Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Riccardo Amorati | -- | 1451 | 2023-10-13 03:49:38 | | | |
| 2 | Rita Xu | Meta information modification | 1451 | 2023-10-13 03:52:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
De Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; De Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Chemistry of Essential Oils. Encyclopedia. Available online: https://encyclopedia.pub/entry/50234 (accessed on 08 March 2026).
De Sousa DP, Damasceno ROS, Amorati R, Elshabrawy HA, De Castro RD, Bezerra DP, et al. Chemistry of Essential Oils. Encyclopedia. Available at: https://encyclopedia.pub/entry/50234. Accessed March 08, 2026.
De Sousa, Damião P., Renan Oliveira S. Damasceno, Riccardo Amorati, Hatem A. Elshabrawy, Ricardo D. De Castro, Daniel P. Bezerra, Vitória Regina V. Nunes, Rebeca C. Gomes, Tamires C. Lima. "Chemistry of Essential Oils" Encyclopedia, https://encyclopedia.pub/entry/50234 (accessed March 08, 2026).
De Sousa, D.P., Damasceno, R.O.S., Amorati, R., Elshabrawy, H.A., De Castro, R.D., Bezerra, D.P., Nunes, V.R.V., Gomes, R.C., & Lima, T.C. (2023, October 13). Chemistry of Essential Oils. In Encyclopedia. https://encyclopedia.pub/entry/50234
De Sousa, Damião P., et al. "Chemistry of Essential Oils." Encyclopedia. Web. 13 October, 2023.
Copy Citation
The term essential oil was created in the 16th century and refers to the theory of “Quinta essentia” proposed by the famous German-Swiss alchemist and physician Paracelsus (1493–1541), born by Theophrastus Philippus Aureolus Bombastus von Hohenheim.
natural products
metabolites
medicinal plants
volatiles
1. Introduction
Paracelsus defined the role of alchemy by developing plant extracts and herbal medicines. He believed that the distillation process extracted the most significant part of the plant, namely, the “quintessence for healing” or “plant’s soul”, separating the “essential” constituents from the “nonessential” [1][2].
Many authors have tried to supply a definition of essential oils. According to the “Association Française de NORmalisation” [3] and to the European Pharmacopoeia (Ph. Eur.), an “essential oil” can be defined as a “product obtained from a natural raw material of plant origin, either by distillation with water or steam, or from the epicarp of Citrus sp. fruits by a mechanical process, or by “dry distillation”. Essential oil is then separated from the aqueous phase by physical means” [3][4].
Plants can synthesize two kinds of oils: fixed and essential oils. Fixed oils are esters of a glycerol molecule attached to three fatty acids, also called triacylglycerols or triglycerides. Essential oils (EOs), also known as essences, volatile oils, etheric oils, or aetheroleum, are complex natural mixtures of volatile, lipophilic, and odoriferous substances commonly found in aromatic plants. The majority of essential oils are colorless or pale yellow, liquid at room temperature, and less dense than water, with very few exceptions (cinnamon, sassafras, and vetiver). Moreover, essential oil chemical constituents have a low molecular weight (below 300), and some of them are optically active, soluble in most organic solvents (ether, alcohol, acetone), and insoluble in water [2][5][6].
EOs have been widely investigated for their therapeutic potential in various pathologies [7][8][9]. Biological and pharmacological tests using EOs and their chemical constituents performed via experimental models at the molecular, cellular, and animal levels have generated promising results in several areas of science [10][11]. Their pharmacological profile includes antimicrobial, anti-inflammatory, antitumor, antioxidant activities, among others [10][12][13][14].
2. Chemistry of Essential Oils
In general, essential oils are composed of approximately 20–60 components at different concentrations, but some of them may contain more than 300 different substances. However, two or three components are usually present in large proportions (20–70%) compared to other constituents present in small concentrations [15]. For example, rotundifolone is the major component (50–65%) of Mentha x villosa Hudson leaf essential oil [16], 1,8-cineole or eucalyptol (70–90%) of Eucalyptus globulus Labill. essential oil [17], and cinnamaldehyde (60–90%) of Cinnamomum zeylanicum Blume bark and leaf essential oil [18]. Typically, the major components of essential oils are the main components responsible for their biological properties. However, minor compounds may also play an important role in bioactivity, either by potentiating the action of major components or through antagonistic or additive effects [19].
Essential oil components possess distinct primary metabolic precursors and are generated through different biosynthetic pathways. They can be divided into two main groups: terpenoids (major group) and non-terpenoids (mainly phenylpropanoids). All of them are hydrocarbons and their oxygenated derivatives, and may exist in the form of several chemical classes, including aldehydes, ketones, alcohols, oxides, esters, amines, amides, phenols, nitrogen and sulfur compounds, and heterocycles [5][20][21][22].
2.1. Terpenes
Terpenes may be considered to be made up of isoprene units and constitute one of the largest and most structurally diverse families (>50,000 molecules) of natural products. Still more numerous than terpenes is a class of compounds named “terpenoids” (or isoprenoids). Terpenes are hydrocarbons, while terpenoids are a modified class of terpenes that present oxygen-containing functional groups, such as ketone, hydroxy, aldehyde, ether, or carboxylic moieties. Chemical structures of terpenes may range from linear to mono- or polycyclic compounds, and their skeleton is formed through the condensation of two to many thousands of 5-carbon-base (C5) units (isoprene units) [23][24][25].
Isopentenyl diphosphate (IPP) and its allylic isomer dimethylallyl diphosphate (DMAPP) are the universal precursors of isoprene molecules [26]. In higher plants, two distinct and independent biochemical routes are involved in isoprenoid biosynthesis: the mevalonate (MVA) pathway, the first and classically acknowledged route for biosynthesis of DMAPP and IPP, and the methylerythritol 4-phosphate (MEP) pathway (non-mevalonate pathway). Moreover, they may also proceed via a combination of both the MVA and the MEP routes. The MVA pathway is localized to the cytosol, whereas the MEP pathway is bound to the plastidic compartment [27][28].
Researchers can classify the terpenes into several categories according to the number of C5 building blocks in their core structure: hemiterpenes (C5H8), monoterpenes (C10H16), sesquiterpenes (C15H24), diterpenes (C20H32), triterpenes (C30H48), tetraterpenes or carotenoids (C40H64), and polyterpenes [(C5H8)n] [29]. Among the terpenic constituents, the mono- and sesquiterpenes are the most volatile and abundant in essential oils [30][31]. Figure 1 contains a general scheme for terpenoid biosynthesis.
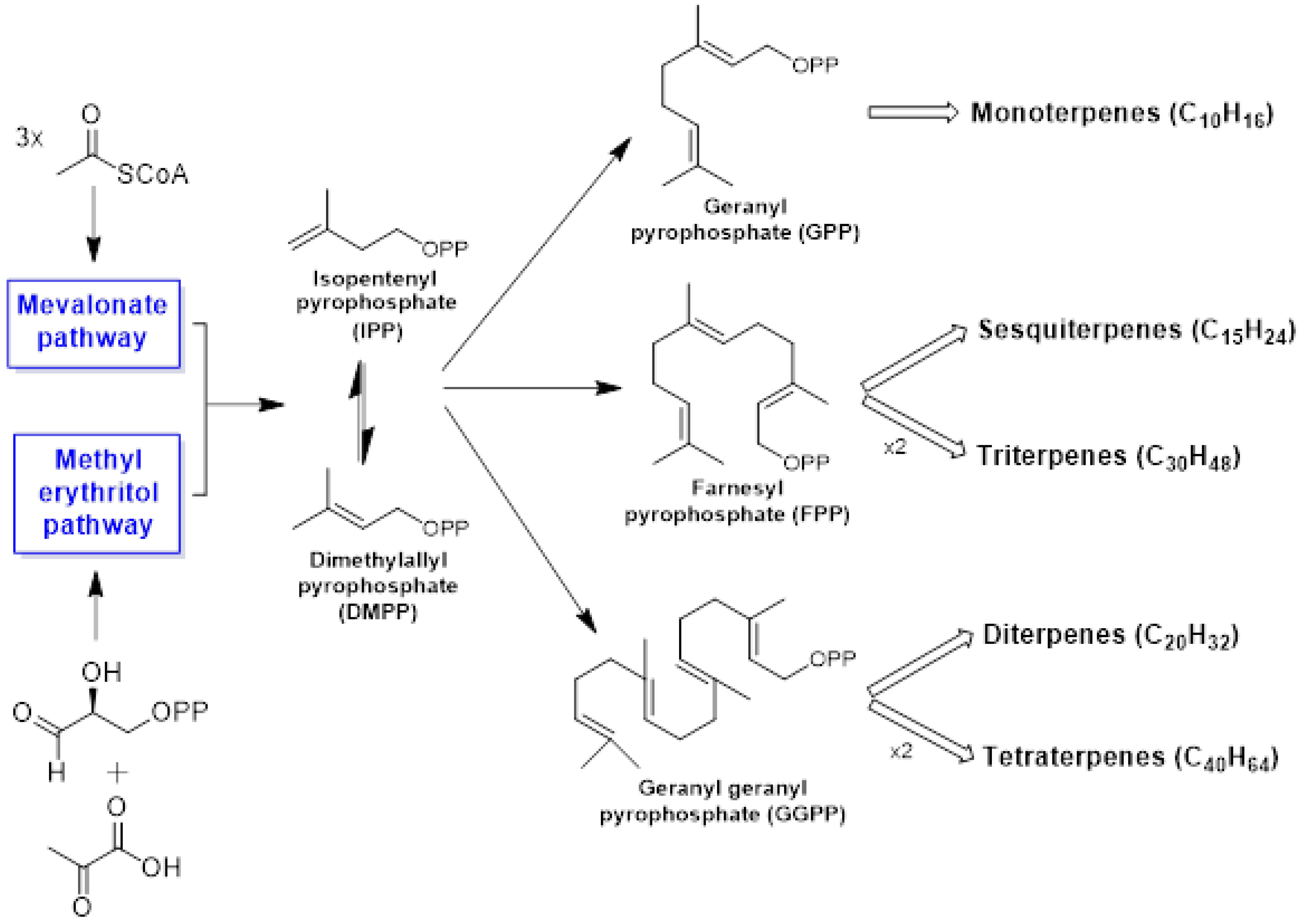
Figure 1. General scheme for the biosynthesis of terpenoids.
Monoterpenes (from the Latin mono, or one) are the most representative and simple terpenes. They are produced from the assembly of two isoprene units (C10), constituting about 90% of plant essential oils and permitting a very large variety of chemical structures (around 1000 metabolites). According to their structural variation, monoterpenes can be divided into different subgroups: acyclics, bornanes, camphanes and isocamphanes, fenchanes, thujanes, p-menthanes, and caranes and pinanes (Figure 2). The variety of possibilities in which these basic skeletons can be rearranged results in amazing structural diversity observed in nature, in which p-menthane-type monoterpenes are the largest group of naturally occurring monoterpenes [21][32].
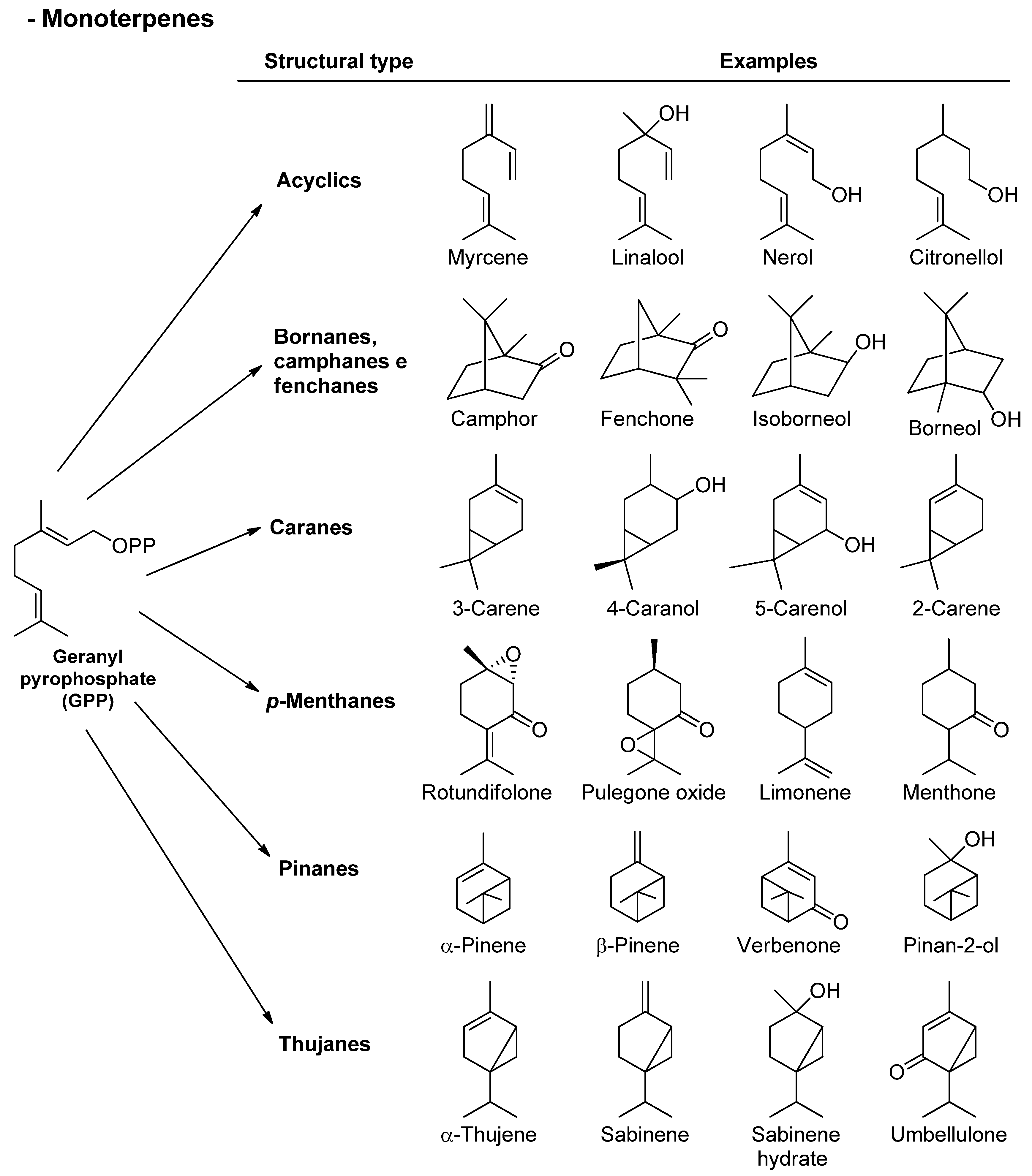
Figure 2. Chemical structures of monoterpenes found in essential oils.
Sesquiterpenes (from the Latin sesqui, or one and a half) are built from the coupling of three isoprene molecules (C15), occurring naturally in insects and higher plants. They are the most diverse group of terpenoids and may have acyclic (linear), monocyclic, bicyclic, or tricyclic frameworks, showing many unique arrangements. Similar to monoterpenes, sesquiterpenes can also occur as hydrocarbons or contain oxygen functionality, including carboxylic acids, lactones, alcohols, aldehydes, ketones, and epoxides. Chain extension with augmentation in a number of cyclizations as well as biochemical modifications (rearrangement or oxidation) allows a good variety of structures from the sesquiterpenes (Figure 3) [21][33].
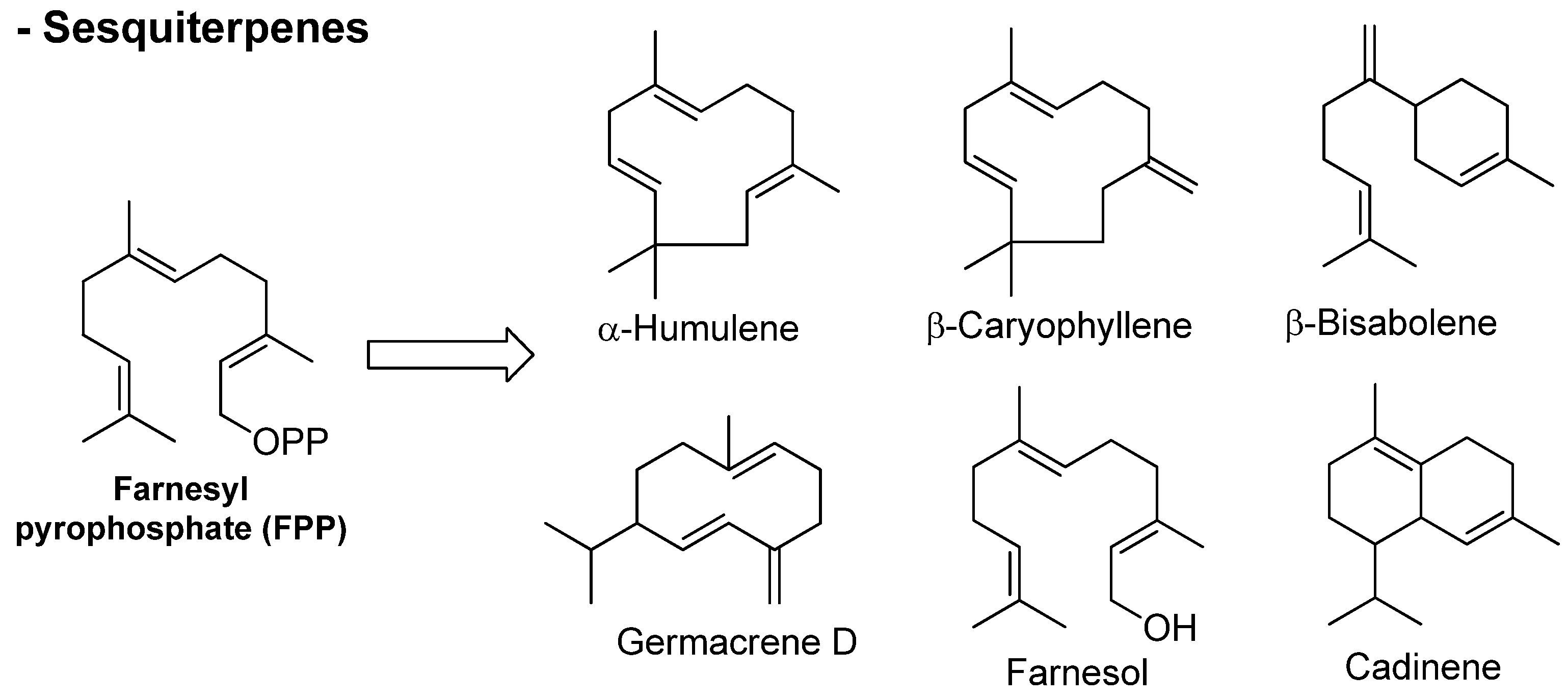
Figure 3. Chemical structures of sesquiterpenes found in essential oils.
2.2. Phenylpropanoids
Phenylpropanoids constitute a group of organic compounds synthesized by plants via the shikimate pathway; the aromatic amino acid L-phenylalanine is their biogenetic precursor. The core of phenylpropanoids consists of a phenyl ring connected to a C3 propane moiety. They are found in the plant kingdom, but are less common than terpenes. The wonderful diversity of phenylpropanoids derives from the efficient modification of a very limited set of core structures. Aromatic compounds originated from the shikimate route (Figure 4) comprise aldehyde, phenol, alcohol, methoxy, and methylenedioxy compounds [34][35]. Chemical structures of different essential oil constituents (mono- and sesquiterpenes, and phenylpropanoids) are depicted in Figure 2, Figure 3 and Figure 4.

Figure 4. Chemical structures of phenylpropanoids found in essential oils.
Generally, essential oils represent a small fraction of plant composition (less than 5% of vegetal dry matter), occurring in specialized secretory structures, such as secretory ducts or cavities, glandular trichomes, and oil cells [36][37]. Volatile oils can be extracted from seeds (Foeniculum vulgare Mill.), flowers (Rosa abietina Gren. ex H. Christ), leaves (Mentha × piperita L.), barks (Cinnamomum cassia (L.) J. Presl), fruits (Citrus sinensis (L.) Osbeck; Spondias mombin L.), grasses (Cymbopogon citratus (DC.) Stapf.), tree blossoms (Cananga odorata (Lam.) Hook. f. & Thomson), rhizomes (Zingiber officinale Roscoe), roots (Vetiveria zizanoides (L.) Nash), woods (Juniperus virginiana L.), gums (Boswellia ameero Balf. f.), and bulbs (Allium sativum L.) [38][39]. Typically, essential oils obtained from different plant organs of the same plant possess specific chemical compositions.
Volatile oils are reasonably widespread in the plant kingdom, being rarely found in both gymnosperms and monocotyledonous angiosperms, and widely distributed in dicotyledonous angiosperms [38]. They are synthesized by more than 17,500 vegetal species, belonging to several genera distributed in approximately 60 families. Some plant families are well-known for their ability to produce aromatic plants rich in essential oils, including Apiaceae (Umbelliferae), Hypericaceae, Myrtaceae, Poaceae, Asteraceae (Compositae), Lamiaceae (Labiatae), Zingiberaceae, Cupressaceae, Lauraceae, Pinaceae, Piperaceae, and Rutaceae [40]. All of the essential oils-producing plant families are rich in terpenoids, while phenylpropanoids are more commonly found in specific plant families, including Apiaceae, Lamiaceae, Myrtaceae, Piperaceae, and Rutaceae [41].
The chemical composition and quality of essential oils may vary due to various factors, such as the number and type of molecules, stereochemistry of constituents, and employed extraction process (hydrodistillation, effleurages, Soxhlet extraction, cold pressing, supercritical fluid extraction). Furthermore, environmental conditions (climate, cultivation, plant organ, harvesting time, light intensity, soil composition, age, and vegetative cycle stage extraction) may also lead to changes in the quality, quantity, and composition of extraction products [42].
Currently, about 3000 aromatic plants are known to produce essential oils as an important part of their secondary metabolism, 300 of which are commercially significant due to their medicinal and industrial values. They are employed in different applications, such as pharmaceuticals, agronomy, sanitary applications, cosmetics, perfumes, dentistry, agriculture, and food [24][43].
References
- Guenther, E. The Essential Oils: Individual Essential Oils of the Plant Families Gramineae, Lauraceae, Burseraceae, Myrtaceae, Umbelliferae and Geraniaceae, 4th ed.; Van Nostrand: Paris, France, 1950; pp. 1–752.
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25.
- Association Française de Normalisation (AFNOR). Huiles Essentielles, Tome 2, Monographies Relatives Aux Huiles Essentielles, 6th ed.; AFNOR, Association Française de Normalisation: Paris, France, 2000.
- Do, T.K.T.; Hadji-Minaglou, F.; Antoniotti, S.; Fernandez, X. Authenticity of essential oils. TrAC Trends Anal. Chem. 2015, 66, 146–157.
- Sell, C. Chemistry of essential oils. In Handbook of Essential Oils. Science, Technology, and Applications; Bașer, K.H., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 121–150.
- Schmidt, E. Production of essential oils. In Handbook of Essential Oils. Science, Technology, and Applications; Bașer, K.H., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 83–119.
- De Cássia da Silveira e Sá, R.; Lima, T.C.; Da Nóbrega, F.R.; De Brito, A.E.M.; De Sousa, D.P. Analgesic-like Activity of Essential Oil Constituents: An Update. Int. J. Mol. Sci. 2017, 18, 2392.
- De Sousa, D.P.; Silva, R.H.N.; Silva, E.F.d.; Gavioli, E.C. Essential Oils and Their Constituents: An Alternative Source for Novel Antidepressants. Molecules 2017, 22, 1290.
- De Sousa, D.P.; Hocayen, P.D.A.S.; Andrade, L.N.; Andreatini, R. A Systematic Review of the Anxiolytic-like Effects of Essential Oils in Animal Models. Molecules 2015, 20, 18620–18660.
- Pandey, V.K.; Tripathi, A.; Srivastava, S.; Dar, A.H.; Singh, R.; Farooqui, A.; Pandey, S. Exploiting the bioactive properties of essential oils and their potential applications in food industry. Food Sci. Biotechnol. 2023, 32, 885–902.
- Sattayakhom, A.; Wichit, S.; Koomhin, P. The Effects of Essential Oils on the Nervous System: A Scoping Review. Molecules 2023, 28, 3771.
- Zhao, Q.; Zhu, L.; Wang, S.; Gao, Y.; Jin, F. Molecular mechanism of the anti-inflammatory effects of plant essential oils: A systematic review. J. Ethnopharmacol. 2023, 301, 115829.
- Machado, T.Q.; da Fonseca, A.C.C.; Duarte, A.B.S.; Robbs, B.K.; de Sousa, D.P. A Narrative Review of the Antitumor Activity of Monoterpenes from Essential Oils: An Update. Biomed. Res. Int. 2022, 2022, 6317201.
- Kong, A.S.-Y.; Maran, S.; Yap, P.S.-X.; Lim, S.-H.E.; Yang, S.-K.; Cheng, W.-H.; Tan, Y.-H.; Lai, K.-S. Anti- and Pro-Oxidant Properties of Essential Oils against Antimicrobial Resistance. Antioxidants 2022, 11, 1819.
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils-Present Status and Future Perspectives. Medicines 2017, 4, 58.
- Lima, T.C.; Silva, T.K.M.; Silva, F.L.; Barbosa Filho, J.M.; Marques, M.O.M.; La Corte, R.; Cavalcanti, S.C.H.; Sousa, D.P. Larvicidal activity of Mentha x villosa Hudson essential oil, rotundifolone and derivatives. Chemosphere 2013, 104, 37–43.
- Boukhatem, M.N.; Boumaiza, A.; Nada, H.G.; Rajabi, M.; Mousa, S.A. Eucalyptus globulus essential oil as a natural food preservative: Antioxidant, antibacterial and antifungal properties in vitro and in a real food matrix (orangina fruit juice). Appl. Sci. 2020, 10, 5581.
- Behbahani, B.A.; Falah, F.; Arab, F.L.; Vasiee, M.; Yazdi, F.T. Chemical Composition and antioxidant, antimicrobial, and antiproliferative activities of Cinnamomum zeylanicum bark essential oil. Evid. Based Complement. Altern. Med. 2020, 2020, 5190603.
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006.
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811.
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475.
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53.
- Dewick, P. The biosynthesis of C5-C25 terpenoid compounds. Nat. Prod. Rep. 2002, 19, 181–222.
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Based Complement. Altern. Med. 2014, 2014, 651593.
- Tian, H.; Zada, B.; Singh, B.H.; Wang, C.; Kim, S.-W. Synthetic Biology Approaches for the Production of Isoprenoids in Escherichia coli. In Current Developments in Biotechnology and Bioengineering, 1st ed.; Singh, S.P., Pandey, A., Du, G., Kumar, S., Eds.; Elsevier: New York, NY, USA, 2020; pp. 311–329.
- Leite, A.C.; Lopes, A.A.; Massuo, J.K.; Bolzani, V.d.S.; Furlan, M. Biosynthetic origin of the isoprene units in chromenes of Piper aduncum (Piperaceae). J. Braz. Chem. Soc. 2007, 18, 1500–1503.
- Lichtenthaler, H.; Rohmer, M.; Schwender, J. Two independent biochemical pathways for isopentenyl diphosphate and isoprenoid biosynthesis in higher plants. Physiol. Plantarum. 2006, 101, 643–652.
- Chatzivasileiou, A.O.; Ward, V.; Edgar, S.M.; Stephanopoulos, G. Two-step pathway for isoprenoid synthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 506–511.
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural products. In Biochemistry and Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; American Society of Plant Physiology: Rockville, MD, USA, 2000; Volume 24, pp. 1250–1318.
- Simões, C.M.O.; Schenkel, E.P.; Gosmann, G.; Mello, J.C.P.; Mentz, L.A.; Petrovik, P.R. Farmacognosia: Da Planta ao Medicamento; Editora UFRGS: Porto Alegre, Brazil, 2010.
- Jaeger, R.; Cuny, E. Terpenoids with Special Pharmacological Significance: A Review. Nat. Prod. Commun. 2016, 11, 1373–1390.
- Mahmoud, S.S.; Croteau, R.B. Strategies for transgenic manipulation of monoterpene biosynthesis in plants. Trends Plant. Sci. 2002, 7, 366–373.
- Avila, C. Terpenoids in Marine Heterobranch Molluscs. Mar. Drugs 2020, 18, 162.
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20.
- Deng, Y.; Lu, S. Biosynthesis and Regulation of Phenylpropanoids in Plants. Crit. Rev. Plant Sci. 2017, 36, 257–290.
- Feijó, E.V.R.S.; Oliveira, R.A.; Costa, L.C.B. Light affects Varronia curassavica essential oil yield by increasing trichomes frequency. Rev. Bras. Farmacogn. 2014, 24, 516–523.
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243.
- Butnariu, M.; Sarac, I. Essential Oils from Plants. J. Biotechnol. Biomed. Sci. 2018, 1, 35–43.
- Ampadu, G.A.A.; Mensah, J.O.; Darko, G.; Borquaye, L.S. Essential Oils from the Fruits and Leaves of Spondias mombin Linn.: Chemical Composition, Biological Activity, and Molecular Docking Study. Evid. Based Complement. Altern. Med. 2022, 2022, 7211015.
- Vigan, M. Essential oils: Renewal of interest and toxicity. Eur. J. Dermatol. 2010, 20, 685–692.
- Chami, F.; Chami, N.; Bennis, S.; Trouillas, J.; Remmal, A. Evaluation of carvacrol and eugenol as prophylaxis and treatment of vaginal candidiasis in an immunosuppressed rat model. J. Antimicrob. Chemother. 2004, 54, 909–914.
- Fornari, T.; Vicente, G.; Vázquez, E.; García-Risco, M.R.; Reglero, G. Isolation of essential oil from different plants and herbs by supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 34–48.
- Saranraj, P.; Devi, V.D. Essential oils and its antibacterial properties—A review. Life Sci. Arch. 2017, 3, 994–1011.
More
Information
Subjects:
Chemistry, Medicinal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.4K
Revisions:
2 times
(View History)
Update Date:
13 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





