Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paola Savoia | -- | 4169 | 2023-10-11 11:58:55 | | | |
| 2 | Lindsay Dong | Meta information modification | 4169 | 2023-10-13 03:04:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Savoia, P.; Azzimonti, B.; Rolla, R.; Zavattaro, E. Role of the Microbiota in Skin Neoplasms. Encyclopedia. Available online: https://encyclopedia.pub/entry/50117 (accessed on 01 March 2026).
Savoia P, Azzimonti B, Rolla R, Zavattaro E. Role of the Microbiota in Skin Neoplasms. Encyclopedia. Available at: https://encyclopedia.pub/entry/50117. Accessed March 01, 2026.
Savoia, Paola, Barbara Azzimonti, Roberta Rolla, Elisa Zavattaro. "Role of the Microbiota in Skin Neoplasms" Encyclopedia, https://encyclopedia.pub/entry/50117 (accessed March 01, 2026).
Savoia, P., Azzimonti, B., Rolla, R., & Zavattaro, E. (2023, October 11). Role of the Microbiota in Skin Neoplasms. In Encyclopedia. https://encyclopedia.pub/entry/50117
Savoia, Paola, et al. "Role of the Microbiota in Skin Neoplasms." Encyclopedia. Web. 11 October, 2023.
Copy Citation
The skin and the gut are regularly colonized by a variety of microorganisms capable of interacting with the immune system through their metabolites and influencing the balance between immune tolerance and inflammation. Alterations in the composition and diversity of the skin microbiota have been described in various cutaneous diseases, including skin cancer, and the actual function of the human microbiota in skin carcinogenesis, such as in progression and metastasis, is currently an active area of research.
microbiota
gut–skin axis
skin cancer
melanoma
1. Introduction
The intestinal microbiota, i.e., the whole of microorganisms (bacteria, viruses, fungi, and protozoa) that colonize the gastro-enteric tract, has a broad and complex interaction with the immune system through metabolites able to influence the balance between the immune tolerance and the inflammatory state [1]. Also, it communicates with the skin, as an important regulator of the gastrointestinal–skin axis system (“gut–skin axis”) [1].
Recent studies have demonstrated, in fact, that the intestinal microbiota can influence the skin pathophysiology and its corresponding immune response through the cutaneous migration of microorganisms and their metabolites. These ones can enter the bloodstream, through a damaged gastrointestinal barrier and reach the skin, causing distant effects and contributing to the pathogenesis of numerous chronic inflammatory diseases, such as psoriasis and atopic dermatitis. Increasing evidence has also shown a putative role of microorganisms in the pathogenesis and progression of skin cancer, through both a direct influence on cell proliferation and death processes and indirect effects on host immunity and metabolism [2]. Moreover, microbiota may affect the response to immunotherapy and its tolerability in patients affected by skin malignancies [3].
2. The Human Microbiota and the Gut–Skin Axis
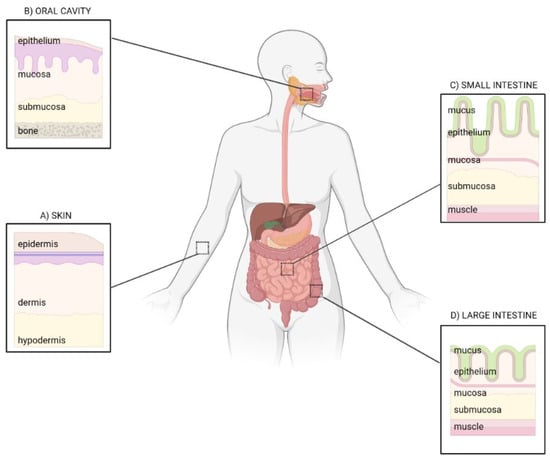
The intestine and the skin share a number of common features: they are border barriers between the external and internal environment, both possessing an epithelial surface of about 30 m2 that, in the epidermis, is mainly determined by the presence of hair follicles, apocrine/eccrine ducts, and sebaceous glands [4] (Figure 1).

Figure 1. Epithelial barriers: from skin to gut. Common and specific traits of the skin (A), oral cavity (B), small (C) and large intestine (D). Image created with BioRender.com.
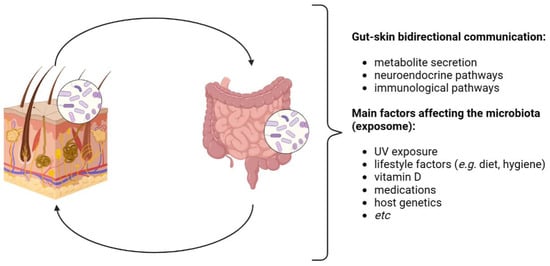
The skin and the gut also share common neuroendocrine properties, driven by gut microbes that produce neurotransmitters such as acetylcholine and serotonin, able to stimulate, via the neural system, the secretion of hormones from specialized intestinal cells, which finally determine systemic and inflammatory effects also involving the skin [4] (Figure 2). Beyond these peculiarities, these districts also show a key role in the mediation of inflammatory conditions and immune development, since, starting from early childhood, they identify novel antigens daily to distinguish whether and how to tolerate them [5].

Figure 2. Gut–skin axis (GSA). The GSA is bidirectional: it is involved in the host’s homeostasis through immunological and neuroendocrine pathways. Gut and skin microbiota eubiosis is affected by several factors, generally recognized as the exposome. Image created with BioRender.com.
To this aim, and to guarantee the host’s allostasis and homeostasis, they cooperate in the production of anti-microbial agents and/or specific nutritional sources [6][7]. By selecting the exogenous and endogenous microbial colonizers that compete for the epithelial surfaces, they prevent the possible numerical prevarication and virulence of primary and/or opportunistic pathogens [1][4][5]. From the skin side, this task is also fulfilled by flaking, which, together with keratin and skin components such as the sebum, allows the epithelial renewal process and protection from weak acids/bases and various antigen types; conversely, from the intestinal mucosa part, the same is done by the mucin glycoproteic components within the epithelial villi [8][9].
Moreover, these two districts are not distinct; they are one the continuation of the other, intimately connected and perfused by the bloodstream. They are two faces of the same coin, “separated” on one side by the mouth and the perineum and, on the other, by the nasal pits and the skin pores, respectively, the “entry” and “exit” routes of the digestive and respiratory systems.
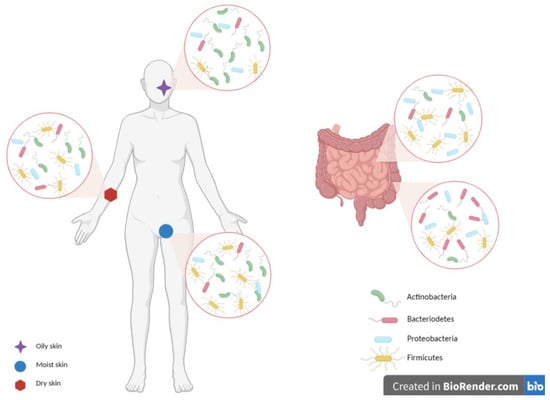
Each one of them possesses a peculiar microbiota, mainly identified through the 16S ribosomal RNA (rRNA) gene sequencing, which finely orchestrates a two-way collaborative relationship [10][11]. A lot of evidence underlines this unequivocal connection and the involvement of the immune and endocrine systems. The skin microbial community is composed of a collection of microorganisms that, with the sole exception of the core microbiota that remains constant over time, vary during life, conditioned as they are by influencers such as hormones, anatomical distribution, pH, and hygiene habits, which determine their peculiar distribution in the diverse dry, moist, and sebaceous areas.
The gut, mostly the large intestine, is indeed partially colonized by the same Actinobacteria (with Bifidobacteria inducing anti-inflammatory Treg cell accumulation at the basis of the immune tolerance) and Firmicutes, both dominant (90% of gut microbiota). Proteobacteria, Bacteroidetes, Fusobacteria, Verrucomicrobia, DNA/RNA viruses, Candida, and protozoa are also key representatives [12]. The gut residents, whose distribution and abundance depend on the anatomical region, pH, and O2 values, also have a metabolic role, with the production of short-chain fatty acid (SCFA) bio-products: in fact, the acetate, butyrate, and propionate, which cooperate for the epithelial barrier integrity preservation, prevent or solve local and systemic inflammatory and immune effects [4][12][13][14][15] (Figure 3).

Figure 3. Skin and gut microbiota composition. The skin and gut are mainly colonized by Firmicutes, Actinobacteria, Proteobacteria, and Bacteroidetes, with a peculiar composition for each single niche. Moist sites are colonized mainly by Firmicutes and Actinobacteria, oily ones by Actinobacteria, while dry areas by diverse microbial populations. Gut microbiota differs between the small and large intestines. Firmicutes and Proteobacteria are the most dominant phyla in the small intestine, while Bacteroidetes dominate the anaerobic environment of the large intestine. Image created with BioRender.com.
3. Experimental Evidence of the Role of Microbiota in Skin Carcinogenic Processes
The role of the microbiota in skin carcinogenesis is an active area of research, even if it still remains unclear. The cutaneous microbiota has been found to play a role in maintaining skin barrier function, modulating the immune system, and defending against pathogens [16]. However, alterations in its composition and diversity have been associated with various skin diseases, including skin cancer, as demonstrated by many experimental studies.
Staphylococcus epidermidis (S. epidermidis) occurs naturally on healthy skin, where it plays a protective and antitumor role by activating the immune system to fight cancer cells and where it competes with other potentially harmful bacteria such as Staphylococcus aureus (S. aureus) [17].
In response to adverse external stimuli, the skin microbiota can become unbalanced, leading to a decrease in the presence of S. epidermidis and an increase in pathogenic S. aureus. Disturbances of the cutaneous microbiota are frequently observed in tumor patients undergoing radiotherapy, chemotherapy, and probiotics [18][19]. Several studies have demonstrated the association between S. aureus and increased susceptibility to skin cancer [20]. Specifically, the presence of S. aureus is strongly associated with SCC. Compared to healthy individuals, S. aureus is significantly more prevalent in the group of patients with SCC of the oral cavity [21]. The prevalence of S. aureus in the skin has also been found to be associated with cutaneous T-cell lymphoma [22].
Several mechanisms have been proposed through which the skin microbiota may influence skin cancer development: inflammation and immune modulation are the main involved. Dysbiosis, the imbalance in the skin microbiota, can lead to chronic inflammation, and this is a known risk factor for cancer development as it can promote DNA damage, cell proliferation, and immune dysfunction, each one of them contributing to carcinogenesis. The skin microbiota also interacts with the immune system, influencing its response. Particularly, dysbiosis can disrupt immune homeostasis and impair the immune response to cancer cells.
As is well known, the commensal and pathogenic skin microbiota regulates innate local immunity through keratinocytes, dendritic cells, mast cells, endothelial cells, fibroblasts, neutrophils, and macrophages [23]. As the first line of defense, the skin is constantly exposed to pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). After microbial stimulation, keratinocytes upregulate the production of antimicrobial lipids and antimicrobial peptides, and PAMPs and/or DAMPs are recognized by pattern recognition receptors (PRRs) [24]. Toll-like receptors (TLRs) are the major class of PRRs involved in detecting invading pathogens in the skin and play a vital role in the initial stage of cutaneous innate immune response by recognizing PAMPs and initiating immune signaling pathways. TLRs are expressed on many different cell types in the skin, including keratinocytes, melanocytes, and Langerhans cells in the epidermis. PRR activation triggers downstream immune signaling pathways, the activation of the innate immune system, and subsequent adaptive immune responses, leading to the clearance of invading pathogens [25].
Aberrant expression or the persistent activation of TLRs by pathogenic skin microbiota may promote chronic inflammation and can contribute to the generation and progression of many skin immune disorders, such as systemic lupus erythematosus, cryopyrin-associated periodic syndrome, and primary inflammatory skin diseases, including psoriasis, atopic dermatitis, and also cancer [26]. In particular, the relationship between various TLRs and skin cancer has been extensively studied [27].
The overexpression of TLR4 has also been observed in malignant melanoma (MM), and TLR4 expression is negatively associated with recurrence-free survival [28][29]. Furthermore, studies have suggested that TLR4 signaling may be associated with the epithelial–mesenchymal transition (EMT), a process that allows epithelial cells to acquire invasive properties. EMT can, in fact, contribute to the metastatic process in melanoma [30][31][32]. Commensal skin microorganisms not only induce innate immune responses but also regulate the cutaneous adaptive immune system and the action of T lymphocytes. Recent studies have highlighted the importance of T-helper-type 17 lymphocytes and effector cytokines in cutaneous inflammation and microbiota-mediated skin carcinogenesis. In particular, IL-17 and IL-22 have been shown to be key factors in skin cancer progression, as they can induce cell proliferation in NMSC cells and promote the migration of human basal cell carcinoma (BCC) and SCC cell lines in vitro. Additionally, IL-17 and IL-22 stimulate tumor growth in mice injected with an SCC cell line, CAL27. However, further studies are needed to identify the specific skin microbiota that enhances the response of Th17 cells in the skin [33].
4. Microbiota and Melanoma
Bacteria and fungi can, through specific wall components, activate the host’s immune system, contributing to the maintenance of a pro-inflammatory and pro-oncogenic state; in melanoma, differences in microbiota composition based on the different stages may therefore underlie the dissimilar prognosis and disease course.
Studies conducted on melanoma animal models revealed some differences in microbe composition and microbial diversity with respect to normal skin [34][35]. In a porcine model, the 16s RNA sequencing demonstrated statistically significant differences in microbiota diversity and richness between melanoma tissue and healthy skin and between the fecal microbiota of MeLiM (Melanoma-Bearing Libechov Minipig) and control piglets [35]. In detail, the abundance of Fusobacterium, Trueperella, Staphylococcus, Streptococcus, and Bacteroides was the distinguishing feature of the melanoma microbiota, while Bacteroides, Fusobacterium, and Escherichia-Shigella were characteristics of the fecal microbiota of MeLiM [35][36]. Also, an abundance of Prevotella copri, Clostridium IV, Holdemania, Anaerofustis, and Saccharomycetales yeasts was demonstrated in patients affected by melanoma, with changes in the composition of gut microbiota between early-stage and invasive melanomas [37][38]. In detail, major differences (n = 180) in microbial communities were found comparing in situ and invasive melanoma; also, differences (n = 23) were observed between regressed and non-regressed melanomas [37]. Overall, the progression of melanoma from in situ to invasive forms is associated with a pauperization of the gut microbiota, with a decrease in alpha diversity.
In the last decade, immunotherapy has revolutionized melanoma treatment, both in the adjuvant and advanced disease setting, greatly improving the prognosis of patients affected by this tumor. In this context, the microbiota controls the response and tolerability in patients treated with immunological checkpoint inhibitors (ICI). Indeed, antibiotic exposure has been related to worse prognosis in patients treated with ICI [39], even if a more recent study [40] on 169 melanoma patients treated with anti-PD1 did not show any association between antibiotic therapy, PFS, and OS. Also, a putative role of diet has been supposed.
Recently, a clinical trial evaluated the safety and efficacy of the transplantation of responder-derived fecal microbiota together with anti-PD-1 in a small group of patients affected by PD-1–refractory melanoma [41][42]. This combination was well tolerated, with an objective response in 3/15 patients and a durable stable disease in three others. The authors reported a persistent microbiota perturbation in responder patients, which exhibited an increased abundance of taxa that were previously shown to be associated with response to anti-PD-1, together with increased CD8+ T cell activation and a decreased frequency of interleukin-8-expressing myeloid cells. Differences and the rate of change in microbial communities were evaluated using multidimensional Euclidean distance; even if the limited size of the sample did not allow the authors to reach a statistical significance, they report that Euclidean distance notably separated responder from non-responder.
5. Microbiota and Non-Melanoma Skin Cancer
NMSC represents the most common kind of tumor affecting human beings and is mostly represented among Caucasian patients. NMSCs include different clinical entities, often occurring in sun-exposed cutaneous sites in fair-skinned subjects. The most frequent NMSCs are represented by keratinocyte carcinomas, namely BCC and SCC, unless other types can also be seen, such as keratoacanthoma, Bowen’s disease, and their precursor actinic keratosis (AK). Both BCC and SCC arise from keratinocytes, but they present some pivotal differences, as they originate from the diverse cellular layers of the epidermis (keratinocytes from the basal layer in BCC, and the spinous layer in SCC), and, most importantly, they recognize different clinical features and behavior, with a moderate/strong aggressiveness and a tendency to metastasize in SCC, which is extremely rare in BCC [43]. AK is a very common cutaneous lesion affecting numerous patients worldwide, and it typically develops on sun-exposed sites and in the context of the so-called field cancerization (FC), i.e., a cutaneous area in which clinical and subclinical actinic damage coexist, possibly with DNA damage, with a high risk of developing AKs and SCCs. AK is commonly considered as an SCC precursor into a possible continuum towards an invasive carcinoma unless it is currently impossible to predict which AK will progress into the invasive form [44][45].
On the other side, it is well known that UV exposure, old age, pale skin, and immuno-suppression represent important factors in favoring the development of AK and, possibly, its transformation. In this regard, solid organ transplant recipients (SOTRs) have up to 250-fold higher risk of developing NMSC, mainly SCC, when compared to immunocompetent subjects, and this has been explained by several factors: immunosuppressant drug intake, which induces photosensitivity and favors cutaneous UV-mediated DNA damage; a decreased capability to counteract the carcinogenetic process; and, not least of all, microbiome changes [46][47].
When considering the microbiota’s role in NMSC’s development in AK, the role of the HPVs belonging to the beta genus (namely ßHPVs) must be carefully evaluated. ßHPVs are a part of the virota and are typically harmless in the general population unless in the presence of certain predisposing factors (i.e., immunosuppression), in which they can promote cutaneous carcinogenesis; such a process has been first described in Epidermodysplasia Verruciformis (EV) patients. EV is a rare, genetically inherited disease characterized by abnormal susceptibility to ßHPVs, which can induce the development of multiple NMSCs early in life through the suppression of the apoptosis of UV-damaged cells [48]. Whether EV has been formerly considered a natural model to investigate the effect of ßHPVs in skin carcinogenesis, more recently, the same process occurring in immunocompromised patients (either for HIV infection or upon chronic immunosuppressive drug intake) led to the coining of the term “acquired EV” [49][50]. On this basis, ßHPV genomes were repeatedly detected either in malignant skin lesions, in perilesional healthy skin, or in plucked eyebrows from SOTRs (up to 85% of the considered samples), and an association between the viruses and AKs/SCCs was also reported, thus validating their role in skin carcinogenesis [51][52].
Another possible explanation for viral-mediated skin carcinogenesis was suggested by Strickley et al. [53] through a murine model in which mice harboring a specific immunity towards MmuPV1 were infected with that viral genotype, and, surprisingly, they were protected against UV-induced skin cancer with a CD8 T cell-dependent mechanism. Accordingly, they demonstrated the existence of an adaptative immunity towards HPVs in normal skin from healthy subjects, thus pointing out the possible use of T cell-based vaccines against commensal HPVs. On the other side, ßHPVs DNA has been detected not only in samples from malignant lesions but also in plucked eyebrows collected from healthy individuals; hence, the persistence of the same type of specific ßHPVs in time was found, and thus the exact role of such viruses in NMSC remains controversial [54][55].
Conversely, it has been reported that some commensal skin bacteria might protect against NMSC development, and this is of great importance in terms of prevention and potential treatments in high-risk subjects. Indeed, the detection of a particular strain of commensal S. epidermidis was found to be associated with a protective effect against skin cancer development. This was explained by the capability of the bacteria to produce 6-N-hydroxyaminopurine (6-HAP), a chemical compound exerting antiproliferative activity against neoplastic cell lines [56].
6. Microbiota and Other Cutaneous Tumors
6.1. Cutaneous T-Cell Lymphomas
The etiopathogenesis of cutaneous lymphomas, which are rare and heterogeneous neoplasms of the lymphoid compartment in which the skin represents the first site of involvement, remains incompletely clarified. It has been hypothesized that various triggering factors can facilitate chronic inflammation and subsequent neoplastic transformation, and among them, a role could be played by the microbiota.
In mouse models of cutaneous T-cell lymphoma (CTCL), the putative influence of microbiota has been supposed through an observation regarding a less severe disease in mice raised in germ-free conditions [57]. So, several studies focused on the possible distinctive characteristics of cutaneous microbiota in CTCL patients, and on dissimilarity between lesional and non-lesional skin.
More interesting are the data obtained by Dehner et al. [58], who revealed the presence of Bacillus safensis in seven patients affected by MF. This bacterium, not evident on the skin of healthy control subjects, has been shown to be able to stimulate T-cell proliferation in vitro through the production of cytokines and growth factors and could confirm that microbes can act as trigger factors for tumorigenesis.
In 2021, a case-control study published by Hooper et al. [59] demonstrated a gut dysbiosis correlated with the disease stage in 38 CTCL patients, compared to age- and geographically matched healthy controls. In detail, CTCL patients were characterized by significantly lower α-diversity and by a loss of butyrate producers (i.e., Bifidobacterium and Anaerotruncus, and Lactobacillus in subjects with advanced disease). These observations also create the foundation for the possible use of FMT in the treatment of this neoplasia.
6.2. Merkel Cell Carcinoma
Merkel Cell Carcinoma (MCC) is a rare skin tumor arising on sun-exposed sites, mainly in immunocompromised and elderly patients, and with aggressive behavior. Despite the possible origin of MCC from its related Merkel cell (located in the basal layer of the epidermidis, thus providing a mechanoreceptor function) still being under debate, the so-called Merkel cell polyomavirus (MCPyV) has been found in a high percentage of the corresponding tumor (ranging from 58% to 88% of cases, according to the different detection techniques), thus confirming its causal role in MCC development [60]. Indeed, it has recently been proposed that MCC can recognize different cellular origins: from dermal fibroblasts and from epidermal keratinocytes, and the difference between those target cells is due to MCPyV’s presence or not. More in detail, two MCC entities can be described: the virus-positive MCC-targeting fibroblasts, and the virus-negative MCC arising from the keratinocytes. MCPyV is a part of the human skin microbiota able to encode for two oncoproteins (LT: large tumor antigen; sT: small tumor antigen) that are implicated in carcinogenesis through viral integration into the host genome in dermal fibroblasts, the inhibition of pRb1, and the evasion of the immune response. Conversely, in virus-negative MCCs, the target cell is represented by the epidermal keratinocytes in which the DNA damage and somatic mutations are multiple, thus resembling those occurring in the case of chronic UV exposure [61].
6.3. Kaposi’s Sarcoma
The pathogenesis of Kaposi’s sarcoma (KS) is notoriously related to the Kaposi’s sarcoma-associated herpesvirus (KSHV), also known as herpesvirus-8, which is one of the seven viruses classified as human carcinogens by the International Agency for Research on Cancer [62]. KSHV is a DNA virus that is capable of infecting not only endothelial cells, from which KS originates, but also monocytes and B cells, acting on cellular metabolism, upregulating the survival pathways, and stimulating angiogenesis and inflammation.
7. Potential Therapeutic Implications
7.1. Microbiota Modulation
Prebiotics and/or probiotics can be used to promote the growth of beneficial bacteria while suppressing the overgrowth of potentially harmful ones. This approach aims to restore microbial balance and enhance the skin’s natural defense mechanisms against cancerous cells [63].
Probiotics such as Lactobacillus and Bifidobacterium genera are live microorganisms that can exert beneficial effects not only on the well-studied and documented gut microbiota but also on the skin. They can inhibit the growth of pathogenic microorganisms and promote an anti-inflammatory phenotype of the epithelium [63]. The oral and topical administration of probiotics appear to be effective for the treatment of various inflammatory skin diseases and dermatological conditions, including atopic dermatitis, acne, and psoriasis, and are also showing a promising role in wound healing and skin cancer [64][65][66][67].
7.2. Immunomodulation by Microbiota
The skin microbiota interacts with the immune system, and its modulation can influence the immune response. Certain microbial species or metabolites produced by the microbiota can enhance immune surveillance against cancer cells or regulate inflammation, which plays a critical role in tumor development. Harnessing these immunomodulatory properties could help improve therapeutic outcomes in skin cancer.
Supplementation with highly active strains of Lactococcus and Lactobacillus has demonstrated immunomodulatory, anti-inflammatory, antiallergic, and antitumor properties. L. lactis has been shown to produce anti-inflammatory compounds, such as bacteriocins and bioactive peptides that can help to reduce inflammation in the body. These compounds can inhibit the production of pro-inflammatory cytokines, modulating both Th1 and Th2 immune cell responses [68]. This ability to regulate T-cell activity is essential for maintaining immune balance and preventing excessive immune responses, inflammation, and carcinogenesis.
7.3. Bacterial Anticancer Metabolites
It is known that some bacteria, even within the skin microbiota—for instance, certain strains of Staphylococcus—have been found to produce metabolites with potential anticancer properties, suggesting a potential therapeutic benefit in cancer treatment. Streptozotocin, a natural compound produced by the bacterium Streptomyces achromogenes, is commonly used as a chemotherapeutic agent in the treatment of pancreatic neuroendocrine tumors. Rapamycin is another natural metabolite produced by the bacterium Streptomyces hygroscopicus. It has immunosuppressive and anticancer properties and is used in cancer treatment, especially in combination with other drugs. Also, bleomycin is an antibiotic produced by Streptomyces verticillus, widely used in cancer chemotherapy, especially in the treatment of SCC [69].
7.4. TLR-Targeted Therapy
We described that when Gram-negative bacteria infect the host, the LPS component of their cell wall highly activates TLR4. The activation of TLR4 by LPS leads to a signaling cascade that triggers the production of pro-inflammatory cytokines, chemokines, and other immune mediators. This robust immune response is critical for the host’s defense against invading pathogens. However, the dysregulated or excessive activation of TLR4 by LPS can lead to harmful effects, such as excessive inflammation and tissue damage. The prolonged activation of TLR4 has been associated with various inflammatory diseases, including sepsis, inflammatory bowel disease, autoimmune conditions, and cancer [70].
Targeting TLR4 signaling may hold promise for managing inflammatory conditions and controlling bacterial infections caused by Gram-negative bacteria. Research in this area continues to provide valuable insights into the complexities of innate immunity and its role not only in host defense but also in inflammatory diseases and cancer.
7.5. Antibiotics
We described above that Staphylococcus species are a group of bacteria that naturally occur on the skin surface and are considered part of the normal skin microbiota. Staphylococcus is one of the predominant genera in the skin microbial community. While S. epidermidis is considered a beneficial member of the skin microbiota, S. aureus can be more opportunistic and cause skin infections and skin cancer. The S. aureus eradication in CTCL patients has been demonstrated to be associated with clinical improvement [71][72][73].
8. Conclusions
The skin microbiota plays an important role in maintaining skin health and protecting the body from dangerous pathogens. It helps to keep the local pH balance, prevents the growth of harmful bacteria, and promotes the production of natural antimicrobial peptides. The composition of the skin microbiota can vary depending on factors such as age, gender, ethnicity, and lifestyle. In addition, changes in the skin microbiota can be influenced by environmental factors such as diet, hygiene practices, and exposure to UV and pollutants.
The emerging knowledge about the involvement of microbiota not only in inflammatory processes but also in cell differentiation, proliferation, and migration phenomena also raised awareness of its potential role in cancer. This could be of even greater importance in the skin because of the possible direct interaction between microbiota and environmental carcinogens, such as UV radiation. The characterization of the skin microbiota could be helpful for identifying patients at greater risk of developing skin neoplasms or also have a prognostic value, defining subcategories of patients more responsive to specific treatments or more prone to treatment-related adverse events.
References
- Mahmud, M.R.; Akter, S.; Tamanna, S.K.; Mazumder, L.; Esti, I.Z.; Banerjee, S.; Akter, S.; Hasan, M.R.; Acharjee, M.; Hossain, M.S.; et al. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 2022, 14, 2096995.
- Xia, C.; Su, J.; Liu, C.; Mai, Z.; Yin, S.; Yang, C.; Fu, L. Human microbiomes in cancer development and therapy. Med. Comm. 2020, 26, e221.
- Bibbò, S.; Ianiro, G.; Giambò, F.; Settanni, C.R.; Cammarota, G.; Gasbarrini, A. Role of gut microbiome on immunotherapy efficacy in melanoma. Hum. Vaccin. Immunother. 2022, 18, 1926759.
- Sinha, S.; Lin, G.; Ferenczi, K. The skin microbiome and the gut-skin axis. Clin. Dermatol. 2021, 39, 829–839.
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353.
- Garcia-Gutierrez, E.; Mayer, M.J.; Cotter, P.D.; Narbad, A. Gut microbiota as a source of novel antimicrobials. Gut Microbes 2019, 10, 1–21.
- Schauber, J.; Gallo, R.L. Antimicrobial peptides and the skin immune defense system. J. Allergy Clin. 2008, 122, 261–266.
- Veniaminova, N.A.; Jia, Y.; Hartigan, A.M.; Huyge, T.J.; Tsai, S.Y.; Grachtchouk, M.; Nakagawa, S.; Dlugosz, A.A.; Atwood, S.X.; Wong, S.Y. Distinct mechanisms for sebaceous gland self-renewal and regeneration provide durability in response to injury. Cell Rep. 2023, 42, 113121.
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243.
- Li, Y.; Jin, Y.; Zhang, J.; Pan, H.; Wu, L.; Liu, D.; Liu, J.; Hu, J.; Shen, J. Recovery of human gut microbiota genomes with third-generation sequencing. Cell Death Dis. 2021, 12, 569.
- Ederveen, T.H.A.; Smits, J.P.H.; Boekhorst, J.; Schalkwijk, J.; van den Bogaard, E.H.; Zeeuwen, P.L.J.M. Skin microbiota in health and disease: From sequencing to biology. J. Dermatol. 2020, 47, 1110–1118.
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14.
- Cao, Z.; Sugimura, N.; Burgermeister, E.; Ebert, M.P.; Zuo, T.; Lan, P. The gut virome: A new microbiome component in health and disease. EBioMedicine 2022, 81, 104113.
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018, 9, 1459.
- Pérez, J.C. Fungi of the human gut microbiota: Roles and significance. Int. J. Med. Microbiol. 2021, 311, 151490.
- Hazrat, B.; Yuanyuan, X.; Muhammad Nadeem, K.; Chen, J.; Wang, Y.; Zeng, Y.; Lin, X. Stabilization of Acne Vulgaris-Associated Microbial Dysbiosis with 2% Supramolecular Salicylic Acid. Pharmaceuticals 2023, 16, 87.
- Severn, M.M.; Horswill, A.R. Staphylococcus epidermidis and its dual lifestyle in skin health and infection. Nat. Rev. Microbiol. 2023, 21, 97–111.
- Squarzanti, D.F.; Zavattaro, E.; Pizzimenti, S.; Amoruso, A.; Savoia, P.; Azzimonti, B. Non-Melanoma Skin Cancer: News from microbiota research. Crit. Rev. Microbiol. 2020, 46, 433–449.
- Squarzanti, D.F.; Zanetta, P.; Azzimonti, B. Non-melanoma skin cancer and the cutaneous microbiota network. Biol. Med. 2020, 12, 1b.
- Wei, Y.; Sandhu, E.; Yang, X.; Yang, J.; Ren, Y.; Gao, X. Bidirectional Functional Effects of Staphylococcus on Carcinogenesis. Microorganisms 2022, 10, 2353.
- Kullander, J.; Forslund, O.; Dillner, J. Staphylococcus aureus and squamous cell carcinoma of the skin. Cancer Epidemiol. Biomark. Prev. 2009, 18, 472–478.
- Fujii, K. Pathogenesis of cutaneous T cell lymphoma: Involvement of Staphylococcus aureus. J. Dermatol. 2022, 49, 202–209.
- Patra, V.; Wagner, K.; Arulampalam, V.; Wolf, P. Skin Microbiome Modulates the Effect of Ultraviolet Radiation on Cellular Response and Immune Function. iScience 2019, 15, 211–222.
- Woo, Y.R.; Cho, S.H.; Lee, J.D.; Kim, H.S. The Human Microbiota and Skin Cancer. Int. J. Mol. Sci. 2022, 23, 1813.
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066.
- Vijay, K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int. Immunopharmacol. 2018, 59, 391–412.
- Zhao, Q.; Wang, Q.; Wang, T.; Xu, J.; Li, T.; Liu, Q.; Yao, Q.; Wang, P. Pattern Recognition Receptors (PRRs) in Macrophages Possess Prognosis and Immunotherapy Potential for Melanoma. Front. Immunol. 2021, 12, 765615.
- Shomali, N.; Hatamnezhad, L.S.; Tarzi, S.; Tamjidifar, R.; Xu, H.; Shotorbani, S.S. Heat Shock Proteins Regulating Toll-like Receptors and the Immune System could be a Novel Therapeutic Target for Melanoma. Curr. Mol. Med. 2021, 21, 15–24.
- Theivanthiran, B.; Yarla, N.; Haykal, T.; Nguyen, Y.V.; Cao, L.; Ferreira, M.; Holtzhausen, A.; Al-Rohil, R.; Salama, A.K.S.; Beasley, G.M.; et al. Tumor-intrinsic NLRP3-HSP70-TLR4 axis drives premetastatic niche development and hyperprogression during anti-PD-1 immunotherapy. Sci. Transl. Med. 2022, 14, eabq7019.
- Jun, Y.K.; Kwon, S.H.; Yoon, H.T.; Park, H.; Soh, H.; Lee, H.J.; Im, J.P.; Kim, J.S.; Kim, J.W.; Koh, S.J. Toll-like receptor 4 regulates intestinal fibrosis via cytokine expression and epithelial-mesenchymal transition. Sci. Rep. 2020, 10, 19867.
- Chen, M.C.; Chang, W.W.; Kuan, Y.D.; Lin, S.T.; Hsu, H.C.; Lee, C.H. Resveratrol inhibits LPS-induced epithelial-mesenchymal transition in mouse melanoma model. Innate Immun. 2012, 18, 685–693.
- Eiro, N.; Ovies, C.; Fernandez-Garcia, B.; Álvarez-Cuesta, C.; González, L.; González, L.; Vizoso, F. Expression of TLR3, 4, 7 and 9 in cutaneous malignant melanoma: Relationship with clinicopathological characteristics and prognosis. Arch. Dermatol. Res. 2013, 305, 59–67.
- McAllister, F.; Kolls, J.K. Th17 cytokines in non-melanoma skin cancer. Eur. J. Immunol. 2015, 45, 692–694.
- Fu, C.; Yang, Z.; Yu, J.; Wei, M. The interaction between gut microbiome and anti-tumor drug therapy. Am. J. Cancer Res. 2021, 11, 5812–5832.
- Mekadim, C.; Kupcova Skalnikova, H.; Cizkova, J.; Cizkova, V.; Palanova, A.; Horak, V.; Mrazek, J. Dysbiosis of skin microbiome and gut microbiome in melanoma progression. BMC Microbiol. 2022, 22, 63.
- Mrázek, J.; Mekadim, C.; Kučerová, P.; Švejstil, R.; Salmonová, H.; Vlasáková, J.; Tarasová, R.; Čížková, J.; Červinková, M. Melanoma-related changes in skin microbiome. Folia Microbiol. 2019, 64, 435–442.
- Vitali, F.; Colucci, R.; Di Paola, M.; Pindo, M.; De Filippo, C.; Moretti, S.; Cavalieri, D. Early melanoma invasivity correlates with gut fungal and bacterial profiles. Br. J. Dermatol. 2022, 186, 106–116.
- Makaranka, S.; Scutt, F.; Frixou, M.; Wensley, K.E.; Sharma, R.; Greenhowe, J. The gut microbiome and melanoma: A review. Exp. Dermatol. 2022, 31, 1292–1301.
- Pinato, J.D.; Gramenitskaya, D.; Altmann, D.M.; Boyton, R.J.; Mullish, B.H.; Marchesi, J.R.; Bower, M. Antibiotic therapy and outcome from immune-checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 287.
- Swami, U.; Chennamadhavuni, A.; Borcherding, N.; Bossler, A.D.; Mott, S.L.; Garje, R.; Zakharia, Y.; Milhem, M. Multivariable Analysis of 169 Cases of Advanced Cutaneous Melanoma to Evaluate Antibiotic Exposure as Predictor of Survival to Anti-PD-1 Based Immunotherapies. Antibiotics 2020, 9, 740.
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602.
- Barbosa, E.C.; Bucar, E.E.C.; Jubé, G.R.; Silveira, L.B.; Silva, N.C.D.; Faria, P.C.C.; Ramos, P.L.C.; Moraes, V.R.Y.; Barros, J.O.B. Fecal microbiota transplantation and its repercussions in patients with melanoma refractory to anti-PD-1 therapy: Scope review. Rev. Col. Bras. Cir. 2023, 50, e20233490.
- Samarasinghe, V.; Madan, V. Nonmelanoma skin cancer. J. Cutan. Aesthet. Surg. 2012, 5, 3–10.
- Willenbrink, T.J.; Ruiz, E.S.; Cornejo, C.M.; Schmults, C.D.; Arron, S.T.; Jambusaria-Pahlajani, A. Field cancerization: Definition, epidemiology, risk factors, and outcomes. J. Am. Acad. Dermatol. 2020, 83, 709–717.
- Fernandez Figueras, M.T. From actinic keratosis to squamous cell carcinoma: Pathophysiology revisited. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 5–7.
- Mittal, A.; Colegio, O. Skin cancers in Organ Transplant Recipients. Am. J. Transplant. 2017, 17, 2509–2530.
- Wood, D.L.A.; Lachner, N.; Tan, J.M.; Tang, S.; Angel, N.; Laino, A.; Linedale, R.; Lê Cao, K.A.; Morrison, M.; Frazer, I.H.; et al. A natural history of actinic keratosis and cutaneous squamous cell carcinoma microbiomes. mBio 2018, 9, e01432-18.
- Majewski, S.; Jablonska, S. Epidermodysplasia verruciformis human papillomavirusese contribute to malignant and benign epidermal proliferations? Arch. Dermatol. 2002, 138, 649–654.
- Patel, T.; Morrison, L.K.; Rady, P.; Tyring, S. Epidermodysplasia verruciformis and susceptibility to HPV. Dis. Markers 2010, 29, 199–206.
- Rogers, H.D.; Macgregor, J.L.; Nord, K.M.; Tyring, S.; Rady, P.; Engler, D.E.; Grossman, M.E. Acquired epidermodysplasia verruciformis. J. Am. Acad. Dermatol. 2009, 60, 315–320.
- Meyer, T.; Arndt, R.; Nindl, I.; Ulrich, C.; Christophers, E.; Stockfleth, E. Association of human papillomavirus infections with cutaneous tumors in immunosuppressed patients. Transpl. Int. 2003, 16, 146–153.
- Borgogna, C.; Lanfredini, S.; Peretti, A.; De Andrea, M.; Zavattaro, E.; Colombo, E.; Quaglia, M.; Boldorini, R.; Miglio, U.; Doorbar, J.; et al. Impreved detection reveals active ß-papillomavirus infection in skin lesions from kidney transplant recipients. Mod. Pathol. 2014, 27, 1101–1115.
- Strickley, J.D.; Messerschmidt, J.L.; Awad, M.E.; Tancheng, L.; Hasegawa, T.; Thinh Ha, D.; Nabeta, H.W.; Bevins, P.A.; Ngo, K.H.; Asgari, M.M.; et al. Immunity to commensal papillomaviruses protects against skin cancer. Nature 2019, 575, 519–522.
- Neagu, N.; Dianzani, C.; Venuti, A.; Bonin, S.; Voidãzan, S.; Zalaudek, I.; Conforti, C. The role of HPV in keratinocyte skin cancer development: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 40–46.
- de Koning, M.N.C.; Struijk, L.; Bouwes Bavinck, J.N.; Kleter, B.; Ter Schegget, J.; Quint, W.G.V.; Feltkamp, M.C.W. Betapapillomaviruses frequently persist in the skin of healthy inidividuals. J. Gen. Virol. 2007, 88, 1489–1495.
- Nakatsuji, T.; Chen, T.H.; Butcher, A.M.; Trzoss, L.L.; Nam, S.J.; Shirakawa, K.T.; Zhou, W.; Oh, J.; Otto, M.; Fenical, W.; et al. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci. Adv. 2019, 4, eaao4502.
- Wu, X.; Hwang, S.T. A Microbiota-Dependent, STAT3-Driven Mouse Model of Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2018, 138, 1022–1026.
- Dehner, C.A.; Ruff, W.E.; Greiling, T.; Pereira, M.S.; Redanz, S.; McNiff, J.; Girardi, M.; Kriegel, M.A. Malignant T Cell Activation by a Bacillus Species Isolated from Cutaneous T-Cell Lymphoma Lesions. JID Innov. 2022, 2, 100084.
- Hooper, M.J.; LeWitt, T.M.; Pang, Y.; Veon, F.L.; Chlipala, G.E.; Feferman, L.; Green, S.J.; Sweeney, D.; Bagnowski, K.T.; Burns, M.B.; et al. Gut dysbiosis in cutaneous T-cell lymphoma is characterized by shifts in relative abundances of specific bacterial taxa and decreased diversity in more advanced disease. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1552–1563.
- Leroux-Kozal, V.; Lévêque, N.; Brodard, V.; Lesage, C.; Dudez, O.; Makeieff, M.; Kanagaratnam, L.; Diebold, M.B. Merkel cell carcinoma: Histopathologic and prognostic features according to the immunohistochemical expression of Merkel cell polyomavirus large T antigen correlated with viral load. Hum. Pathol. 2015, 46, 443–453.
- Hernandez, L.E.; Mohsin, N.; Yaghi, M.; Frech, F.S.; Dreyfuss, I.; Nouri, K. Merkel cell carcinoma: An updated review of pathogenesis, diagnosis and treatment options. Dermatol. Ther. 2022, 35, e15292.
- Bhutani, M.; Polizzotto, M.N.; Uldrick, T.S.; Yarchoan, R. Kaposi sarcoma-associated herpesvirus-associated malignancies: Epidemiology, pathogenesis, and advances in treatment. Semin. Oncol. 2015, 42, 223–246.
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521.
- Fang, Z.; Li, L.; Zhang, H.; Zhao, J.; Lu, W.; Chen, W. Gut Microbiota, Probiotics, and Their Interactions in Prevention and Treatment of Atopic Dermatitis: A Review. Front. Immunol. 2021, 12, 720393.
- Goodarzi, A.; Mozafarpoor, S.; Bodaghabadi, M.; Mohamadi, M. The potential of probiotics for treating acne vulgaris: A review of literature on acne and microbiota. Dermatol. Ther. 2020, 33, e13279.
- Rigon, R.B.; de Freitas, A.C.P.; Bicas, J.L.; Cogo-Müller, K.; Kurebayashi, A.K.; Magalhães, R.F.; Leonardi, G.R. Skin microbiota as a therapeutic target for psoriasis treatment: Trends and perspectives. J. Cosmet. Dermatol. 2021, 20, 1066–1072.
- Yu, Y.; Dunaway, S.; Champer, J.; Kim, J.; Alikhan, A. Changing our microbiome: Probiotics in dermatology. Br. J. Dermatol. 2020, 182, 39–46.
- Valdez, J.; Peral, M.; Rachid, M.; Santana, M.; Perdigon, G. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: The potential use of probiotics in wound treatment. Clin. Microbiol. Infect. 2005, 11, 472–479.
- Huang, H.C.; Lee, I.J.; Huang, C.; Chang, T.M. Lactic Acid Bacteria and Lactic Acid for Skin Health and Melanogenesis Inhibition. Curr. Pharm. Biotechnol. 2020, 21, 566–577.
- Hosseini, S.; Imenshahidi, M.; Hosseinzadeh, H.; Karimi, G. Effects of plant extracts and bioactive compounds on attenuation of bleomycin-induced pulmonary fibrosis. Biomed. Pharmacother. 2018, 107, 1454–1465.
- Le, M.; Ghazawi, F.M.; Netchiporouk, E.; Litvinov, I.V. The Novel Role of Antibiotic Treatment in the Management of Cutaneous T-Cell Lymphoma (CTCL) Patients. J. Cutan. Med. Surg. 2020, 24, 410–411.
- Lindahl, L.M.; Willerslev-Olsen, A.; Gjerdrum, L.M.R.; Nielsen, P.R.; Blümel, E.; Rittig, A.H.; Celis, P.; Herpers, B.; Becker, J.C.; Stausbøl-Grøn, B.; et al. Antibiotics Inhibit Tumor and Disease Activity in Cutaneous T-Cell Lymphoma. Blood 2019, 134, 1072–1083.
- Lindahl, L.M.; Iversen, L.; Ødum, N.; Kilian, M. Staphylococcus aureus and Antibiotics in Cutaneous T-Cell Lymphoma. Dermatology 2021, 238, 3.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
744
Revisions:
2 times
(View History)
Update Date:
13 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





