Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marco Zaffanello | -- | 1561 | 2023-10-13 22:56:00 | | | |
| 2 | Jason Zhu | Meta information modification | 1561 | 2023-10-17 03:16:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zaffanello, M.; Piacentini, G.; Nosetti, L.; Zoccante, L. Sleep Disordered Breathing in Autism Spectrum Disorder. Encyclopedia. Available online: https://encyclopedia.pub/entry/50293 (accessed on 07 February 2026).
Zaffanello M, Piacentini G, Nosetti L, Zoccante L. Sleep Disordered Breathing in Autism Spectrum Disorder. Encyclopedia. Available at: https://encyclopedia.pub/entry/50293. Accessed February 07, 2026.
Zaffanello, Marco, Giorgio Piacentini, Luana Nosetti, Leonardo Zoccante. "Sleep Disordered Breathing in Autism Spectrum Disorder" Encyclopedia, https://encyclopedia.pub/entry/50293 (accessed February 07, 2026).
Zaffanello, M., Piacentini, G., Nosetti, L., & Zoccante, L. (2023, October 13). Sleep Disordered Breathing in Autism Spectrum Disorder. In Encyclopedia. https://encyclopedia.pub/entry/50293
Zaffanello, Marco, et al. "Sleep Disordered Breathing in Autism Spectrum Disorder." Encyclopedia. Web. 13 October, 2023.
Copy Citation
Sleep-disordered breathing is a significant problem affecting the pediatric population. These conditions can affect sleep quality and children’s overall health and well-being. Difficulties in social interaction, communication, and repetitive behavioral patterns characterize autism spectrum disorder. Sleep disturbances are common in children with ASD.
autism spectrum disorder
children
sleep apnea
sleep-disordered breathing
1. Introduction
Sleep-disordered breathing (SDB) represents a significant issue affecting the pediatric population [1][2]. Within the broader pediatric population, obstructive sleep apnea (OSA) ranges from 2% to 5%, although in specific medical contexts, its prevalence can be significantly higher [3]. These conditions can have severe consequences on the health and well-being of children [1][4][5].
In the broader context, independent risk factors for OSA encompass persistent snoring for ≥3 months, tonsillar and adenoid hypertrophy, and obesity (Xu et al., 2020). Moreover, frequent respiratory infections [6] may amplify the impact, particularly when coupled with factors such as tonsillar and adenoid hypertrophy or obesity. Muscle hypotonia in children with genetic comorbidities [7][8][9] may serve as concurrent catalysts exacerbating SDB. The intricate interplay of SDB can orchestrate disruptions in sleep patterns and intermittent hypoxia, exerting a discernible impact on the cognitive and behavioral faculties of children [10][11][12]. Consequently, delving into the long-term trajectories of these disorders and maintaining vigilant follow-up mechanisms becomes imperative [1][13].
Neurodevelopmental disorders constitute a wide-ranging category of medical conditions that profoundly impact the intricate development of the nervous system, particularly during the critical phases of brain maturation. The delicate interplay of factors shaping proper nervous system development intertwines with the multifaceted complexities characterizing the manifestation of autism spectrum disorder (ASD) during the crucial stages of childhood or infancy [14][15][16], exploring the nuanced aspects of the condition. The resulting impact reverberates across a comprehensive spectrum of domains, encompassing the intricate threads of communication, the fabric of learning, the dance of social behavior, and the tapestry of motor skills. While ASD maintains its distinct identity with unique attributes, acknowledging the latent potential for multiple neurodevelopmental disorders to converge within the presentation of children remains of paramount significance [17][18][19].
2. Sleep Disordered Breathing in Children
Research on the effects of SDB or OSA in children with ASD has revealed significant insights (Figure 1). One study demonstrated that 34% of children with ASD (n = 53, age 7.5 [4.8, 12.8] years) were diagnosed with OSA through PSG [20]. Another study reported that children with ASD (age 4.7 ± 1.14 years) who snore occasionally account for 25.4%, and 5.1% snore constantly. Children experiencing sleep apnea occasionally account for 3.5%, and those experiencing it frequently represent 0.6%, according to the CSHQ [21]. An elevated susceptibility to the development of sleep disorders, encompassing OSA, has been documented. The risk of sleep disorders in autistic children is increased by 96% compared to non-autistic children. Furthermore, children with ASD are also at a higher risk of undergoing PSG (increased by 274% compared to the control group) and ENT surgery (increased by 50% compared to the control group) [22]. Among 45 children with ASD (age 6.1 ± 2.8 years), 58% had OSA diagnosed through PSG, and 33% were obese [23]. In summary, these studies bring attention to the health issue of the elevated occurrence of SDB in children with ASD. The rising prevalence of children with ASD undergoing PSG underscores the growing demand for diagnostic and therapeutic interventions in this population [24] and exceptional attention to weight management [23]. Treatments for these disorders may encompass behavioral therapies, surgical interventions [22], medications, or a combination of these approaches.
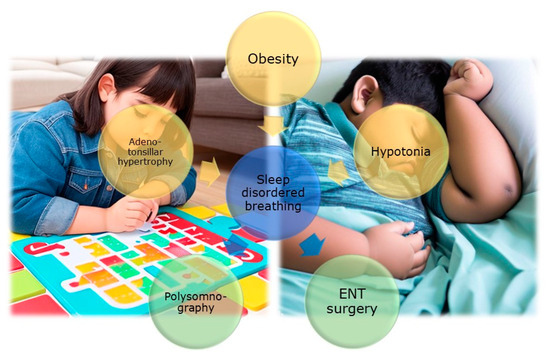
Figure 1. The figure (AI image generator https://dream.ai/create (accessed on 29 August 2023)) illustrates the association between autism and SDB in children. Key risk factors such as muscle hypotonia, obesity, and adenotonsillar hypertrophy are highlighted. Additionally, the figure underscores the importance of PSG in recognizing and treating SDB in autistic children. Lastly, the significance of otorhinolaryngological surgical intervention as an effective therapeutic option to alleviate symptoms of SDB and enhance the quality of life for children with autism is emphasized.
The incidence of obesity in children with ASD is at least as high as, or even higher than, in the general population of children. Risk factors for a high BMI are advanced child age, high maternal BMI, low physical activity, and an increased likelihood of food selectivity [25]. Obesity has been identified as a predictive factor for SDB (OR 1.04, 95% CI 1.0–1.08, p = 0.02), especially for severe OSA, in children with ASD [23]. Moreover, the risk and rate of obesity in pediatric individuals with ASD are elevated [26], with causes primarily being attributed to sedentary lifestyles and improper dietary habits [27]. A study found that ferritin levels and BMI in children with ASD (n = 53, age 7.5 [IQR 4.8, 12.8] years) were not significantly correlated with OSA, suggesting the presence of other influential factors [20]. Another study indicated that BMI and age of autism diagnosis could independently impact the generation of OSA diagnosis [28].
The treatment of OSA (diagnosed through PSG) with A&T has proven to be essential in enhancing behavioral outcomes, irrespective of the child’s obesity and age [24]. In general, there have been reports of behavioral and cognitive improvements following A&T therapy in pediatric OSA, with authors consistently observing significant score enhancements in nearly all studies [29]. It is essential to conduct in-depth investigations to examine the advantages of A&T treatment in pediatric patients who have both ASD and OS.
OSA negatively impacts behaviors and overall well-being in children with ASD [24]. A study found a correlation between the number of pain-related behaviors exhibited in the previous week. It increased overall sleep-related problems, specifically shorter sleep duration, parasomnias (sleepwalking or nightmares), and SDB [30]. Some researchers have concluded that children with ASD are more likely to receive a diagnosis of sleep disorders, including SDB, and are more inclined to undergo related diagnostic and surgical procedures than individuals without ASD as controls [22].
Investigations are underway to explore the potential connection between connective tissue irregularities and ASD. However, the precise relationship between these two factors remains incompletely understood. Altered connectivity can give rise to heightened sensitivity to environmental stimuli. In ASD, there is a higher prevalence of asthma and allergic rhinitis, with a ratio of 5:1 compared to healthy subjects [31]. Frequent episodes of interstitial inflammation, immune-mediated forms of allergic asthma, bronchial hyperreactivity, nasal secretion, and a sensation of nasal obstruction have been observed following exposure to environmental allergens [32][33]. Very young children with common ear and upper respiratory symptoms appear to have an elevated risk of subsequently receiving an autism diagnosis or exhibiting high levels of autism-related traits [34]. However, it is important to note that a straightforward linear correlation between the degree of nasal obstruction and the severity of SDB is not consistently evident. In most cases of moderate or severe OSA, nasal obstruction is not the primary underlying factor [35].
Joint hypermobility has quite a high prevalence in ASD, so authors tend to include autism among hypermobility spectrum disorders (HSDs) [33]. Recognizing the differences between muscle weakness (hypotonia), tendon laxity, and joint hypermobility is complex, especially when dealing with individuals with autism, where it becomes an important issue [33][36]. However, hypotonia/joint hypermobility is also a recognizable marker of ASD [37]. Hypotonia/ligament laxity is when children have very “soft” or flaccid muscle tone [33]. The hypotonia/joint hypermobility observed was classified as mild to moderate and exhibited a widespread distribution across the entire body. Hypotonia was the most common motor symptom in a cohort of 154 children with ASD (51%), and it appears to improve over time. In the 2–6-year-old group, the prevalence of hypotonia was approximately 63% [36] at the age in which there is a high prevalence of SDB, mainly due to adenotonsillar hypertrophy [38][39]. In general, the atonia of skeletal muscles present during REM sleep might be exacerbated by the underlying hypotonia in children [40], and in ASD might increase the risk of OSA. When muscles are not adequately toned, the airways can become more collapsible. Identifying these joint-related issues paves the way for future research and the investigation of interventions designed to address joint hypermobility [33], hypotonia, and their effects on the respiratory health of children with ASD. It is important to emphasize that a customized physiotherapy approach can play a pivotal role in a comprehensive treatment plan for children with ASD who experience muscle hypotonia and are at risk of OSA.
As obesity has been acknowledged as a contributing factor for SDB in pediatric individuals with ASD, it becomes essential to implement effective weight monitoring and management strategies tailored to this specific population. Addressing this concern may involve implementing lifestyle modification programs or targeted dietary interventions. While most participants in a study agreed that pediatricians should take the lead in managing obesity in children with ASD, only a few reported receiving adequate training for this role. As a result, they were more inclined to refer children with ASD to specialized services, such as dietitians or developmental–behavioral pediatricians [41]. Recognizing obesity as a predictive factor for SDB in children with ASD is crucial. Physicians should pay special attention to obese children with ASD because of their heightened risk of SDB, which can enable earlier diagnosis and treatment.
Children diagnosed with ASD might experience challenges in tolerating PSG [42][43] or discomfort sleeping in a different environment from their bed. However, some steps can help reduce anxiety during the PSG procedure, such as providing a detailed explanation of the procedure, identifying the child’s strengths, using accessories that reflect the child’s interests, and allowing family members to be present during the process to reduce anxiety, as well as paying close attention to the child’s needs. It is important to note that if a child cannot undergo PSG due to their condition, there are alternative methods for assessing SDB for the development of an appropriate therapeutic plan [43][44][45].
References
- Nosetti, L.; Zaffanello, M.; Katz, E.S.; Vitali, M.; Agosti, M.; Ferrante, G.; Cilluffo, G.; Piacentini, G.; La Grutta, S. Twenty-year follow-up of children with obstructive sleep apnea. J. Clin. Sleep Med. 2022, 18, 1573–1581.
- Nosetti, L.; Zaffanello, M.; De Bernardi, F.; Piacentini, G.; Roberto, G.; Salvatore, S.; Simoncini, D.; Pietrobelli, A.; Agosti, M. Age and Upper Airway Obstruction: A Challenge to the Clinical Approach in Pediatric Patients. Int. J. Environ. Res. Public Health 2020, 17, 3531.
- Piotto, M.; Gambadauro, A.; Rocchi, A.; Lelii, M.; Madini, B.; Cerrato, L.; Chironi, F.; Belhaj, Y.; Patria, M.F. Pediatric Sleep Respiratory Disorders: A Narrative Review of Epidemiology and Risk Factors. Children 2023, 10, 955.
- Brockmann, P.E.; Gozal, D. Neurocognitive Consequences in Children with Sleep Disordered Breathing: Who Is at Risk? Children 2022, 9, 1278.
- Zaffanello, M.; Piacentini, G.; La Grutta, S. The cardiovascular risk in paediatrics: The paradigm of the obstructive sleep apnoea syndrome. Blood Transfus. 2020, 18, 217.
- Nino, G.; Restrepo-Gualteros, S.M.; Gutierrez, M.J. Pediatric sleep apnea and viral respiratory infections: What do clinicians need to know? Expert. Rev. Respir. Med. 2022, 16, 253–255.
- Khayat, A.; Bin-Hassan, S.; Al-Saleh, S. Polysomnographic findings in infants with Pierre Robin sequence. Ann. Thorac. Med. 2017, 12, 25–29.
- Schaefer, J.; Davey, M.J.; Nixon, G.M. Sleep-disordered breathing in school-aged children with Prader-Willi syndrome. J. Clin. Sleep Med. 2022, 18, 1055–1061.
- Hill, C.M.; Evans, H.J.; Elphick, H.; Farquhar, M.; Pickering, R.M.; Kingshott, R.; Martin, J.; Reynolds, J.; Joyce, A.; Rush, C.; et al. Prevalence and predictors of obstructive sleep apnoea in young children with Down syndrome. Sleep Med. 2016, 27–28, 99–106.
- Hunter, S.J.; Gozal, D.; Smith, D.L.; Philby, M.F.; Kaylegian, J.; Kheirandish-Gozal, L. Effect of Sleep-disordered Breathing Severity on Cognitive Performance Measures in a Large Community Cohort of Young School-aged Children. Am. J. Respir. Crit. Care Med. 2016, 194, 739–747.
- Menzies, B.; Teng, A.; Burns, M.; Lah, S. Neurocognitive outcomes of children with sleep disordered breathing: A systematic review with meta-analysis. Sleep Med. Rev. 2022, 63, 101629.
- Zaffanello, M.; Ferrante, G.; Zoccante, L.; Ciceri, M.L.; Nosetti, L.; Tenero, L.; Piazza, M.; Piacentini, G. Predictive Power of Oxygen Desaturation Index (ODI) and Apnea-Hypopnea Index (AHI) in Detecting Long-Term Neurocognitive and Psychosocial Outcomes of Sleep-Disordered Breathing in Children: A Questionnaire-Based Study. J. Clin. Med. 2023, 12, 3060.
- Harris, V.C.; Links, A.R.; Kim, J.M.; Walsh, J.; Tunkel, D.E.; Boss, E.F. Follow-up and Time to Treatment in an Urban Cohort of Children with Sleep-Disordered Breathing. Otolaryngol. Head Neck Surg. 2018, 159, 371–378.
- Zaffanello, M.; Zamboni, G.; Fontana, E.; Zoccante, L.; Tatò, L. A case of partial biotinidase deficiency associated with autism. Child Neuropsychol. 2003, 9, 184–188.
- Noto, A.; Fanos, V.; Barberini, L.; Grapov, D.; Fattuoni, C.; Zaffanello, M.; Casanova, A.; Fenu, G.; De Giacomo, A.; De Angelis, M.; et al. The urinary metabolomics profile of an Italian autistic children population and their unaffected siblings. J. Matern. Fetal Neonatal Med. 2014, 27 (Suppl. S2), 46–52.
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Transl. Pediatr. 2020, 9, S55–S65.
- Morris-Rosendahl, D.J.; Crocq, M.A. Neurodevelopmental disorders-the history and future of a diagnostic concept. Dialogues Clin. Neurosci. 2020, 22, 65–72.
- Posar, A.; Visconti, P. Sleep Problems in Children with Autism Spectrum Disorder. Pediatr. Ann. 2020, 49, e278–e282.
- Schwichtenberg, A.J.; Janis, A.; Lindsay, A.; Desai, H.; Sahu, A.; Kellerman, A.; Chong, P.L.H.; Abel, E.A.; Yatcilla, J.K. Sleep in Children with Autism Spectrum Disorder: A Narrative Review and Systematic Update. Curr. Sleep Med. Rep. 2022, 8, 51–61.
- Youssef, J.; Singh, K.; Huntington, N.; Becker, R.; Kothare, S.V. Relationship of serum ferritin levels to sleep fragmentation and periodic limb movements of sleep on polysomnography in autism spectrum disorders. Pediatr. Neurol. 2013, 49, 274–278.
- Johnson, C.R.; DeMand, A.; Lecavalier, L.; Smith, T.; Aman, M.; Foldes, E.; Scahill, L. Psychometric properties of the children’s sleep habits questionnaire in children with autism spectrum disorder. Sleep Med. 2016, 20, 5–11.
- Elrod, M.G.; Nylund, C.M.; Susi, A.L.; Gorman, G.H.; Hisle-Gorman, E.; Rogers, D.J.; Erdie-Lalena, C. Prevalence of Diagnosed Sleep Disorders and Related Diagnostic and Surgical Procedures in Children with Autism Spectrum Disorders. J. Dev. Behav. Pediatr. 2016, 37, 377–384.
- Tomkies, A.; Johnson, R.F.; Shah, G.; Caraballo, M.; Evans, P.; Mitchell, R.B. Obstructive Sleep Apnea in Children With Autism. J. Clin. Sleep Med. 2019, 15, 1469–1476.
- Murata, E.; Mohri, I.; Kato-Nishimura, K.; Iimura, J.; Ogawa, M.; Tachibana, M.; Ohno, Y.; Taniike, M. Evaluation of behavioral change after adenotonsillectomy for obstructive sleep apnea in children with autism spectrum disorder. Res. Dev. Disabil. 2017, 65, 127–139.
- Kamal Nor, N.; Ghozali, A.H.; Ismail, J. Prevalence of Overweight and Obesity Among Children and Adolescents With Autism Spectrum Disorder and Associated Risk Factors. Front. Pediatr. 2019, 7, 38.
- Veronese, S.; Zoccante, L.; Smania, N.; Sbarbati, A. Stretch marks: A visible expression of connective’s involvement in autism spectrum disorders. Front. Psychiatry 2023, 14, 1155854.
- Eow, S.Y.; Gan, W.Y.; Lim, P.Y.; Awang, H.; Mohd Shariff, Z. Parental Feeding Practices and Child-Related Factors are Associated with Overweight and Obesity in Children and Adolescents with Autism Spectrum Disorder. J. Autism Dev. Disord. 2022, 52, 3655–3667.
- Santapuram, P.; Chen, H.; Weitlauf, A.S.; Ghani, M.O.A.; Whigham, A.S. Investigating differences in symptomatology and age at diagnosis of obstructive sleep apnea in children with and without autism. Int. J. Pediatr. Otorhinolaryngol. 2022, 158, 111191.
- Di Mauro, P.; Cocuzza, S.; Maniaci, A.; Ferlito, S.; Rasà, D.; Anzivino, R.; Vicini, C.; Iannella, G.; La Mantia, I. The Effect of Adenotonsillectomy on Children’s Behavior and Cognitive Performance with Obstructive Sleep Apnea Syndrome: State of the Art. Children 2021, 8, 921.
- Tudor, M.E.; Walsh, C.E.; Mulder, E.C.; Lerner, M.D. Pain as a predictor of sleep problems in youth with autism spectrum disorders. Autism 2015, 19, 292–300.
- Zerbo, O.; Leong, A.; Barcellos, L.; Bernal, P.; Fireman, B.; Croen, L.A. Immune mediated conditions in autism spectrum disorders. Brain Behav. Immun. 2015, 46, 232–236.
- Tonacci, A.; Billeci, L.; Ruta, L.; Tartarisco, G.; Pioggia, G.; Gangemi, S. A systematic review of the association between allergic asthma and autism. Minerva. Pediatr. 2017, 69, 538–550.
- Zoccante, L.; Ciceri, M.L.; Gozzi, L.A.; Gennaro, G.D.; Zerman, N. The "Connectivome Theory": A New Model to Understand Autism Spectrum Disorders. Front. Psychiatry 2021, 12, 794516.
- Hall, A.; Maw, R.; Iles-Caven, Y.; Gregory, S.; Rai, D.; Golding, J. Associations between autistic traits and early ear and upper respiratory signs: A prospective observational study of the Avon Longitudinal Study of Parents and Children (ALSPAC) geographically defined childhood population. BMJ Open 2023, 13, e067682.
- Georgalas, C. The role of the nose in snoring and obstructive sleep apnoea: An update. Eur. Arch. Otorhinolaryngol. 2011, 268, 1365–1373.
- Ming, X.; Brimacombe, M.; Wagner, G.C. Prevalence of motor impairment in autism spectrum disorders. Brain Dev. 2007, 29, 565–570.
- Gabis, L.V.; Shaham, M.; Leon Attia, O.; Shefer, S.; Rosenan, R.; Gabis, T.; Daloya, M. The Weak Link: Hypotonia in Infancy and Autism Early Identification. Front. Neurol. 2021, 12, 612674.
- Nosetti, L.; Zaffanello, M.; De Bernardi di Valserra, F.; Simoncini, D.; Beretta, G.; Guacci, P.; Piacentini, G.; Agosti, M. Exploring the Intricate Links between Adenotonsillar Hypertrophy, Mouth Breathing, and Craniofacial Development in Children with Sleep-Disordered Breathing: Unraveling the Vicious Cycle. Children 2023, 10, 1426.
- Kang, K.T.; Chou, C.H.; Weng, W.C.; Lee, P.L.; Hsu, W.C. Associations between adenotonsillar hypertrophy, age, and obesity in children with obstructive sleep apnea. PLoS ONE 2013, 8, e78666.
- Strollo, P.J., Jr.; Rogers, R.M. Obstructive sleep apnea. N. Engl. J. Med. 1996, 334, 99–104.
- Walls, M.; Broder-Fingert, S.; Feinberg, E.; Drainoni, M.L.; Bair-Merritt, M. Prevention and Management of Obesity in Children with Autism Spectrum Disorder Among Primary Care Pediatricians. J. Autism Dev. Disord. 2018, 48, 2408–2417.
- Asmika, A.; Oktafiani, L.D.A.; Kusworini, K.; Sujuti, H.; Andarini, S. Autistic Children Are More Responsive to Tactile Sensory Stimulus. Iran. J. Child Neurol. 2018, 12, 37–44.
- Moore, M.; Evans, V.; Hanvey, G.; Johnson, C. Assessment of Sleep in Children with Autism Spectrum Disorder. Children 2017, 4, 72.
- Villa, M.P.; Pietropaoli, N.; Supino, M.C.; Vitelli, O.; Rabasco, J.; Evangelisti, M.; Del Pozzo, M.; Kaditis, A.G. Diagnosis of Pediatric Obstructive Sleep Apnea Syndrome in Settings With Limited Resources. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 990–996.
- Chervin, R.D.; Weatherly, R.A.; Garetz, S.L.; Ruzicka, D.L.; Giordani, B.J.; Hodges, E.K.; Dillon, J.E.; Guire, K.E. Pediatric sleep questionnaire: Prediction of sleep apnea and outcomes. Arch. Otolaryngol.-Head Neck Surg. 2007, 133, 216–222.
More
Information
Subjects:
Health Care Sciences & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
881
Revisions:
2 times
(View History)
Update Date:
17 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




