
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aiham Qdaisat | -- | 3315 | 2023-10-11 19:39:24 | | | |
| 2 | Camila Xu | Meta information modification | 3315 | 2023-10-12 02:53:08 | | |
Video Upload Options
Hemoptysis in cancer patients is a potentially serious symptom that requires detailed evaluation by oncologists and emergency department physicians. Timely diagnosis and appropriate management are crucial to address both the immediate concern of bleeding and the broader implications for the patient's cancer care. As hemoptysis in cancer patients indicates the presence of complications or progression of the disease, investigating the underlying cause using appropriate diagnostic procedures such as imaging studies (CT scans, bronchoscopy) and laboratory tests is vital, as it can significantly impact treatment choices and potentially alter the patient's overall prognosis. Risk stratification for cancer patients presenting with hemoptysis will support a personalized treatment approach that ensures that each patient receives tailored and effective care and identifies patients who are at a higher risk of deterioration, warranting more aggressive diagnostic and treatment plans and close, continuous monitoring for these patients.
1. Definition
2. Pathophysiology
3. Etiology
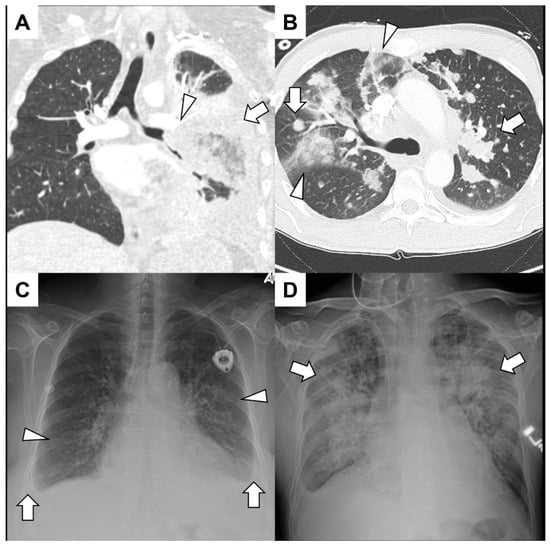
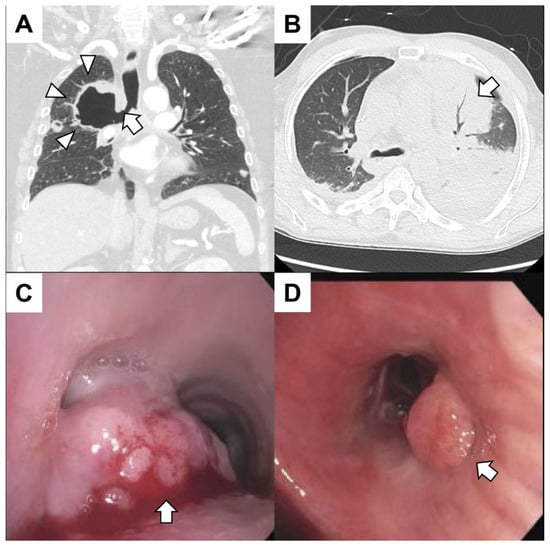
| Category | Disease |
|---|---|
| Pulmonary | Neoplasm *, e.g., bronchogenic carcinoma (second most common cause) Broncho-vascular fistula resulting from bronchopleural fistula [32], causing massive hemoptysis Tumors infiltrating the bronchial wall [33] Transformation of adenocarcinoma into squamous cell carcinoma [34] Endobronchial tumors (may present with hemoptysis in 15% of patients) Infection Tuberculosis (most common cause worldwide) Acute viral or bacterial infection Mucormycosis [35] Staphylococcus aureus [36] Invasive aspergillosis [35] Aspergillus mycetoma (fungus ball) Strongyloidiasis [37] Nontuberculous mycobacterial (Ntm) [38] Coccidioidomycosis [39] Actinomycosis [40] Pyogenic lung abscess [41] Cystic fibrosis Bronchiectasis * Lupus pneumonitis due to systemic lupus erythematosus |
| Cardiac | Mitral stenosis * Congestive heart failure * Pulmonary hypertension Congenital heart disease * |
| Vascular | Pulmonary embolism Vasculitis Goodpasture syndrome Behcet disease Granulomatosis with polyangiitis Pulmonary vascular fistula Vascular malformation (e.g., pulmonary artery aneurysm, arteriovenous malformations) * Pulmonary artery rupture Tracheoinnominate artery fistula Hereditary hemorrhagic telangiectasia |
| DAH * [42][43][44][45] | Typically presents with dyspnea, cough, hemoptysis and new alveolar infiltrates on chest imaging Hemoptysis is common but may be absent in up to one-third of patients |
| Hematologic | Anticoagulant use Coagulopathy Thrombocytopenia |
| Trauma | Airway trauma Lung contusion Foreign body |
| Iatrogenic | Injury to structures * during: Pulmonary artery catheterization Lung biopsy Airway stenting Right heart catheterization |
| Drugs and toxins | Penicillamine [46][47] Crack cocaine [22][48] Bevacizumab [13] |
| Other | Thoracic endometriosis (catamenial hemoptysis) Cryptogenic: no identified cause on CT or bronchoscopy (50% of cases in high-income countries) Idiopathic pulmonary hemosiderosis |
4. Epidemiology of Hemoptysis
5. Presentations and Investigations for Hemoptysis in Cancer Patients
5.1. Primary Lung Cancer
5.2. Pulmonary Metastasis
6. Risk stratification of hemoptysis in patients with cancer
| Study/Author(s) | Cohort | Risk Factors for Mortality, Prognosis, Other Findings |
|---|---|---|
| Grosu et al. [58] | Retrospective Patients with solid organ tumors and mild hemoptysis N = 112 |
Upon multivariate analysis, factors independently associated with improved survival had higher hemoglobin values (HR 0.78; 95% CI, 0.67–0.91) and cessation of hemoptysis without recurrence at 48 h (HR 0.43; 95% CI 0.22–0.84). Variables independently associated with worse survival were disease stage (HR 10.8; 95% CI, 2.53–46.08) and active bleeding with endobronchial lesion (HR 3.20; 95% CI 1.74–5.89). |
| Fartoukh et al. [3] | Retrospective Consecutive patients admitted to ICU with hemoptysis N = 1087 |
Independent predictors of mortality were mechanical ventilation at the time of referral, cancer diagnosis, aspergillosis, chronic alcoholism, pulmonary artery involvement and infiltrates involving two or more quadrants upon admission. A model-based score for prognosis was developed that assigned 1 point for chronic alcoholism, pulmonary artery involvement and radiographic patterns and 2 points for cancer, aspergillosis and mechanical ventilation. |
| Hirshberg et al., Vanni et al., Soares et al., and Uzun et al. [6][62][63][64] | Analytical cohort studies | Malignancy was a leading cause of hemoptysis, with a decrease in mortality related to bronchiectasis, lower respiratory tract infections and other less frequent causes. |
| Uzun et al. and Tsoumakidou et al. [64][65] | Analytical cohort studies | Malignancy was a leading underlying cause of hemoptysis with mortality rates ranging from 19.5% to 22%. |
| Soares et al., Petersen et al., and Abdulmalak et al. [60][61][62] | Analytical cohort studies | Lung cancer was the primary cause. Reported mortality rates varied significantly, ranging from 5.9% to 27%. |
| Petersen et al. [60] | Retrospective Consecutive patients with no malignancy suspected on chest CT N = 609 |
Predictors of mortality were advanced age, a previous lung cancer diagnosis, a current or previous smoking history, and concurrent lung diseases. |
| Mondoni et al. [59][66] | 2019 study: secondary analysis of an observational multicenter study N = 486 2021 study: prospective multicenter study N = 606 |
Recurrences indicated previously undetected pathological findings, as there was a recurrence of hemoptysis in 7 patients, of whom 3 were found to have lung cancer upon further investigation. Pulmonary neoplasms were the primary cause of death, and the overall mortality rate was 13.7%. |
| Tsoumakidou et al. [65] | Prospective cohort N = 184 |
No patients initially diagnosed with an etiology other than lung cancer were found to have lung cancer upon further investigation. |
| Abdulmalak et al. [61] | A 5-year retrospective cohort study N = 81,572 |
An initial diagnosis of respiratory infection with highest lung cancer detection rate (10.4%) during the follow-up, and lung cancer was the cause in 17.4% of patients. |
| Majhail et al. [67] | Prospective data review of patients who had hematopoietic stem cell transplantation (HSCT) with alveolar hemorrhage N = 116 |
Advanced age, utilization of an allogeneic donor source, administration of a myeloablative conditioning regimen and the occurrence of acute severe graft-versus-host-disease were identified as independent predictors associated with a heightened risk of alveolar hemorrhage following HSCT. The probability of 60-day survival from the onset of hemorrhage was determined to be 16% in the diffuse alveolar hemorrhage group and 32% for the idiopathic alveolar hemorrhage group. With the exception of 20 patients, all individuals in this study received a standard regimen of high-dose corticosteroids; among the patients who received corticosteroids, the 60-day survival rate was found to be 26%, while those who did not receive corticosteroids exhibited a 60-day survival rate of 25%. |
References
- Corder, R. Hemoptysis. Emerg. Med. Clin. N. Am. 2003, 21, 421–435.
- Khalil, A.; Soussan, M.; Mangiapan, G.; Fartoukh, M.; Parrot, A.; Carette, M.-F. Utility of high-resolution chest CT scan in the emergency management of haemoptysis in the intensive care unit: Severity, localization and aetiology. Br. J. Radiol. 2007, 80, 21–25.
- Fartoukh, M.; Khoshnood, B.; Parrot, A.; Khalil, A.; Carette, M.-F.; Stoclin, A.; Mayaud, C.; Cadranel, J.; Ancel, P.Y. Early Prediction of In-Hospital Mortality of Patients with Hemoptysis: An Approach to Defining Severe Hemoptysis. Respiration 2012, 83, 106–114.
- Sakr, L.; Dutau, H. Massive Hemoptysis: An Update on the Role of Bronchoscopy in Diagnosis and Management. Respiration 2010, 80, 38–58.
- Crocco, J.A.; Rooney, J.J.; Fankushen, D.S.; Di Benedetto, R.J.; Lyons, H.A. Massive hemoptysis. Arch. Intern. Med. 1968, 121, 495–498.
- Hirshberg, B.; Biran, I.; Glazer, M.; Kramer, M.R. Hemoptysis: Etiology, Evaluation, and Outcome in a Tertiary Referral Hospital. Chest 1997, 112, 440–444.
- Ibrahim, W.H. Massive haemoptysis: The definition should be revised. Eur. Respir. J. 2008, 32, 1131–1132.
- Dweik, R.A.; Stoller, J.K. Role of Bronchoscopy in Massive Hemoptysis. Clin. Chest Med. 1999, 20, 89–105.
- Ong, T.-H.; Eng, P. Massive hemoptysis requiring intensive care. Intensive Care Med. 2003, 29, 317–320.
- Bruzzi, J.F.; Rémy-Jardin, M.; Delhaye, D.; Teisseire, A.; Khalil, C.; Rémy, J. Multi–Detector Row CT of Hemoptysis. RadioGraphics 2006, 26, 3–22.
- Jougon, J. Massive hemoptysis: What place for medical and surgical treatment. Eur. J. Cardio Thorac. Surg. 2002, 22, 345–351.
- Kvale, P.A.; Selecky, P.A.; Prakash, U.B.S. Palliative Care in Lung Cancer. Chest 2007, 132, 368S–403S.
- Cho, Y.-J.; Murgu, S.D.; Colt, H.G. Bronchoscopy for bevacizumab-related hemoptysis. Lung Cancer 2007, 56, 465–468.
- Je, Y.; Schutz, F.A.; Choueiri, T.K. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: A systematic review and meta-analysis of clinical trials. Lancet Oncol. 2009, 10, 967–974.
- Sonpavde, G.; Bellmunt, J.; Schutz, F.; Choueiri, T.K. The Double Edged Sword of Bleeding and Clotting from VEGF Inhibition in Renal Cancer Patients. Curr. Oncol. Rep. 2012, 14, 295–306.
- Wong, J.; Gutierrez, C.; Shannon, V.R.; Eapen, G.A.; Faiz, S.A. Bronchomediastinal fistula after endobronchial ultrasound-guided transbronchial needle aspiration. Am. J. Respir. Crit. Care Med. 2016, 194, 114–115.
- Barber, N.A.; Ganti, A.K. Pulmonary toxicities from targeted therapies: A review. Target. Oncol. 2011, 6, 235–243.
- Susanto, I.; Bando, J.M. Assessment of haemoptysis. BJM Best Pract. 2019.
- Chalmers, J.D.; Polverino, E.; Aliberti, S. (Eds.) Bronchiectasis; European Respiratory Society: Sheffield, UK, 2018.
- He, R.; Hu, C.; Tang, Y.; Yang, H.; Cao, L.; Niu, R. Report of 12 cases with tracheobronchial mucormycosis and a review. Clin. Respir. J. 2018, 12, 1651–1660.
- Earwood, J.S.; Thompson, T.D. Hemoptysis: Evaluation and management. Am. Fam. Physician 2015, 91, 243–249.
- Jiménez-Zarazúa, O.; López-García, J.A.; Arce-Negrete, L.R.; Vélez-Ramírez, L.N.; Casimiro-Guzmán, L.; Mondragón, J.D. Alveolar hemorrhage associated with cocaine consumption. Hear. Lung 2018, 47, 525–530.
- Dhamija, E.; Meena, P.; Ramalingam, V.; Sahoo, R.; Rastogi, S.; Thulkar, S. Chemotherapy-induced pulmonary complications in cancer: Significance of clinicoradiological correlation. Indian J. Radiol. Imaging 2020, 30, 20–26.
- Shannon, V.R.; Subudhi, S.K.; Huo, L.; Faiz, S.A. Diffuse alveolar hemorrhage with nivolumab monotherapy. Respir. Med. Case Reports 2020, 30, 101131.
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060.
- Struble, R.; Koppurapu, V.; Berger, H. Diffuse Alveolar Hemorrhage Following Dual Checkpoint Inhibitor Therapy. Chest 2019, 156, A1460.
- Shijubou, N.; Sawai, T.; Hatakeyama, T.; Munakata, S.; Yamazoe, M. Alveolar Hemorrhage Caused by the Combination of Immune Checkpoint Inhibitors (ICIs) and Angiogenesis Inhibitors: The Underlying Long-Term Vascular Endothelial Growth Factor (VEGF) Inhibition. Cureus 2022, 14, e23272.
- Stephenson, B.W.; Roberts, A.H.; Read, C.A. Diffuse alveolar hemorrhage in critically ill cancer patients. Oncol. Crit. Care 2019, 583–592.
- Radchenko, C.; Alraiyes, A.H.; Shojaee, S. A systematic approach to the management of massive hemoptysis. J. Thorac. Dis. 2017, 9, S1069–S1086.
- Ittrich, H.; Bockhorn, M.; Klose, H.; Simon, M. The Diagnosis and Treatment of Hemoptysis. Dtsch. Arztebl. Int. 2017, 114, 371–381.
- Gagnon, S.; Quigley, N.; Dutau, H.; Delage, A.; Fortin, M. Approach to Hemoptysis in the Modern Era. Can. Respir. J. 2017, 2017, 1565030.
- Zhang, Z.; Wang, Y. Clinical experiences of bronchopleural fistula-related fatal hemoptysis after the resection of lung cancer: A report of 7 cases. Zhongguo Fei Ai Za Zhi 2012, 15, 39–43.
- Aldiani, A.; Alatas, M.F. Pathophysiology of Haemoptysis in Lung Disease. J. Respirologi Indones. 2023, 43, 58–65.
- Shao, Y.; Zhong, D.-S.; Guan, S.-S. Histologic transformation of lung adenocarcinoma to squamous cell carcinoma after chemotherapy: Two case reports. Transl. Cancer Res. 2020, 9, 388–393.
- Klimko, N.; Khostelidi, S.; Shadrivova, O.; Volkova, A.; Popova, M.; Uspenskaya, O.; Shneyder, T.; Bogomolova, T.; Ignatyeva, S.; Zubarovskaya, L.; et al. Contrasts between mucormycosis and aspergillosis in oncohematological patients. Med. Mycol. 2019, 57, S138–S144.
- von Ranke, F.M.; Zanetti, G.; Hochhegger, B.; Marchiori, E. Infectious Diseases Causing Diffuse Alveolar Hemorrhage in Immunocompetent Patients: A State-of-the-Art Review. Lung 2013, 191, 9–18.
- Shrestha, P.; O’Neil, S.E.; Taylor, B.S.; Bode-Omoleye, O.; Anstead, G.M. Hemoptysis in the Immunocompromised Patient: Do Not Forget Strongyloidiasis. Trop. Med. Infect. Dis. 2019, 4, 35.
- Przybylski, G.; Bukowski, J.; Kowalska, W.; Pilaczyńska-Cemel, M.; Krawiecka, D. Trends from the Last Decade with Nontuberculous Mycobacteria Lung Disease (NTM-LD): Clinicians’ Perspectives in Regional Center of Pulmonology in Bydgoszcz, Poland. Pathogens 2023, 12, 988.
- Kooner, L.; Munoz, A.; Garcia, A.; Kaur, A.; Sharma, R.; Bustamante, V.; Narang, V.; Thompson, G.R.; Kuran, R.; Berjis, A.; et al. Coccidioidal Pulmonary Cavitation: A New Age. J. Fungi 2023, 9, 561.
- Zhao, X.; Huang, J.; Wang, L.; Guo, C.; Di, J.; Xiong, Y.; Huang, W.; Ma, J.; Wang, G. Severe Hemoptysis Secondary to Actinomycosis: A Case Report. Infect. Drug Resist. 2023, 16, 2933–2937.
- Montmeat, V.; Bonny, V.; Urbina, T.; Missri, L.; Baudel, J.-L.; Retbi, A.; Penaud, V.; Voiriot, G.; Cohen, Y.; De Prost, N.; et al. Epidemiology and clinical patterns of Lung Abscesses in ICU: A French multicenter retrospective study. Chest 2023.
- Sonye, K.; Danoff, R.H. The Diffuse Alveolar Hemorrhage Syndromes. 2023. Available online: https://www.uptodate.com/contents/the-diffuse-alveolar-hemorrhage-syndromes (accessed on 20 August 2023).
- Lee, J. Diffuse Alveolar Hemorrhage. 2022. Available online: https://www.merckmanuals.com/professional/pulmonary-disorders/diffuse-alveolar-hemorrhage-and-pulmonary-renal-syndrome/diffuse-alveolar-hemorrhage (accessed on 20 August 2023).
- Park, M.S. Diffuse Alveolar Hemorrhage. Tuberc. Respir. Dis. 2013, 74, 151.
- de Prost, N.; Parrot, A.; Picard, C.; Ancel, P.Y.; Mayaud, C.; Fartoukh, M.; Cadranel, J. Diffuse alveolar haemorrhage: Factors associated with in-hospital and long-term mortality. Eur. Respir. J. 2010, 35, 1303–1311.
- Sternlieb, I. D-Penicillamine Induced Goodpasture’s Syndrome in Wilson’s Disease. Ann. Intern. Med. 1975, 82, 673.
- Thomas, A. Management of Massive Haemoptysis Anaesthesia. World Fed. Soc. Anaesthesiol. 2011, 1–9.
- Suess, C.; Schwartz, M.; Hausmann, R. Pulmonary capillaritis as a cause of lethal diffuse alveolar hemorrhage. Int. J. Leg. Med. 2023, 137, 1481–1487.
- Mondoni, M.; Carlucci, P.; Job, S.; Parazzini, E.M.; Cipolla, G.; Pagani, M.; Tursi, F.; Negri, L.; Fois, A.; Canu, S.; et al. Observational, multicentre study on the epidemiology of haemoptysis. Eur. Respir. J. 2018, 51, 1701813.
- Miller, R.R.; McGregor, D.H. Hemorrhage from carcinoma of the lung. Cancer 1980, 46, 200–205.
- Ito, M.; Niho, S.; Nihei, K.; Yoh, K.; Ohmatsu, H.; Ohe, Y. Risk factors associated with fatal pulmonary hemorrhage in locally advanced non-small cell lung cancer treated with chemoradiotherapy. BMC Cancer 2012, 12, 27.
- Corey, R.; Hla, K.M. Major and Massive Hemoptysis: Reassessment of Conservative Management. Am. J. Med. Sci. 1987, 294, 301–309.
- Razazi, K.; Parrot, A.; Khalil, A.; Djibre, M.; Gounant, V.; Assouad, J.; Carette, M.F.; Fartoukh, M.; Cadranel, J. Severe haemoptysis in patients with nonsmall cell lung carcinoma. Eur. Respir. J. 2015, 45, 756–764.
- Murgu, S.D.; Egressy, K.; Laxmanan, B.; Doblare, G.; Ortiz-Comino, R.; Hogarth, D.K. Central Airway Obstruction: Benign Strictures, Tracheobronchomalacia, and Malignancy-related Obstruction. Chest 2016, 150, 426–441.
- Folch, E.; Mehta, A. Airway Interventions in the Tracheobronchial Tree. Semin. Respir. Crit. Care Med. 2008, 29, 441–452.
- Arooj, P.; Bredin, E.; Henry, M.T.; Khan, K.A.; Plant, B.J.; Murphy, D.M.; Kennedy, M.P. Bronchoscopy in the investigation of outpatients with hemoptysis at a lung cancer clinic. Respir. Med. 2018, 139, 1–5.
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203.
- Grosu, H.B.; Casal, R.F.; Morice, R.C.; Nogueras-González, G.M.; Eapen, G.A.; Ost, D.; Sarkiss, M.G.; Jimenez, C.A. Bronchoscopic findings and bleeding control predict survival in patients with solid malignancies presenting with mild hemoptysis. Ann. Am. Thorac. Soc. 2013, 10, 342–349.
- Mondoni, M.; Carlucci, P.; Cipolla, G.; Pagani, M.; Tursi, F.; Fois, A.; Pirina, P.; Canu, S.; Gasparini, S.; Bonifazi, M.; et al. Long-term prognostic outcomes in patients with haemoptysis. Respir. Res. 2021, 22, 219.
- Petersen, C.L.; Weinreich, U.M. Five-year follow-up of hemoptysis with no malignancy suspected on chest computed tomography: Recurrence, lung cancer and mortality. Eur. Clin. Respir. J. 2019, 6, 1616519.
- Abdulmalak, C.; Cottenet, J.; Beltramo, G.; Georges, M.; Camus, P.; Bonniaud, P.; Quantin, C. Haemoptysis in adults: A 5-year study using the French nationwide hospital administrative database. Eur. Respir. J. 2015, 46, 503–511.
- Pires, F.S.; Teixeira, N.; Coelho, F.; Damas, C. Hemoptysis—Etiology, evaluation and treatment in a university hospital. Rev. Port. Pneumol. 2011, 17, 7–14.
- Vanni, S.; Bianchi, S.; Bigiarini, S.; Casula, C.; Brogi, M.; Orsi, S.; Acquafresca, M.; Corbetta, L.; Grifoni, S. Management of patients presenting with haemoptysis to a Tertiary Care Italian Emergency Department: The Florence Haemoptysis Score (FLHASc). Intern. Emerg. Med. 2017, 13, 397–404.
- Uzun, O.; Atasoy, Y.; Findik, S.; Atici, A.G.; Erkan, L. A prospective evaluation of hemoptysis cases in a tertiary referral hospital. Clin. Respir. J. 2009, 4, 131–138.
- Tsoumakidou, M.; Chrysofakis, G.; Tsiligianni, I.; Maltezakis, G.; Siafakas, N.M.; Tzanakis, N. A Prospective Analysis of 184 Hemoptysis Cases—Diagnostic Impact of Chest X-Ray, Computed Tomography, Bronchoscopy. Respiration 2006, 73, 808–814.
- Mondoni, M.; Carlucci, P.; Cipolla, G.; Fois, A.; Gasparini, S.; Marani, S.; Centanni, S.; Sotgiu, G. Bronchoscopy to assess patients with hemoptysis: Which is the optimal timing? BMC Pulm. Med. 2019, 19, 36.
- Majhail, N.S.; Parks, K.; Defor, T.E.; Weisdorf, D.J. Diffuse Alveolar Hemorrhage and Infection-Associated Alveolar Hemorrhage following Hematopoietic Stem Cell Transplantation: Related and High-Risk Clinical Syndromes. Biol. Blood Marrow Transplant. 2006, 12, 1038–1046.




