrients.
1. Introduction
Having access to balanced food resources is crucial for the development, health and fitness of bees – which are essential pollinators that provide valuable ecosystem services [1] [2]. Alarmingly, wild bee populations are in decline due to the loss of floral diversity and a subsequent decrease in the nutritional quality of floral resources, such as pollen [1]. Pollen provides bees their primary source of nutrients and, therefore, is key for bee larval development, immunity, physiology, and adult reproduction [2]. Pollen quality, however, varies widely across space and time and among plant species, and bees must select pollen that meets their nutritional demands [2]. Similarly, the nutritional needs of different bee species and between larvae and adults vary extensively, which imply different nutritional constraints across species and along bee ontogeny [3] [4].
2. The Goal of the Project
Although floral diversity has been identified as an important factor for the fitness and population growth of pollinators, not all plants produce pollen that satisfies the nutritional requirements of bee larvae, and only some key pollen host plants provide balanced larval diets [3] [4]. The goal of the project is to determine the nutritional limitations imposed on wild bees by the lack of nutritionally balanced pollen. Therefore, one may (i) determine the nutritional value of the pollen produced by various plant species and (ii) estimate the nutritional needs of different species of wild bees. Finally, the combination of both research aims will enable to (iii) assess nutritional bottlenecks as determinants of the biodiversity of wild bee species.
3. Bee Nutritional Demands: Nutritional Ecology Approach
Nectar-feeding adult bees are energetically limited, whereas larvae are limited by the availability of body-building nutrients in their food, namely, pollen. Although floral diversity has been identified as an important factor that promotes the fitness and population growth of bees, it may act indirectly, i.e., via among-species differences in pollen nutritional quality [3] [4]. Bee larvae are highly vulnerable to changes in pollen due to their reliance on a fixed quantity and quality of food provided to them by adults. Therefore, even if adult bees have unlimited access to energy-rich nectar, bee populations and communities are shaped during the larval life stage by potential nutritional limitations that result from an unbalanced nutritional composition of pollen. Despite the importance of bee larvae-pollen quality interactions, the studies that consider bee nutrition have focused mostly on adults [2] [4]. Moreover, as studies of pollinators have mainly considered the domesticated bee, Apis mellifera L., the data related to wild species are limited, and a focus on the drivers of the current decline in the populations of wild native pollinators is needed [1] [5] [6].
The framework of ecological stoichiometry is a promising conceptual approach that helps to address an underlying mechanism of the nutritional ecology of bees, i.e., balancing the larval diet to enable larval growth, development and pupation into the adult body equipped with all the structures needed for maximal fitness [2]. The growth and development of every cell, tissue, organism and population is subject to the law of conservation of mass. Thus, organisms build their bodies by relying on thousands of chemical reactions, all of which must be chemically balanced. Therefore, an insufficient supply of certain elements in the food will likely prevent the production of tissues and physiologically important molecules that are constructed from this food either by the consumer itself or by its microbiota [2]. The demand for the resources used for growth and development is reflected in the stoichiometry of living organisms [7], i.e., the elemental phenotype of an organism [8]. Organismal stoichiometry is regulated homeostatically and is species-specific because it is a consequence of the particular structure, physiology and metabolism of different taxa [9] [10][11]. Consequently, a mismatch between the elemental phenotype of an organism’s adult body and its larval food is expected to have negative fitness consequences [7] [12]. This phenomenon is called stoichiometric mismatch and is mathematically understood as much lower proportions of Carbon:nutrient in the consumer’s body than in its food [11][13].
As living organisms require a diversity of nutrients to perform their biological functions, these functions can be limited by multiple nutrients simultaneously, where a shortfall of more than one chemical element can limit individual performance, population growth, and ecosystem function [14]. Ecological stoichiometry is a multivariate approach that uses multiple chemical elements as a metric [11]. Therefore, ecological stoichiometry can provide additional predictive power and complement traditional approaches to understand the role of nutrition in consumer-resource interactions [11]. However, after 30 years of the development of the framework of ecological stoichiometry and almost 20 years after the seminal book of Sterner and Elser [11] was published, the data on elemental phenotypes of a diversity of organisms are scarce and lag far behind the data on their genomes [14].
One way to understand the role of nutrient demands by consumers and supply by their resources is to use the approaches proposed by the biogeochemical niche and stoichiometric niche related to ecological stoichiometry [10] [15]. The stoichiometric niche is defined as the region of multivariate stoichiometric space (i.e., multiple elements) occupied by a group of individuals and/or species that helps to define their niche [10] [15]. As consumer species differ in their stoichiometries, to obtain stoichiometrically balanced food, they should prefer specific food sources that supply nutrients in the correct stoichiometric proportion. The current knowledge on pollen stoichiometry is based on only 14 studies on 85 pollen species that have been investigated over the last 80 years [3] [4]. From these 14 studies, only 5 contained data on hand-collected pollen (the majority of the studies considered honey bee-collected pollen pellets that contained pollen “contaminated” with nectar). Furthermore, the carbon (C) concentration, which is crucial for organisms to obtain energy and in stoichiometry, to quantify energy-nutrient imbalances, has never been reported for pollen apart from our own studies on polyfloral pollen loads collected by O. bicornis and A. mellifera [3][4][16]. The elemental phenotypes of only two bee species, namely, domesticated A. mellifera and wild O. bicornis, have been studied to date [3][4][16] and provided valuable new knowledge applicable to bee conservation efforts [3][4][16]. However, this is only a drop in the bucket compared with the amount of data that is needed to understand the nutritional ecology of bees.
Here, I propose to study the stoichiometry of chosen atoms in the biomass (body and cocoon) of of wild bees (i.e., their elemental phenotype; [8]), in two sexes, and in a diversity of pollen species. Accordingly, I propose to use the multidimensional stoichiometric approach to analyze which species of pollen are nutritionally limiting for specific bee species. The selection scheme should consider the data on the food sources preferred by particular bee species, the seasonality of bees and their potential food sources.
The following hypotheses might be tested:
1. Pollen stoichiometry vary among plant species and populations but will differ more widely among species than within different populations of the same species.
2. The stoichiometry of bees will vary substantially among bee species and between sexes within a species, which suggests the existence of different nutritional demands. Therefore, it is expected that the stoichiometric mismatches experienced by bees will vary in a species-specific and sex-specific manner.
3. For a given bee species, specific pollen species allow the overcoming of stoichiometric mismatches and will balance the diet. Accordingly, it is expected that flora diversity and, thus, pollen diversity matches the stoichiometric niches of bees.
3. Ecological Stoichiometry: Development, Current Knowledge, and A Brief Literature Overview
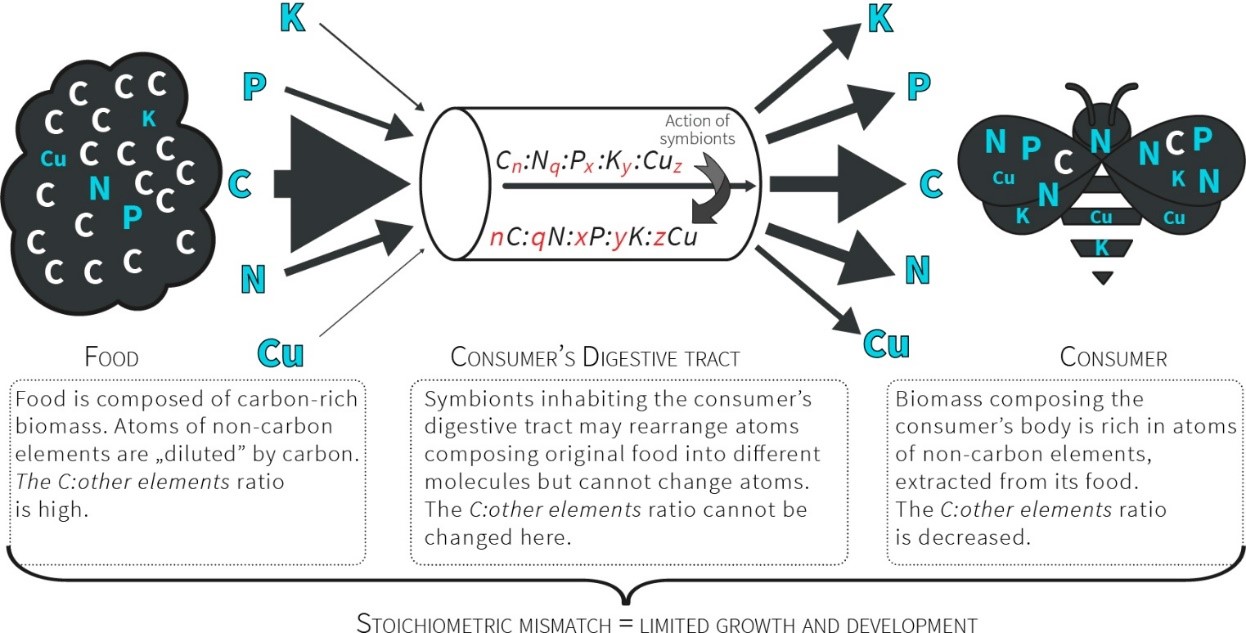
Consumers, especially herbivores, are faced with a high threshold of stoichiometric incompatibility between the chemical composition of their tissues and their food (Fig. 1). This observation has attracted the attention of ecologists and resulted in the development of a new research field, ecological stoichiometry [11]. Ecological stoichiometry raises questions concerning the strategies of crossing this threshold (the advantages and costs involved) and how these strategies affect the structure of communities and ecosystem functioning. The strategies for crossing stoichiometric thresholds are still poorly understood.
The atoms of chemical elements build the tissues and bodies of organisms in various proportions. This fact is crucial for ecological interactions and may shape the functioning of entire ecosystems [11]. The most fundamental feature of the elements, enabling their use in ecological stoichiometry, is that specific atoms cannot be transformed into different atoms by the organism. This feature distinguishes atoms from organic compounds. Within this context, ecological stoichiometry provides a common currency that links the ecology of organisms to life history trade-offs and evolutionary processes entrenched in the biogeochemical economy of life [7]. This currency is the ratio of atoms that compose the bodies of organisms and their food [11][17].
To date, ecological stoichiometry has provided data on elemental composition patterns in different trophic levels, with non-C element body contents tending to be the lowest in autotrophs, relatively low in herbivores and the highest in predators. Stoichiometric homeostasis has been shown be weaker in autotrophs and stronger in heterotrophs [11]. Ecological stoichiometry has also revealed stoichiometric mismatches at various trophic levels and has elucidated nutrient cycling in food webs [11][18][19]. However, these studies were conducted almost exclusively in aquatic ecosystems [20][21][22][23][24]. Recent studies have researched nutrient cycling in soil ecosystems and the relationship between stoichiometry and biodiversity [25][26][27][28][29][30], and considerable efforts have been made in the field of consumer-driven nutrient recycling theory (e.g., [31][32][33][34]). Nevertheless, much still needs to be done, since only C, N and P have been studied in this respect (e.g., [35]; see [22] for a review). The few papers on the stoichiometry beyond C:N:P almost exclusively concern freshwater systems [36][37][38][39], although limited data are available for marine ecosystems [40].
It was noted recently that it is easier to find in the scientific literature a population’s genome than its elemental composition [14]. At the same time, the concept of stoichiometric niche was introduced and defined as the region of multivariate niche space occupied by a group of individuals where the axes represent their elemental content [10][15]. If a consumer is unable to find food that fits its stoichiometric niche, a limitation will be imposed on its growth and development that negatively influences its fitness, and the entire population may be negatively impacted in this way [7][12]. By using this concept within the framework of the current project, one might provide a useful tool based on ecological stoichiometry that allows us to predict the impact of changes in floral composition on populations of wild bees. To this end, one need to fill a knowledge gap concerning the elemental compositions of and stoichiometric relationships between wild bees and their potential foods.
4. The Framework of Ecological Stoichiometry: Overview
Limitations are imposed on organisms by the scarcity of organic substances in food, including specific amino acids [41]. However, it is practically impossible to incorporate into a single study the abundance of organic components that make food nutritionally balanced because of their wide diversity. Therefore, previous studies have focused on either (1) a specific group of organic substances (e.g., PLFAs or amino acids) or (2) the total concentrations of carbohydrates, proteins and lipids (reviews in [64–66]). Approach (1) provides only limited information on the nutritional ecology of bees, and method (2) overlooks the limiting effects of the scarcity of specific substances in food on bees that may exist even if the food contains a large amount of proteins, sugars or lipids. An alternative approach is to study the constraints imposed on organismal growth and development by unbalanced food within the framework of ecological stoichiometry [11]. This approach capitalizes on organisms that comprise identical building blocks – the atoms of chemical elements – although these building blocks create a remarkable diversity of molecules that have various functions [7][11][12]. During larval development, organisms assimilate all the building blocks needed to compose the adult body. The body is built of atoms in a taxonomically specific proportion known as the organismal stoichiometry [7]. The demand for resources gathered during larval growth is reflected in the organismal stoichiometry of the adult body, and a stoichiometric mismatch may occur between the atomic composition of the body and the larval food [11]. Toxic effects may, in fact, be caused by such a stoichiometric mismatch rather than truly toxic substances [42]. Adult food, which is rich in energy, has different characteristics than larval food, which is rich in body-building matter. Wild bees may have an almost infinite access to energy-rich food (nectar) that meets the nutritional needs of the energetically limited adults. However, the quantity of food for bee larvae (pollen) cannot be substituted for its quality, and a poor nutritional balance in the pollen consumed may limit the growth and development of individuals, which negatively influences their populations [11][41].
Fig.1. Consumers ingest a prepackaged ratio of atoms. For herbivores, their food contains more C relative to other atoms; therefore, they must manage a diet that presents a stoichiometric mismatch through excess C that is further exacerbated by the unbalanced relationships between non-C elements (due to the exceptional scarcity of some of them).
5. Trophic Stoichiometric Ratio (TSR) – the Measure of Stoichiometric Mismatch for Bees that Feed on Various Pollen Species
The fundamental index in ecological stoichiometry is the threshold elemental ratio (TER; [11][13][23]). This index allows for the calculation of the limiting effect imposed on an organism by stoichiometric mismatches. The threshold elemental ratio is the lowest atomic ratio of C:other element in food at which the consumer’s development is not limited by the scarcity of C (i.e., energy) but is limited by the scarcity of the non-C element in the food. The basis for calculating the TER represents the consumer’s requirement for any non-C element during growth and development. This requirement is represented by utilizing the consumer’s consumption rates, assimilation rates and respiration rates of C and the non-C element. Therefore, the TER considers both (1) the energy budget, which is measured as the C balance, and (2) the budget of any non-C element.
The TER is understood as follows:
TERx = (GGEx / GGEc)
where GGEx is the gross growth efficiency of element x, GGEC is the gross growth efficiency of carbon, i is the trophic level, C is the concentration of carbon, and X is the concentration of element x.
If:
(C:X) ≥ TERx
then element x may become a limiting factor for growth at trophic level i+1.
The TER for any C:X ratio, where X is any element other than C, may be calculated as follows:
TERx = {Ax / [(IcAc - Rc) / Ic]}
where AX and AC are the assimilation rates for elements C and X, respectively, IC is the C ingestion rate, RC is the C respiration rate, and (C:X)i+1 is the atomic ratio of C:X in the consumer’s body.
However, in the case of herbivorous invertebrates, utilizing this index for certain elements is technically impossible. The gross growth efficiencies should be experimentally measured by using laboratory feeding trials in growing animals. Such data are extremely scarce, particularly for elements other than N and P. In practical terms, the TER index for invertebrates can only be estimated with arbitrary assumptions [35][43][43]. To allow for the identification of multiple elements that co-limit the development of an organism and facilitate comparisons between various taxa, habitats, food and life histories, the trophic stoichiometric ratio (TSR) was developed. The TSR is a simplified version of the TER that solely utilizes the data on the elemental composition of the organism and its food, and feeding experiments are not required [43][44]. The TSR is based on the following:
(C:X)i / (C:X)i+1 ≥ GGEx / GGEc
The minimum balanced GGEx / GGEC ratio can be estimated as 1/0.25 = 4, assuming that 75% of the consumed carbon is released as CO2 while the other consumed elements are incorporated with a 100% efficiency. Accordingly, it is conservatively assumed that for (C:X)i / (C:X)i+1 ≥ 4.0, element x may impose a constraint on growth [43][44]. Therefore, the TSR is calculated as follows[43][44]:
TSRx = (C:X)food / (C:X)consumer
where C is the concentration of carbon and X is the concentration of element x. Therefore, a TSRx ≥ 4 indicates a limitation imposed on the growth and development of an organism that feeds on the given food caused by the scarcity of element x in the food. When the TSR value is higher, the limiting effect is more severe.
Various elements may be differentially acquired, assimilated, reused, and excreted. The TSR index compares the elemental composition of an animal’s body and the food eaten (not the food assimilated). The absorbed matter has a different elemental composition than the ingested matter, but to absorb this and to void the indigestible surplus, the physiological effort must be proportional to the difference between the food eaten and the food assimilated to which the stoichiometric mismatch represented by the TSR index is proportional. Because the TSR index assumes that noncarbon elements are assimilated from food at a maximum rate (100%), the actual mismatches in natural situations can only be greater than the estimated TSR values. Therefore, the TSR index serves as a conservative but convenient tool that facilitates not only the detection of elements that co-limit development but also comparisons of the severity of the limitations imposed by various foods on different consumers.
The TSR index is used in the present project to study stoichiometric mismatches for bees that feed on various pollen species. Thus, it is possible to detect plant species that are stoichiometrically adequate/inadequate for specific bee species and that therefore positively/negatively influence bee communities and populations.
6. Expected Impact of the Research Project on the Development of Science
The link between floral diversity/composition and bee performance is unclear. We do not yet understand the differential contribution of various nutrients to bees’ growth and development. Are there species-specific key nutrients or nutrient ratios linked to development? Are such key nutrients associated with specific key plant species? Accordingly, can increased bee fitness (which is typically found in, for example, more diverse environments) be better predicted by optimal ratios of nutrients? Answering these questions will shed light on the mechanisms that underlie the known positive correlation between floral biodiversity and bee prosperity. However, the project has wider significance, as ecological stoichiometry aims to explain the adaptive strategies that compensate for stoichiometric mismatches, the costs and benefits involved, and how these strategies affect the structure of biotic communities and ecosystem function. The strategies used to overcome these mismatches by various herbivorous taxa remain poorly understood, especially considering terrestrial ecosystems. The proposed research will help to address this gap.
References
- IPBES. Assessment Report on Pollinators, Pollination and Food Production. UNEP/GRID Europe. 2016. doi:10.1007/s00442-010-1809-8; https://www.ipbes.net/assessment-reports/pollinators
- Michał Filipiak; A Better Understanding of Bee Nutritional Ecology Is Needed to Optimize Conservation Strategies for Wild Bees—The Application of Ecological Stoichiometry. Insects 2018, 9, 85, 10.3390/insects9030085.
- Michał Filipiak; Karolina Kuszewska; Michel Asselman; Bożena Denisow; Ernest Stawiarz; Michał Woyciechowski; January Weiner; Ecological stoichiometry of the honeybee: Pollen diversity and adequate species composition are needed to mitigate limitations imposed on the growth and development of bees by pollen quality. PLOS ONE 2017, 12, e0183236, 10.1371/journal.pone.0183236.
- Michał Filipiak; Key pollen host plants provide balanced diets for wild bee larvae: A lesson for planting flower strips and hedgerows. Journal of Applied Ecology 2019, 56, 1410-1418, 10.1111/1365-2664.13383.
- Denise Margaret S. Matias; Christian Borgemeister; Henrik Von Wehrden; Thinking beyond Western commercial honeybee hives: towards improved conservation of honey bee diversity. Biodiversity and Conservation 2017, 26, 3499-3504, 10.1007/s10531-017-1404-y.
- Romina Rader; Ignasi Bartomeus; Lucas A. Garibaldi; Michael Garratt; Brad Howlett; Rachael Winfree; Saul Cunningham; Margaret. M. Mayfield; Anthony D. Arthur; Georg K. S. Andersson; et al.Riccardo BommarcoClaire BrittainLuísa G. CarvalheiroNatacha P. ChacoffMartin H. EntlingBenjamin FoullyBreno M. FreitasBarbara Gemmill-HerrenJaboury GhazoulSean R. GriffinCaroline L. GrossLina HerbertssonFelix HerzogJuliana HipólitoSue JaggarFrank JaukerAlexandra-Maria KleinDavid KleijnSmitha KrishnanCamila Q. LemosSandra A. M. LindströmYael MandelikVictor M. MonteiroWarrick NelsonLovisa NilssonDavid E. PattemoreNatália De O. PereiraGideon PisantySimon G. PottsMenno ReemerMaj RundlöfCory SheffieldJeroen ScheperChristof SchüeppHenrik G. SmithDara A. StanleyJane C. StoutHajnalka SzentgyörgyiHisatomo TakiCarlos H. VergaraBlandina F. VianaMichal Woyciechowski Non-bee insects are important contributors to global crop pollination.. Proceedings of the National Academy of Sciences 2015, 113, 146-51, 10.1073/pnas.1517092112.
- Adam D Kay; Isabel W. Ashton; Elena Gorokhova; Andrew J. Kerkhoff; Antonia Liess; Elena Litchman; Toward a stoichiometric framework for evolutionary biology. Oikos 2005, 109, 6-17, 10.1111/j.0030-1299.2005.14048.x.
- Puni Jeyasingh; Rickey D. Cothran; Michael Tobler; Testing the ecological consequences of evolutionary change using elements. Ecology and Evolution 2014, 4, 528-538, 10.1002/ece3.950.
- Angélica L. González; Régis Céréghino; Olivier Dézerald; Vinicius F. Farjalla; Céline Leroy; Michael J. Richardson; Michael J. Richardson; Gustavo Q. Romero; Diane S. Srivastava; Ecological mechanisms and phylogeny shape invertebrate stoichiometry: A test using detritus‐based communities across Central and South America. Functional Ecology 2018, 32, 2448-2463, 10.1111/1365-2435.13197.
- Josep Penuelas; Marcos Fernández Martinez; Philippe Ciais; David Jou; Shilong Piao; Michael Obersteiner; Sara Vicca; Ivan A. Janssens; Jordi Sardans; The bioelements, the elementome, and the biogeochemical niche.. Ecology 2019, 100, e02652, 10.1002/ecy.2652.
- Sterner, RW; Elser, JJ. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, 2002; pp. 464.
- A.D. Kay; T. Vrede; Evolutionary and Biochemical Aspects. Encyclopedia of Ecology 2008, 1, 1472-1481, 10.1016/b978-008045405-4.00305-0.
- Robert F. Denno; William F. Fagan; MIGHT NITROGEN LIMITATION PROMOTE OMNIVORY AMONG CARNIVOROUS ARTHROPODS?. Ecology 2003, 84, 2522-2531, 10.1890/02-0370.
- Michael Kaspari; Jennifer S Powers; Marlene Zuk; Biogeochemistry and Geographical Ecology: Embracing All Twenty-Five Elements Required to Build Organisms. The American Naturalist 2016, 188, S62-S73, 10.1086/687576.
- Angélica L. González; Olivier Dézerald; Pablo A. Marquet; Gustavo Q. Romero; Diane S. Srivastava; The Multidimensional Stoichiometric Niche. Frontiers in Ecology and Evolution 2017, 5, na, 10.3389/fevo.2017.00110.
- Michał Filipiak; January Weiner; Plant–insect interactions: the role of ecological stoichiometry. Acta Agrobotanica 2017, 70, na, 10.5586/aa.1710.
- Mehdi Cherif; Biological Stoichiometry: The Elements at the Heart of Biological Interactions. Stoichiometry and Research - The Importance of Quantity in Biomedicine 2012, na, na, 10.5772/34528.
- Mehdi Cherif; Carolyn Faithfull; Junwen Guo; Cedric Leo Meunier; Judith Sitters; Wojciech Uszko; Francisco Rivera Vasconcelos; An Operational Framework for the Advancement of a Molecule-to-Biosphere Stoichiometry Theory. Frontiers in Marine Science 2017, 4, na, 10.3389/fmars.2017.00286.
- Nina Welti; Maren Striebel; Amber J. Ulseth; Wyatt F. Cross; Stephen Devilbiss; P. M. Glibert; Laodong Guo; Andrew G. Hirst; Jim Hood; John S. Kominoski; et al.Keeley L. MacNeillAndrew S. MehringJill R. WelterHelmut Hillebrand Bridging Food Webs, Ecosystem Metabolism, and Biogeochemistry Using Ecological Stoichiometry Theory. Frontiers in Microbiology 2017, 8, 1298, 10.3389/fmicb.2017.01298.
- S. J. Moe; Robert S. Stelzer; M. Rebecca Forman; W. Stanley Harpole; Tanguy Daufresne; Takehito Yoshida; Recent advances in ecological stoichiometry: insights for population and community ecology. Oikos 2005, 109, 29-39, 10.1111/j.0030-1299.2005.14056.x.
- C. A. Klausmeier; Elena Litchman; T. Daufresne; Simon A. Levin; Phytoplankton stoichiometry. Ecological Research 2008, 23, 479-485, 10.1007/s11284-008-0470-8.
- Jordi Sardans; Albert Rivas-Ubach; Josep Penuelas; The elemental stoichiometry of aquatic and terrestrial ecosystems and its relationships with organismic lifestyle and ecosystem structure and function: a review and perspectives. Biogeochemistry 2011, 111, 1-39, 10.1007/s10533-011-9640-9.
- Dag O. Hessen; James Elser; Robert Sterner; Jotaro Urabe; Ecological stoichiometry: An elementary approach using basic principles. Limnology and Oceanography 2013, 58, 2219-2236, 10.4319/lo.2013.58.6.2219.
- Nathan P. Lemoine; Sean T. Giery; D. E. Burkepile; Differing nutritional constraints of consumers across ecosystems. Oecologia 2014, 174, 1367-1376, 10.1007/s00442-013-2860-z.
- Thomas Schneider; Katharina Keiblinger; Emanuel Schmid; Katja Sterflinger-Gleixner; Günther Ellersdorfer; Bernd Roschitzki; Andreas Richter; Leo Eberl; Sophie Zechmeister-Boltenstern; Katharina Riedel; et al. Who is who in litter decomposition? Metaproteomics reveals major microbial players and their biogeochemical functions. The ISME Journal 2012, 6, 1749-62, 10.1038/ismej.2012.11.
- Maike Abbas; Anne Ebeling; Yvonne Oelmann; Robert Ptacnik; Christiane Roscher; Alexandra Weigelt; Wolfgang W. Weisser; Wolfgang Wilcke; Helmut Hillebrand; Biodiversity Effects on Plant Stoichiometry. PLOS ONE 2013, 8, e58179, 10.1371/journal.pone.0058179.
- David Ott; Christoph Digel; Björn C. Rall; Mark Maraun; Stefan Scheu; Ulrich Brose; Unifying elemental stoichiometry and metabolic theory in predicting species abundances. Ecology Letters 2014, 17, 1247-1256, 10.1111/ele.12330.
- Anton M. Potapov; Irina I. Semenyuk; Alexei Tiunov; Seasonal and age-related changes in the stable isotope composition (15N/14N and 13C/12C) of millipedes and collembolans in a temperate forest soil. Pedobiologia 2014, 57, 215-222, 10.1016/j.pedobi.2014.09.005.
- Jofre Carnicer; Jordi Sardans; Constantí Stefanescu; Andreu Ubach; Mireia Bartrons; Dolores Asensio; Josep Penuelas; Global biodiversity, stoichiometry and ecosystem function responses to human-induced C-N-P imbalances.. Journal of Plant Physiology 2014, 172, 82-91, 10.1016/j.jplph.2014.07.022.
- Aleksandra M. Lewandowska; Antje Biermann; E. T. Borer; Miguel Ángel Cebrián-Piqueras; Steven A. J. Declerck; Luc De Meester; Ellen Van Donk; Lars Gamfeldt; Daniel S. Gruner; Nicole Hagenah; et al.W. Stanley HarpoleKevin KirkmanChristopher A. KlausmeierMichael KleyerJohannes M. H. KnopsPieter LemmensEric LindElena LitchmanJasmin Mantilla-ContrerasKoen MartensSandra MeierVanessa MindenJoslin L. MooreHarry Olde VenterinkE. W. SeabloomUlrich SommerMaren StriebelAnastasia TrenkampJuliane TrinoggaJotaro UrabeWim VyvermanDedmer B. Van De WaalClaire E. WiddicombeHelmut Hillebrand The influence of balanced and imbalanced resource supply on biodiversity–functioning relationship across ecosystems. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 2016, 371, 20150283, 10.1098/rstb.2015.0283.
- James J. ElserJotaro Urabe; The Stoichiometry of Consumer-Driven Nutrient Recycling: Theory, Observations, and Consequences. Ecology 1999, 80, 735-751, 10.2307/177013.
- Michelle A. Evans-White; Gary A. Lamberti; Stoichiometry of consumer-driven nutrient recycling across nutrient regimes in streams. Ecology Letters 2006, 9, 1186-1197, 10.1111/j.1461-0248.2006.00971.x.
- Antonia Liess; Jens Olsson; Mario Quevedo; Peter Eklöv; Tobias Vrede; Helmut Hillebrand; Food web complexity affects stoichiometric and trophic interactions. Oikos 2006, 114, 117-125, 10.1111/j.2006.0030-1299.14517.x.
- Lesley B. Knoll; Peter B. McIntyre; Michael J. Vanni; Alexander S. Flecker; Feedbacks of consumer nutrient recycling on producer biomass and stoichiometry: separating direct and indirect effects. Oikos 2009, 118, 1732-1742, 10.1111/j.1600-0706.2009.17367.x.
- P. C. Frost; Jonathan P. Benstead; Wyatt F. Cross; Helmut Hillebrand; James H. Larson; Marguerite A. Xenopoulos; Takehito Yoshida; Threshold elemental ratios of carbon and phosphorus in aquatic consumers. Ecology Letters 2006, 9, 774-779, 10.1111/j.1461-0248.2006.00919.x.
- Roxanne Karimi; Carol L. Folt; Beyond macronutrients: element variability and multielement stoichiometry in freshwater invertebrates. Ecology Letters 2006, 9, 1273-1283, 10.1111/j.1461-0248.2006.00979.x.
- Antonietta Quigg; A. Irwin; Zoe V. Finkel; Evolutionary inheritance of elemental stoichiometry in phytoplankton. Proceedings of the Royal Society of London. Series B: Biological Sciences 2010, 278, 526-534, 10.1098/rspb.2010.1356.
- C. Bradshaw; Ulrik Kautsky; L. Kumblad; Ecological Stoichiometry and Multi-element Transfer in a Coastal Ecosystem. Ecosystems 2012, 15, 591-603, 10.1007/s10021-012-9531-5.
- Jared M. Goos; Rickey D. Cothran; Puni Jeyasingh; Within-population variation in the chemistry of life: the stoichiometry of sexual dimorphism in multiple dimensions. Evolutionary Ecology 2017, 31, 635-651, 10.1007/s10682-017-9900-9.
- B. S. Twining; Stephen B. Baines; The Trace Metal Composition of Marine Phytoplankton. Annual Review of Marine Science 2013, 5, 191-215, 10.1146/annurev-marine-121211-172322.
- Slansky F, Rodriguez JG. Nutritional Ecology of Insects, Mites, Spiders, and Related Invertebrates. Wiley-Interscience; 1987
- Ruth Jones; Kevin J. Flynn; Nutritional Status and Diet Composition Affect the Value of Diatoms as Copepod Prey. Science 2005, 307, 1457-1459, 10.1126/science.1107767.
- Michał Filipiak; January Weiner; Nutritional dynamics during the development of xylophagous beetles related to changes in the stoichiometry of 11 elements. Physiological Entomology 2016, 42, 73-84, 10.1111/phen.12168.
- Michał Filipiak; Nutrient Dynamics in Decomposing Dead Wood in the Context of Wood Eater Requirements: The Ecological Stoichiometry of Saproxylophagous Insects. Saproxylic Insects 2018, na, 429-469, 10.1007/978-3-319-75937-1_13.
- Michał Filipiak; Nutrient Dynamics in Decomposing Dead Wood in the Context of Wood Eater Requirements: The Ecological Stoichiometry of Saproxylophagous Insects. Saproxylic Insects 2018, na, 429-469, 10.1007/978-3-319-75937-1_13.