Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Karolina Kula | -- | 1899 | 2023-10-13 11:12:43 | | | |
| 2 | Jason Zhu | Meta information modification | 1899 | 2023-10-17 03:36:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kula, K.; Nagatsky, R.; Sadowski, M.; Siumka, Y.; Demchuk, O.M. Synthesis of Arylcyanomethylenequinone Oximes. Encyclopedia. Available online: https://encyclopedia.pub/entry/50257 (accessed on 08 February 2026).
Kula K, Nagatsky R, Sadowski M, Siumka Y, Demchuk OM. Synthesis of Arylcyanomethylenequinone Oximes. Encyclopedia. Available at: https://encyclopedia.pub/entry/50257. Accessed February 08, 2026.
Kula, Karolina, Roman Nagatsky, Mikołaj Sadowski, Yevheniia Siumka, Oleg M. Demchuk. "Synthesis of Arylcyanomethylenequinone Oximes" Encyclopedia, https://encyclopedia.pub/entry/50257 (accessed February 08, 2026).
Kula, K., Nagatsky, R., Sadowski, M., Siumka, Y., & Demchuk, O.M. (2023, October 13). Synthesis of Arylcyanomethylenequinone Oximes. In Encyclopedia. https://encyclopedia.pub/entry/50257
Kula, Karolina, et al. "Synthesis of Arylcyanomethylenequinone Oximes." Encyclopedia. Web. 13 October, 2023.
Copy Citation
Quinone methides are a class of biologically active compounds that can be used in medicine as antibacterial, antifungal, antiviral, antioxidant, and anti-inflammatory agents. In addition, quinone methides have the potential to be used as pesticides, dyes, and additives for rubber and plastics.
quinonemethide
arylcyanomethylenequinone oximes
quinone
1. The Condensation of (Hetero) Arylacetonitriles with Nitro (Hetero) Arenes
There are not many methods applicable to the synthesis of both arylcyanomethylenequinone oximes and other quinone methide oxime derivatives. The analysis of the literature data showed that one of the best approaches for the construction of their backbone is the condensation of benzyl cyanides with 4-unsubstituted nitroarenes or nitroheteroarenes [1], which is a simple way of synthesis of various derivatives of such oximes.
The synthesis of phenylcyanomethylenequinone oxime was first described by Davis et al. [2] in 1960. The authors stated that when benzyl cyanide 1a and nitrobenzene 2a are added to a warm alcoholic solution of potassium hydroxide, the reaction mixture becomes dark red and a like-coloured solid soon precipitates. The solid dissolves in water, and upon acidification with acetic acid solution, a new yellow–orange solid precipitates with a yield of 77%; the latter solid is 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a (Scheme 1).
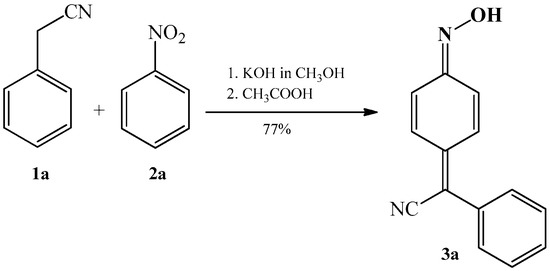
Scheme 1. Condensation reaction of benzyl cyanide 1a with nitrobenzene 2a that produces 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a.
Simultaneously, in that work, the authors also reported that no evidence for the other possible reaction products, except for product 3a, were obtained. Attempts to isolate possible structures 4a, 5a, 6a were unsuccessful (Figure 2).
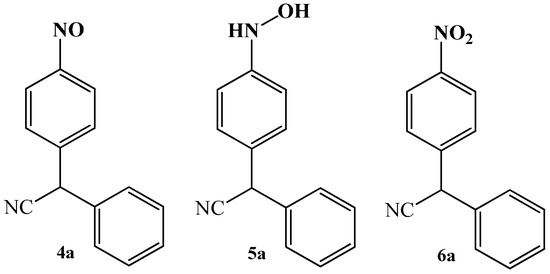
Figure 2. Structures of other potential reaction products of condensation reaction between benzyl cyanide 1a and nitrobenzene 2a.
On the other hand, they have noted that in the reaction between benzyl cyanide 1a and nitrobenzene 2a, it is possible to obtain other products when the methanol solvent was replaced with pyridine: the mixture of products 5a and 6a (Scheme 2) was obtained then.
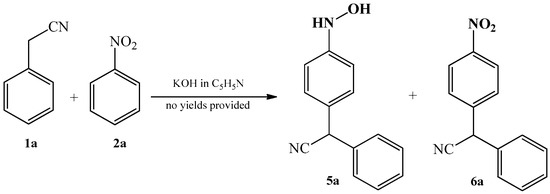
Scheme 2. Condensation reaction of benzyl cyanide 1a with nitrobenzene 2a to produce analogues 5a and 6a of oxime 3a.
In the same paper the authors have also shown, for the first time, that nitrobenzene 2a can react with 4-substituted benzyl cyanides, such as 4-chlorobenzyl cyanide 1b and 4-methoxybenzyl cyanide 1c, as well as with α-naphthylacetonitrile 7a. Reactions took place in a methanolic solution of potassium hydroxide, forming corresponding quinone oximes 3ba, 3ca (Scheme 3), and 8a (Scheme 4).
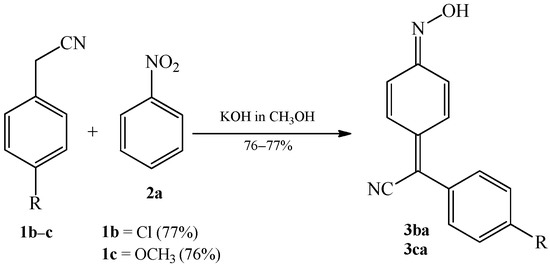
Scheme 3. Condensation reaction of 4-substituted benzyl cyanides 1b–c with nitrobenzene 2a to produce quinone oximes 3ba and 3ca.
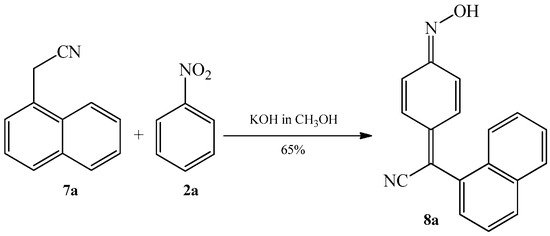
Scheme 4. Condensation reaction of α-naphthylacetonitrile 7a with nitrobenzene 2a to produce quinone oximes 8a.
The mechanism of condensation reaction between benzyl cyanide 1a and nitrobenzene 2a was described on Scheme 5. In the first step, nitrobenzene 2a undergoes nucleophilic attack of the benzyl cyanide anion 1’a at the 4-position, with subsequent addition of a proton to the oxygen of the nitro group to form intermediate 9a, which can also exist as a potassium salt. Further elimination of a water molecule leads to the formation of the nitroso derivative 4a. In turn, compound 4a rapidly transforms into an anion, possessing two alternative forms 10a and 10’a. Finally, acidification of the potassium salt 10’a leads to the formation of phenylcyanomethylenequinone oxime 3a.
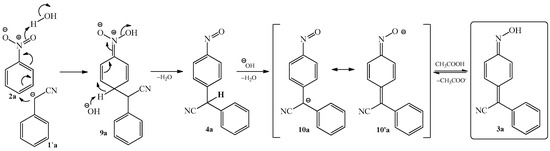
Scheme 5. Mechanism of condensation reaction of nitrobenzene 1a with benzyl cyanide 2a to produce 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a.
In 1962, Lichtenberger and Weiss [3] reproduced the condensation reaction between nitrobenzene 1a and benzyl cyanide 2a, proposed by Davis et al. [2]. The authors reported that they had obtained 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a as the only reaction product, thus confirming previous studies.
In 1961, Davis et al., continuing the study of the course of the condensation of various 4-substituted benzyl cyanides 1a–c with 4-unsubstituted nitroarenes 11a–n (Scheme 6), published the work [4] in which detailed preparations, by the previously shown condensation method, were described. As a result, 34 new arylcyanomethylenequinone oxime analogues 12aa–cn were synthesized (Table 1). The reactions were realized in warm alcoholic solution of potassium hydroxide.
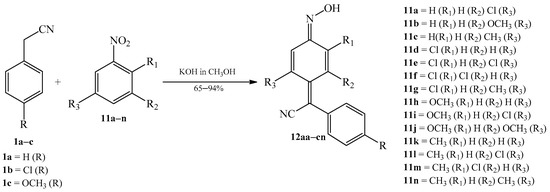
Scheme 6. Condensation reaction of 4-substituted benzyl cyanides 1a–c with 4-unsubstituted analogues of nitrobenzene 11a–n to produce quinone oximes 12aa–cn.
Table 1. Analogues of arylcyanomethylenequinone oximes 12aa–cn.
| Product (Yield [%]) | R | R1 | R2 | R3 | Product (Yield [%]) | R | R1 | R2 | R3 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 12aa | (53) | -H | -H | -H | -Cl | 12be | (94) | -Cl | -Cl | -H | -Cl |
| 12ab | (25) | -H | -H | -H | -OCH3 | 12bf | (92) | -Cl | -Cl | -Cl | -H |
| 12ac | (76) | -H | -H | -H | -CH3 | 12bg | (77) | -Cl | -Cl | -H | -CH3 |
| 12ad | (92) | -H | -Cl | -H | -H | 12bh | (80) | -Cl | -OCH3 | -H | -H |
| 12ae | (93) | -H | -Cl | -H | -Cl | 12bi | (81) | -Cl | -OCH3 | -H | -Cl |
| 12af | (53) | -H | -Cl | -Cl | -H | 12bj | (80) | -Cl | -OCH3 | -H | -OCH3 |
| 12ag | (77) | -H | -Cl | -H | -CH3 | 12bk | (60) | -Cl | -CH3 | -H | -H |
| 12ah | (87) | -H | -OCH3 | -H | -H | 12bm | (80) | -Cl | -CH3 | -Cl | -H |
| 12ai | (82) | -H | -OCH3 | -H | -Cl | 12bn | (43) | -Cl | -CH3 | -H | -CH3 |
| 12aj | (88) | -H | -OCH3 | -H | -OCH3 | 12ce | (91) | -OCH3 | -Cl | -H | -Cl |
| 12ak | (72) | -H | -CH3 | -H | -H | 12cg | (80) | -OCH3 | -Cl | -H | -CH3 |
| 12al | (92) | -H | -CH3 | -H | -Cl | 12cf | (65) | -OCH3 | -Cl | -Cl | -H |
| 12am | (82) | -H | -CH3 | -Cl | -H | 12ci | (84) | -OCH3 | -OCH3 | -H | -Cl |
| 12an | (53) | -H | -CH3 | -H | -CH3 | 12cj | (65) | -OCH3 | -OCH3 | -H | -OCH3 |
| 12ba | (100) | -Cl | -H | -H | -Cl | 12cl | (88) | -OCH3 | -CH3 | -H | -Cl |
| 12bb | (89) | -Cl | -H | -H | -OCH3 | 12cm | (80) | -OCH3 | -CH3 | -Cl | -H |
| 12bd | (100) | -Cl | -Cl | -H | -H | 12cn | (69) | -OCH3 | -CH3 | -H | -CH3 |
In 1964, Davis [5] patented another example of condensation of benzyl cyanide 1a with 4-chloronitrobenzene 2b. The author described that in a course of reaction, the expected arylcyanomethylenequinone oxime is not created but an analogue of isoxazole 13 is formed as the only product (Scheme 7). The reaction was carried out in warm alcoholic solution of potassium hydroxide.
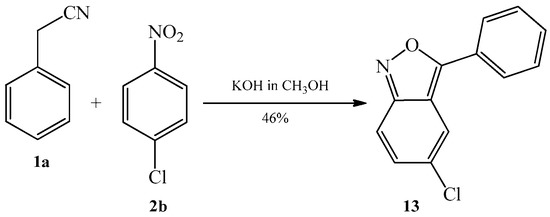
Scheme 7. Condensation reaction of benzyl cyanide 1a with 4-chloronitrobenzene 2b to produce 3-phenyl-5-chloroanthranil 13.
In the same patent, Davis reported that in the reaction between benzyl cyanide 1a and 4-chloronitrobenzene 2b, when the solvent is changed from methanol to pyridine, the 4-chloro substituent is not retained in the product. In turn, 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a is formed (Scheme 8). This variation of the reaction, in contrast to the previous examples, allows to use 4-substituted nitroarenes in the synthesis of arylcyanomethylenequinone oximes.
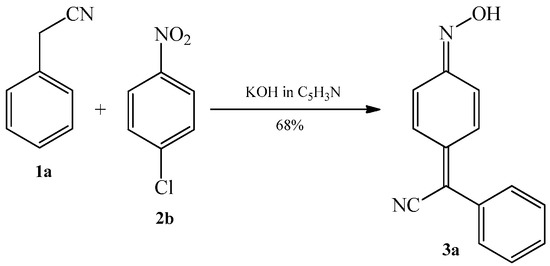
Scheme 8. Condensation reaction of benzyl cyanide 1a with 4-chloronitrobenzene 2b to produce 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a.
In 1965, Marey et al. [6] proposed the use of heteroaromatic analogues of benzyl cyanide 2a such as 3-pyridylacetonitrile 14 and 2-thienylacetonitrile 15 and (hetero)aromatic nitro compounds like 2-substituted nitrobenzene 16a–c and 2-nitrothiophene 17 in the condensation reaction. Reactions took place in warm methanolic solution of potassium hydroxide, forming corresponding heterocyclic analogues of arylcyanomethylenequinone oximes 18–21 (Scheme 9). The authors mentioned that yields of all reactions were >10%.
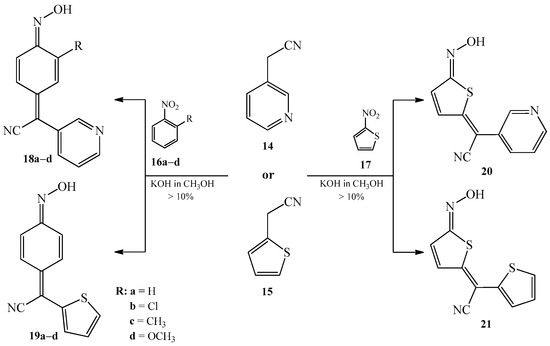
Scheme 9. Condensation reaction of 3-pyridylacetonitrile 14 or 2-thienylacetonitrile 15 with 2-substituted nitrobenzene analogues 16a–c and 2-nitrothiophene 17 to produce oximes 18–21.
Continuing the study of synthesis of such heterocyclic oximes, in 1968, Fournari and Marey [7] reported on the study of reactions between heteroarylacetonitriles 14 and 15 with (hetero)aromatic nitro compounds 17 and 22–24. Reactions took place in a way analogous to the previous one, namely, deploying methanolic solution of potassium hydroxide, forming corresponding oximes 20 and 25–27 (Scheme 10a–d), thereby increasing the range of known oxime derivatives. The compounds were formed in low to moderate yields.
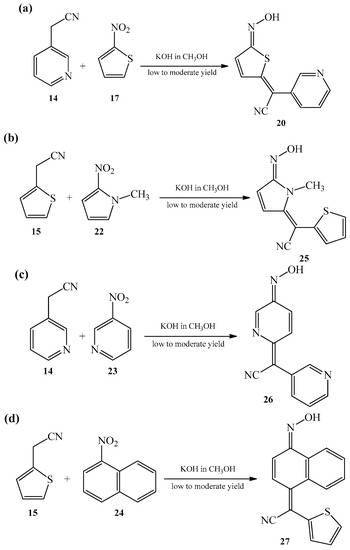
Scheme 10. Condensation reaction between nitriles 14 and 15 and (hetero)aromatic nitro compounds 17, 22–24 forming heterocyclic analogues 20, 25 (a,b), heteroatom containing 26 (c), and bi(carbo)cyclic 27 (d) heteroarylcyanomethylenequinone oximes.
In the same year, Mitchell in two patents [8][9] described synthesis of derivatives of bis (phenylacetonitrile) oxime 29 and 31a–b in a reaction of condensation of benzyl cyanide 1a with 2,2′-dinitrobibenzyl 28 (Scheme 11a) as well as 1,4-bis (cyanomethyl) benzene 30a with nitrobenzene 2a (Scheme 11b) and 1,4-bis (cyanomethyl) diphenyl ether 30b, also, with nitrobenzene 2a (Scheme 11b). All reactions were realized in warm alcoholic solution of potassium hydroxide.
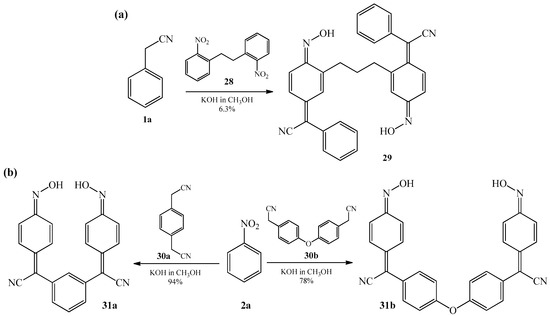
Scheme 11. (a) Condensation reaction of benzyl cyanide 1a with 2,2′-dinitrobibenzyl 28; (b) Condensation reaction of nitrobenzene 2a with 1,4-bis(cyanomethyl)benzene 30a and 1,4-bis (cyanomethyl) diphenyl ether 30b.
In the 1970s, Takahashi et al. began studying the possibility of deploying catalysts and their effect on the course of the condensation reactions. Their research led to forming 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a in a reaction of benzyl cyanide 1a with nitrobenzene 2a mediated by base catalyst [10]. In 1984, Arseniyadis et al. [11] described the mechanisms of addition and substitution reactions of nitrile-stabilized carbanions, including the mechanism of the formation of 4-quinone methide oximes. Later, Freyne and Raeymaekers in 1991 [12], Freyne et al. in 1996 [13], Makosza and Wróbel in 1996 [14], Wróbel in 2000 [15], Yamato et al. in 2000 [16], Suwiński et al. in 2003 [17], Orlov et al. in 2007 [18], Konovalova et al. in 2008 [19], Orlov et al. in 2009 [20], Buehler et al. in 2010 [21], Orlov et al. in 2010 [22], and Hong et al. in 2016 [1] mentioned the synthesis of 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a by the already presented condensation of benzyl cyanide 1a with 4-unsubstituted nitrobenzenes 11a–n with attempts to optimize the conditions of these reactions via the use of catalysts, and changes of solvents, reaction times and temperature regimes, among other modifications.
In 2015, Kouakou et al. [23] used the already known approach of regioselective nucleophilic substitution of various 4-substituted benzyl cyanides 1a–d with N-alkyl-7-nitroindazoles 32a–b (Scheme 12). Reactions took place in methanolic solution of potassium hydroxide, forming corresponding 2-(7-hydroxyimino-1-alkyl-1,7-dihydroindazol-4-ylidene)-2-arylacetonitriles 33aa–db (Table 2).
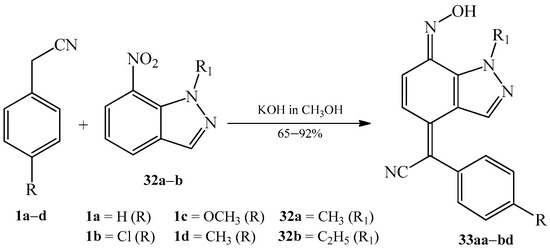
Scheme 12. Condensation reaction of various 4-substituted benzyl cyanides 1a–d with N-alkyl-7-nitroindazoles 32a–b to produce quinone oximes 33aa–db.
Table 2. Analogues of 2-(7-hydroxyimino-1-alkyl-1,7-dihydroindazol-4-ylidene)-2-arylacetonitriles 33aa–db.
| Product (Yield (%)) |
R | R1 | Product (Yield (%)) |
R | R1 | ||
|---|---|---|---|---|---|---|---|
| 33aa | (67) | -H | -CH3 | 33ca | (65) | -OCH3 | -CH3 |
| 33ab | (69) | -H | -C2H5 | 33cb | (70) | -OCH3 | -C2H5 |
| 33ba | (86) | -Cl | -CH3 | 33da | (90) | -CH3 | -CH3 |
| 33bb | (76) | -Cl | -C2H5 | 33db | (92) | -CH3 | -C2H5 |
Alternative approaches to the synthesis of quinone methide oxime derivatives are severely underrepresented in the literature.
2. The Conversion of Quinone Methide Derivatives Using Hydroxylamine
As an alternative approach to the synthesis of quinone methide derivatives of oximes, the reaction of the corresponding quinone methide derivatives with the addition of hydroxylamine should be considered. This method has been known for a long time and has become more common in recent years for the conversion of a keto group into an oxime [24][25][26][27]. As an alternative transformation, which was not used for the synthesis of phenylcarboxymethylomethylenecyclohexa-2,5-dien-1-one oxime core, was presented by Wróbel in 2000 [15]. In that approach, the reaction between the nitroarene and phenylacetic ester furnished the nitroso compound which was in equilibrium with the oxime form.
In 2017, Piazza et al. [28] patented the synthesis of quinone methide oximes 36a–d. The processes were two-stepped. The first stage consisted of conversion of the corresponding hydroxyl derivatives 34a–d to quinone methides 35a–d. The reactions were realized using 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ). Dichloromethane (DCM) was used as a solvent. The reactions took place at 0 °C. As the last step, the quinone methides 35a–d were converted to quinone methide oximes 36a–d by the Gilman reagent ((CH3)2CuLi) and hydroxylamine (NH2OH), under reflux, with methanol as a solvent (Scheme 13).
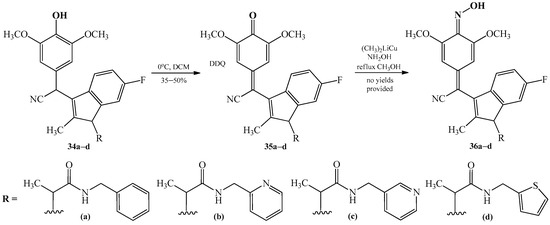
Scheme 13. Two-stepped transformation reaction of hydroxylamine 34a–d to produce quinone methide oximes 36a–d. The structures of substituents R represent drawings (a–d).
Methods applying quinone methides in obtaining arylcyanomethylenequinone oximes are virtually absent. This is due to the multitude of synthesis steps required in those type of processes leading to arylcyanomethylenequinone [29][30] (Scheme 14). In the presence of one-step condensation reaction of benzyl cyanide derivatives with 4-unsubstituted nitroarenes or their analogues, which is easier to adapt, the absence of processes deploying quinone methides is understandable.
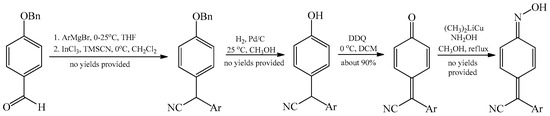
Scheme 14. A concept of multistep transformation of 4-alkoxybenzaldehydes to produce arylcyanomethylene quinone oximes.
References
- Hong, Z.; Li, J.J.; Chen, G.; Jiang, H.J.; Yang, X.F.; Pan, H.; Su, W.K. Solvent-free mechanochemical synthesis of arylcyanomethylenequinone oximes from phenylacetonitriles and 4-unsubstituted nitroaromatic compounds using KF/nano-gamma-Al2O3 as catalyst. RSC Adv. 2016, 6, 13581–13588.
- Davis, R.B.; Pizzini, L.C.; Benigni, J.D. The condensation of aromatic nitro compounds with arylacetonitriles. I. Nitrobenzene. J. Am. Chem. Soc. 1960, 82, 2913–2915.
- Lichtenberger, J.; Weiss, F. Derivatives of phenylfiuoroform. I. Trifiuoromethylbanzophenones. Bull. Soc. Chim. Fr. 1962, 1, 587–593.
- Davis, R.B.; Pizzini, L.C.; Bara, E.J. The condensation of aromatic nitro compounds with arylacetonitriles. III. Some ortho- and meta-substituted nitrobenzenes. J. Org. Chem. 1961, 26, 4270–4274.
- Davis, R.B. Arylcyanomethylenequinone Oximes. U.S. Patent Publication No. US3156704A, 10 November 1964.
- Marey, T.; Fournari, P.; Tirouflet, J. Condensation of nitriles with aromatic and heterocyclic nitro derivatives. Compt. Rend. 1965, 260, 4340–4342.
- Fournari, P.; Marey, T. Synthesis and spectroscopic results of the products resulting from the condensation of aromatic or heterocyclic nitro-derivatives with acetonitrile substituted with an aromatic or a heterocyclic group. Bull. Soc. Chim. Fr. 1968, 8, 3223–3233.
- Mitchell, C.D. Phenylacetonitrile Oximes. U.S. Patent Publication No. US3374248A, 19 March 1968.
- Mitchell, C.D. Bis(phenylacetonitrile oximes) as Ultraviolet Light Absorbers in Plastics. U.S. Patent Publication No. US3374249A, 19 March 1968.
- Takahashi, K.; Tsuboi, T.; Kazutoshi, Y.; Hirotada, I. Studies on the base-catalyzed reaction. VIII. Reactions of cyanophenylmethanide ion with nitrobenzene. Nippon Kagaku Kaishi 1976, 1, 144–147.
- Arseniyadis, S.; Kyler, K.S.; Watt, D.S. Addition and substitution reactions of nitrile-stabilized carbanions. Org. React. 1984, 31, 1–364.
- Freyne, E.J.E.; Raeymaekers, A.H.M. Positive Inotropic and Lusitropic 3,5-dihydroimidazoquinazolin-2(1H)-one Derivatives, Compositions and Use. U.S. Patent Publication No. US5043327A, 27 August 1991.
- Freyne, E.J.E.; Raeymaekers, A.H.M.; De Chaffoy, D.C.D. 1,3-Dihydro-2H-imidazoquinolin-2-one Derivatives as Inhibitors of Phosphodiesterase III and IV. U.S. Patent Publication No. US5521187A, 28 May 1996.
- Makosza, M.; Wróbel, Z. Conversion of 1-(o-nitroaryl) methyl p-tolyl sulfones into anthranilic ester analogues. Acta Chem. Scand. 1996, 50, 646–648.
- Wróbel, Z. Synthesis of 4-arylsulfonylquinolines by double condensation of allyl aryl sulfones with nitroarenes. Eur. J. Org. Chem. 2000, 3, 521–525.
- Yamato, H.; Asakura, T.; Birbaum, J.L.; Kurt, D. Oximes and Use Thereof as Latent Acids for Photoresists. International Patent Publication No. WO2000026219A1, 11 May 2000.
- Suwiński, J.; Świerczek, K.; Wagner, P.; Kubicki, M.; Borowiak, T.; Stowikowska, J. Oximes as intermediates or final products in reactions of nitroheteroarenes with nucleophiles in the presence of sodium methoxide-methanol system. J. Heterocycl. Chem. 2003, 40, 523–528.
- Orlov, V.Y.; Bazlov, D.A.; Ganzha, V.V.; Kotov, A.D.; Rusakov, A.I. Direction of the nucleophilic substitution reaction of hydrogen in nitrobenzene by arylacetonitrile carbanion. Bashk. Khim. Zh. 2007, 14, 22–25.
- Konovalova, N.V. Aromatic nucleophilic substitution of hydrogen in nitroarenes by phenylacetonitrile carbanion. Bashk. Khim. Zh. 2008, 15, 103–104.
- Orlov, V.Y. Selection of optimal conditions for synthesis of 2arylacetonitriles. Khim. Tekhnolog. 2009, 10, 393–395.
- Buehler, S.; Ott, M.; Pfleiderer, W. Photolabile Protective Groups for Improved Processes to Prepare Oligonucleotide Arrays. U.S. Patent Publication No. US7759513B2, 20 July 2010.
- Orlov, V.Y.; Kotov, A.D.; Bazlov, D.A.; Ganzha, V.V.; Konovalova, N.V. Theoretical calculation of the equilibrium constant of nitroso-oxime tautomerization of phenylcyanomethylene-2,5-cyclohexadien-1-one monooxime. Izv. Vyss. Uchebnykh Zaved. 2010, 53, 124–125.
- Kouakou, A.; Abbassi, N.; Chicha, H.; Ammari, L.E.; Saadi, M.; Rakib, E.M. Synthesis of novel substituted indazoles via nucleophilic substitution of hydrogen (SNH). Heteroat. Chem. 2015, 26, 374–381.
- Li, X.Z.; Jiang, S.Y.; Li, G.Q.; Jiang, Q.R.; Li, J.W.; Li, C.C.; Han, Y.Q.; Song, B.L.; Ma, X.R.; Qi, W.; et al. Synthesis of heterocyclic ring-fused analogs of HMG499 as novel degraders of HMG-CoA reductase that lower cholesterol. Eur. J. Med. Chem. 2022, 236, e114323.
- Qiu, W.; Song, B.; Jiang, Q.; Jiang, S.; Shi, X.; Li, C. Cholic Acid Derivatives as HMG-CoA Reductase Inhibitors and Their Preparation, Pharmaceutical Compositions and Use in the Reducing Cholesterol. China Patent Publication No. CN113,563,405A, 29 October 2021.
- Zheng, X.; Zhang, Y.; Fu, C. Preparation of Antiviral Drug Molnupiravir Using Microchannel Reactor. China Patent Publication No. CN112608357A, 6 April 2021.
- Zhao, P.; Guo, Y.; Luan, X. Total synthesis of dalesconol a by Pd (0)/norbornene-catalyzed three-fold domino reaction and Pd (II)-catalyzed trihydroxylation. J. Am. Chem. Soc. 2021, 143, 21270–21274.
- Piazza, G.A.; Chen, X.; Keeton, A.B.; Boyd, M.R. Compounds, Compositions and Methods of Treating Cancer. International Patent Publication No. WO2017106520A1, 22 June 2017.
- Wang, L.; Wang, N.; Qi, Y.; Sun, S.; Liu, X.; Li, W.; Liu, L. Synthesis of sterically hindered α-aminonitriles through 1,6-aza-conjugate addition of anilines to δ-cyano substituted para-quinone methides. Chin. J. Org. Chem. 2020, 40, 3934–3943.
- Zhu, Y.; Wang, H.; Wang, G.; Wang, Z.; Liu, Z.; Liu, L. Enantioselective construction of single and vicinal all-carbon quaternary stereocenters through ion-pair-catalyzed 1,6-conjugate addition. Org. Lett. 2021, 23, 7248–7253.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
504
Revisions:
2 times
(View History)
Update Date:
17 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




