Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mateusz Zygadło | -- | 4642 | 2023-10-11 10:39:25 | | | |
| 2 | Sirius Huang | Meta information modification | 4642 | 2023-10-12 03:20:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Muszyński, M.; Nowicki, J.; Zygadło, M.; Dudek, G. Chemical Depolymerization Methods of Poly(ethylene terephthalate). Encyclopedia. Available online: https://encyclopedia.pub/entry/50105 (accessed on 13 January 2026).
Muszyński M, Nowicki J, Zygadło M, Dudek G. Chemical Depolymerization Methods of Poly(ethylene terephthalate). Encyclopedia. Available at: https://encyclopedia.pub/entry/50105. Accessed January 13, 2026.
Muszyński, Marcin, Janusz Nowicki, Mateusz Zygadło, Gabiela Dudek. "Chemical Depolymerization Methods of Poly(ethylene terephthalate)" Encyclopedia, https://encyclopedia.pub/entry/50105 (accessed January 13, 2026).
Muszyński, M., Nowicki, J., Zygadło, M., & Dudek, G. (2023, October 11). Chemical Depolymerization Methods of Poly(ethylene terephthalate). In Encyclopedia. https://encyclopedia.pub/entry/50105
Muszyński, Marcin, et al. "Chemical Depolymerization Methods of Poly(ethylene terephthalate)." Encyclopedia. Web. 11 October, 2023.
Copy Citation
The significant amount of waste generated by poly(ethylene terephthalate) (PET) requires the development of a recycling process chain in which chemical recycling plays an important role. On the one hand, it allows the depolymerization of degraded plastics that do not meet the quality requirements to be used in mechanical recycling, and on the other hand, provides an opportunity to process cheap waste and obtain products with greater added value. It can be widely used in the recycling of both packaging plastics and textiles, or other waste generated with PET.
chemical recycling
depolymerization
catalysis
glycolysis
transesterification
hydrolysis
1. Introduction
Plastics are a massively used product in the global economy. It is estimated that about 83,000 million tons of plastics have been produced since 1950 [1]. Plastics are widely used, among others, as construction materials, in the textile industry, as well as in packaging, transport and electronics [2]. The most commonly produced plastics are polyolefins, of which a significant part is poly(ethylene terephthalate) (PET), which is mainly used in the production of packaging and textiles [1]. The mass production of plastics and the lack of proper practices and regulations regarding waste collection and treatment have led to an increasing accumulation of waste in the natural environment. Untreated and poorly deposited waste enters the natural environment, leading to pollution in both land and aquatic environments. The latter has recently been a source of particular interest due to the presence of microplastics in the natural environment, which accumulates in the environment due to poor biodegradability [3][4]. The accumulation of waste and the introduction of legal regulations have resulted in an increase in interest in plastic recycling. Over the years, various methods of recycling plastics have been developed. Among these methods, energy, mechanical and chemical recycling are distinguished. Thermal recycling is used to generate energy and is used for materials that are not suitable for processing using other methods [5]. It is used for degraded plastics that can no longer be recycled, cross-linked polymers and composites whose recycling is not economical. Mechanical recycling is most commonly used for thermoplastics that can be easily recycled and reused. Chemical recycling is the process by which a polymer undergoes a chemical reaction that produces monomers or other valuable chemical compounds. Of the massively used polymers, only some can be chemically recycled. Important factors determining the possibility of chemical recycling of polymers include their susceptibility to depolymerization reaction, availability on the market and cost-effectiveness. The material most often subjected to the depolymerization process is poly(ethylene terephthalate) (PET), although there are also known methods of depolymerization of other materials such as other polyesters [6], polycarbonates [7], and polyurethanes [8]. Poly(ethylene terephthalate) is chemically recycled using such methods as hydrolysis [9], glycolysis [10] and alcoholysis [11].
2. Chemical Recycling
2.1. Hydrolysis
The hydrolytic decomposition of synthetic polymers that provide starting monomers which can be reused for polymer synthesis is one of the most important methods in the chemical recycling of post-consumer polymeric materials [12]. Not every type of polymer can be processed by this method. However, polyethylene terephthalate is one of the polymers for which this processing path is possible to carry out (Figure 1).
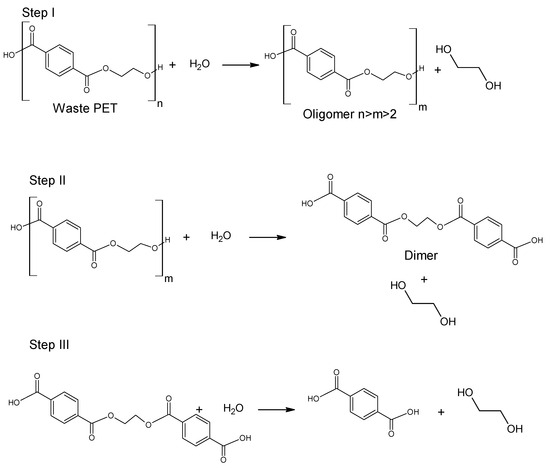
Figure 1. Hydrolysis of poly(ethylene terephthalate).
The hydrolytic decomposition of PET for producing starting monomers was the earlier method of waste PET recycling. From a chemical point of view, the hydrolytic decomposition of PET can be carried out in neutral, alkaline and acidic conditions [13][14]. The disadvantage of this method is the relatively high cost of purifying terephthalic acid (TPA) from the post-reaction mixture, which limits the commercial use of this method for the production of high-quality polymer, e.g., intended for contact with food [14].
The neutral hydrolysis method of PET depolymerization uses water or steam without the addition of an acid or basic catalyst, usually at temperatures between 250–300 °C and pressures between 1.5–4.0 MPa. The weight ratio of PET: water is generally from 1:2 to 1:12. The neutral hydrolysis method leads directly to monomers for their subsequent use in polyester synthesis. It can be carried out both in stationary (batch) and continuous modes. In this method, various metal catalysts are often used, which positively affect the efficiency of the process, but the addition of these catalysts has a negative impact on the separation and purification of monomers, especially terephthalic acid. The literature provides a number of examples describing these types of solutions. Stanica-Ezeanu and Matei described a method of waste PET hydrolysis in neutral conditions carried out in a seawater environment [15]. The process was carried out at a temperature of 215 °C and a pressure of 4 MPa. Under these conditions, only 85–87% conversion was achieved and the TPA yield was only 76–84%.
Under the standard hydrothermal process (250 °C, 39–40 bar, 30 min.), 90–92% PET conversion can be achieved [16][17]. Depolymerization of the colored polymer is slightly less effective. Conversion of PET obtained in this case was only 85% [17]. The process can also be carried out in supercritical conditions (H2O or CO2) [17] and also supported by microwave heating [18][19]. Although this method can be considered as effective and more ecological, it requires the use of more drastic conditions of temperature, pressure and reaction time, which can be up to 5 h.
Based on recent literature, methods of PET depolymerization (hydrolysis) carried out in alkaline conditions, most often using NaOH as an alkaline catalyst, are definitely dominant [20][21][22][23]. Both the PET conversion and TPA yields obtained were typically >90%. There are also known solutions in which the hydrolysis process is carried out in a mixture containing an additional solvent, such as ethanol [24] or γ-valerolactone used as pre-solvent of waste PET [25]. Specific phase transfer catalysts were also used as catalysts supporting the hydrolysis process [26][27][28]. They turned out to be very effective in the tested reaction, as evidenced by the very high PET conversion values (99%) obtained. Both classical ammonium salts and highly specific ammonium phosphotungstates were used as the phase transfer catalyst (Table 1).
Table 1. PET hydrolysis in alkaline process conditions.
| TPA Yield [%] | Reaction Temperature [°C] | Reaction Time [min] | Pressure [bar] | Catalyst | Solvent | Reference |
|---|---|---|---|---|---|---|
| 97 | 120–200 | 60 | n/a | NaOH | Water | [12] |
| 92 | 200 | 25 | n/a | NaOH | Water | [20] |
| 95 | 220 | n/a | 26 | NaOH | Water | [23] |
| 95 | 80 | 20 | 1 | NaOH | Water/Ethanol | [24] |
| 99 | 80 | 20 | 1 | NaOH | Water | [25] |
| 95 | n/a 1 | 60 | 1 | NaOH + TBAJ | Water | [26] 1 |
| 99 | 110 | 300 | n/a | NaOH + [CTA]3PW | Water | [27] 2 |
| 93 | 145 | 120 | n/a | NaOH + [CTA]3PW | Water | [28] 2 |
1 under microwave irradiation, 2 cetyltrimethylammonium phosphotungstate, n/a—not applicable.
In alkaline hydrolysis, separation of terephthalic acid requires an additional precipitation operation using acid solutions, which generates an additional stream of waste inorganic salts. These problems do not occur in hydrolysis processes carried out under acidic conditions. As catalysts, both strong sulfonic acids, such as H2SO4 [28] or p-toluenesulphonic acid, were commonly applied [29]. Some heterogeneous catalysts with acidic properties, such as heteropolyacids [28] and “superacid” type SO42−/TiO2 [30] and WO3/SiO2 [31] were also used as catalysts in PET hydrolysis process. In the case of sulfuric acid, a PET conversion of 83% was obtained with a TPA yield of about 75%. Similar results were obtained for heteropolyacids; however, superior results were obtained for ptc-type quaternary ammonium phosphotungstates, for which PET conversion was even 100% [28]. Yang et al. described an interesting process of PET hydrolysis catalyzed by easily recyclable terephthalic acid [32]. The advantage of this method is that no compounds are introduced into the process that would require removal from the post-synthesis mixture. PET conversion was close to 100% with a TPA yield of 95.5% (Table 2).
Table 2. PET hydrolysis in acidic process conditions.
| TPA Yield [%] | Reaction Temperature [°C] |
Reaction Time [min] | Pressure [bar] | Catalyst | Solvent | Reference |
|---|---|---|---|---|---|---|
| 75 | 180 | 180 | n/a | H2SO4 | Water | [28] |
| 73 | 180 | 180 | n/a | H3PW12O40 | Water | [28] |
| 93 | 180 | 180 | n/a | [CTA]3PW | Water | [28] |
| 96.2 | 150 | 90 | n/a | PTSA | Water | [29] |
| 99.2 | 160 | 12 h | 150 | SO42−/TiO2 | Water | [30] 1 |
| 99.5 | 160 | 15 h | 150 | WO3/SiO2 | Water | [31] 1 |
| 95.5 | 220 | 180 | n/a | TPA | Water | [32] |
Note 1 under supercritical CO2, n/a—not applicable.
2.2. PET Alcoholysis
Alcoholysis of poly(ethylene terephthalate) (Figure 2) is, apart from alkaline hydrolysis, one of the basic methods of chemical depolymerization of waste PET. Alcoholysis involves the degradation of PET in an alcohol environment under conditions of high temperature and pressure. Alcoholysis is considered to be one of the more reliable and effective methods applied for waste PET recycling [33][34]. Alcoholysis processes use a wide group of well-known alcohols, among which the most often used are: methanol, ethanol, butanol and isooctyl alcohol (2-ethylhexanol). Among the described reports in this field, various variants of methanolysis processes definitely dominate, which is related to the key role of dimethyl terephthalate obtained in these processes. As it is known, dimethyl terephthalate is the basic raw material in the synthesis technologies of phthalate polyesters. Alcoholysis using alcohols > C1 has become less common and esters of terephthalic acid and higher alcohols are often used as raw materials in processes other than the synthesis of polyesters.
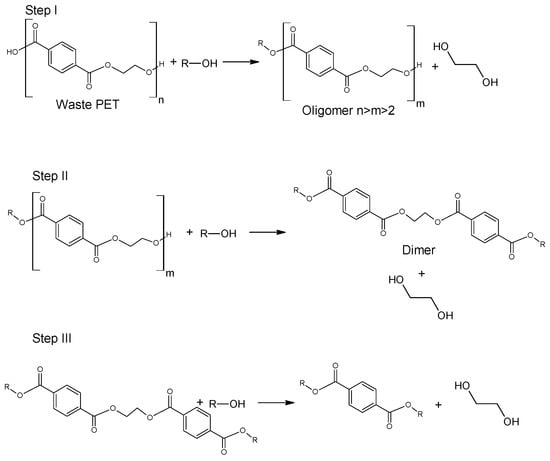
Figure 2. Alcoholysis of poly(ethylene terephthalate).
2.2.1. Methanolysis
Methanolysis process is used to produce dimethylterephthalate (DMT) which then can be applied in poly(ethylene terephthalate). DMT is produced via the reaction of PET with methyl alcohol usually under increased pressure due to the low boiling point of methanol. Reaction is conducted in the presence of various types of catalysts such as Bronsted and Lewis acids, hydroxides, organic bases, oxides and ionic liquids. The process is most often conducted in liquid phase; however, gas-phase methanolysis [35] and processes conducted in supercritical conditions are described in the scientific literature [36]. Separation and repeated usage of catalysts from depolymerization products is an important aspect of designing a viable process that can be implemented as a working chemical technology. Homogenous catalysts can be difficult to remove and to reuse. To remove this obstacle, the use of heterogeneous catalysts in chemical depolymerization of PET is investigated.
One of the catalysts in depolymerization reaction of poly(ethylene terephthalate) is zinc acetate. Its application as a PET depolymerization catalyst was described in numerous scientific publications and is characterized by high activity and its application results in high product yields. Hofmann et al. [37] investigated methanolysis of waste PET using zinc acetate catalyst and waste PET. The process was conducted in the presence of dichloromethane which acted as a solvent. The authors obtained high yields of dimethyl terephthalate which reached 98% after a 20 min time period. However, a large amount of methanol was used in this process. Equivalent methanol-to-PET repeating unit ratio varied from 46.2 to 92.5. Catalyst amount was weight in respect to amount of PET used in the reaction. Interestingly, reaction yield dropped significantly after lowering temperature to 140 °C. In this case, after 20 min reaction yield was under 1%wt and reached 92%wt after 60 min of reaction. Employed catalyst is also susceptible to contaminants in waste PET. When reaction was carried out using dyed bottles, this resulted in lower reaction yield of 38%wt. Investigation of thermodynamics and kinetics of waste PET methanolysis was conducted by Mishra et al. [38] using zinc and lead acetates as catalysts. Influence of PET particle size on reaction kinetics was investigated and reaction was also optimized. Optimal reaction time was determined to be 120 min at temperatures ranging from 130 to 140 °C with PET particle size of 127.5 μm. Zinc acetate was also employed as a catalyst in chemical recycling of mixture of waste PET and polylactic acid (PLA) [37] using methanol as well as a number of other solvents, i.e., ethanol, ethylene glycol, etc. After reaction conducted in 15 h time under boiling point of methanol, there was no discernable effect on PET while all of the PLA was depolymerized. This result is attributed to differences between solubility of tested polymers in methanol. Methanolysis of PET in microwave reactor was investigated in [6] and the process is characterized by a very short reaction time and low amount of catalyst used. Within 10 min of the process with catalyst loading of 0.01 g per 1 g of PET, 88%wt of PET can be converted.
Some inorganic as well as organic catalysts are active at low temperatures in methanolysis of waste poly(ethylene terephthalate). Catalysts like potassium carbonate 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) and potassium methoxide CH3OK exhibit good catalytic properties in methanolysis reaction conducted in low temperatures [39]. Methanolysis conducted at a temperature of 25 °C over a period of 24 h using K2CO3 yielded 93.1%wt dimethyl terephthalate. To obtain such high yields, a large excess of methanol and dichloromethane was used as well as large amounts of catalyst. Implementation of TBD and CH3OK resulted in lower yields of product 89.3 and 85.5%wt, respectively. Interestingly, K2CO3 exhibits highest catalytic activity although it did not completely dissolve in applied solvent. Other catalysts like KHCO3, KOAc, Na2CO3, and CaO among others had given significantly lower concentrations of dimethyl terephthalate.
Calcinated sodium silicate (Na2SiO3) was used in the methanolysis of PET by Tang et al. [40]. The authors investigated catalyst obtained by calcination under different temperatures as well as influence of catalyst concentration, reaction temperature methanol-to-PET weight ratio and reaction time on reaction yield and PET conversion. Applied silicate exhibits good catalytic properties in relatively small catalyst loadings from 3 to 7%wt reaching up to 63% yield and 74% conversion rate using as much as 5% of the catalyst. The process was tested in temperatures ranging from 160 to 200 °C. Interestingly, the authors used relatively low alcohol-to-PET ratio which ranged from 3 to 7. Process conducted under optimized conditions resulted in obtaining dimethyl terephthalate with 95% yield and 100% conversion. Recycling of catalyst was also investigated. Silicate catalyst was reused four times with some loss in activity attributed to adsorption of water. Magnesium phosphate catalyst obtained in the presence of pectin was used in PET methanolysis [41]. The use of pectin resulted in obtaining catalyst with large BET surface area of 19.51 m2/g and average pore size of 26.01, which was significantly higher compared to catalyst obtained without the use of pectin. Process was conducted at 180 °C for 150 min with 3%wt of catalyst achieving yield of 74%wt. MgP catalyst is stable when reused as it was reused four times with low loss in PET conversion. However, a methanol-to-PET weight ratio of 200 was used in this study. Such large alcohol excess will have a negative influence on the overall amount of obtained DMT per synthesis.
Heterogeneous catalysts obtained from bio wastes can also be implemented in methanolysis of waste poly(ethylene terephthalate) [42]. The authors used a catalyst obtained by calcination of bamboo leaf at 700 °C in methanolysis of PET waste. The obtained catalyst was composed mainly of SiO2 and a mixture of various oxides of other metals such as calcium, potassium, iron, manganese, magnesium, etc. Methanolysis reaction using such catalyst allowed for achieving DMT with a yield of 78%wt after two hours in relatively low methanol excess of 7.5 and catalyst loading of 20.8%wt in relation to the mass of PET. Interestingly, increase in catalyst loading, reaction temperature and time resulted in lower yield of DMT. Reusability tests have shown the loss in activity of the catalyst. After four cycles, DMT yield lowered from 78%wt to 67%wt. Nanocatalysts in the form of zinc oxide dispersions are found to be active in methanolysis of poly(ethylene terephthalate) [43]. Depolymerization process conditions were optimized regarding reaction time, methanol-to-PET ratio and catalyst concentration. The tested catalyst exhibits very good activity achieving DMT yield of 97%wt after 15 min at 170 °C and subsequent trials conducted to test the possibility of catalyst reuse have shown a decrease in activity by approximately 20%. Overall, ZnO nanodispersion has proven to be an active catalytic system which allows for obtaining high yields in very short time periods. Heterogeneous hydrotalcite (Mg-Al) has proven to be an effective degradation catalyst when used in conjunction with dimethyl sulfoxide (DMSO) [44]. Degradation process was completed in a 10 min time period obtaining PET oligomer. Obtained product was then reacted with methanol in the presence of sodium hydroxide (NaOH) at 35 °C for 60 min.
Ionic liquids are used as methanolysis catalysts in depolymerization of poly(ethylene terephthalate). Liu et al. [45] tested a series of ionic liquids in methanolysis of various polymers including poly(ethylene terephthalate), polycarbonate, Polyhydroxybutyrate and polylactic acid. [HDBU][Im] and [Bmim]2[CoCl4] were used in PET methanolysis. Reaction was conducted at 170 °C over a period of four hours for [Bmim]2[CoCl4] and 140 °C over a period of three hours for [HDBU][Im]. Reactions yielded 78 and 75%wt, respectively.
Chemical recycling of waste poly(ethylene terephthalate) under supercritical conditions can be employed successfully for PET methanolysis [46]. Process conducted at 298 °C for a duration of 112 min with excess methanol results in DMT yield of 99.79%wt (Table 3).
Table 3. Methanolysis reaction parameters.
| Yield [%] | Alcohol/Polymer Ratio |
Reaction Temperature [°C] |
Reaction Time [min] | Pressure [bar] | Catalyst | Catalyst Amount [%wt.] | Reference |
|---|---|---|---|---|---|---|---|
| 98 | 46.2 | 160 | 20 | n/a | Zn(OAc)2 | 1 | [37] |
| 97.76 | 2.38 | 130 | 120 | 8.5 | Zn(OAc)2/Pb(OAc)2 | 5.1 | [38] |
| 0.0 | 2.16 | 64.7 | 900 | 1 | Zn(OAc)2 | 0.07 | [37] |
| 93.1 | 50 | 25 | 1440 | 1 | K2CO3 | 0.2 a | [39] |
| 89.3 | 50 | 25 | 1440 | 1 | TBD | 0.2 a | [39] |
| 85.5 | 50 | 25 | 1440 | 1 | CH3OK | 0.2 a | [39] |
| 95 | 5 | 200 | 45 | n/a | Na2SiO3 | 5 | [40] |
| 74 | 200 | 180 | 150 | n/a | MgP | 3 | [41] |
| 78 | 7.5 | 200 | 120 | n/a | BLA | 20.8 | [42] |
| 97 | 6 | 170 | 15 | n/a | ZnO nanodispersion | 3.5 | [43] |
| 78 | 5 | 170 | 240 | n/a | [Bmim]2[CoCl4] | 1.6 | [47] |
| 75 | 5 | 140 | 180 | n/a | [HDBU][Im] | 1.6 | [47] |
| 99.79 | 6 | 298 | 112 | n/a | - | - | [48] |
a % of PET degradation; n/a—not applicable.
2.2.2. Glycolysis
Glycolysis is a widely used method for the chemical processing of PET. Most commonly, as the reactive solvent, ethylene glycol (EG) and diethylene glycol (DEG) are used [14]. From a chemical point of view, the glycolysis reaction is the decomposition of PET with glycols, in the presence of transesterification catalysts, where ester linkages break and are replaced with hydroxyl terminals. One of the earliest patents of this process was proposed in 1964 by Ostrysz et al. and concerns the preparation of unsaturated polyester resins (UPR) bearing a structure as presented in Figure 3 [49][50].
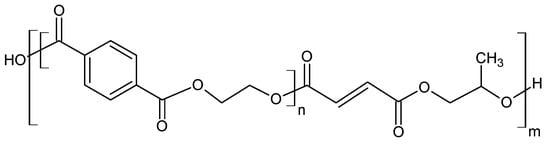
Figure 3. Structure of Thixotropic and Chemoresistant Unsaturated Polyester Resins [3].
Glycolysis proceeds in a several steps as presented in Figure 4. Initially, PET is converted to its dimer form through oligomer molecules at a high rate, and then slow transition takes place. Process is mainly carried out in mild conditions under atmospheric pressure and at a temperature ranging between 180–240 °C. A major advantage of this procedure is the application of less volatile glycols compared to alcohols of similar structure [51][52]. It was also found that specific order of parameters: catalyst concentration > glycolysis temperature > glycolysis time affects the yield of obtained product [53][54]. However, the disadvantage of this process is that the yield is insufficient for industrial application. In order to increase efficiency of the process, catalysts are used [5][55]. The most widely discussed group of catalysts in the recent literature are salts of transition group metals. Zinc (II) Acetate (Zn(OAc)2) had been found to be the most effective catalyst. Zn(OAc)2 was applied for the first time as a catalyst for PET glycolysis in 1989 by Vaidya and Nadkarni; however, other molecules have been topics of interest in recent studies. These are mainly transition metals salts and complexes, ionic liquids and metal oxides [5][56].
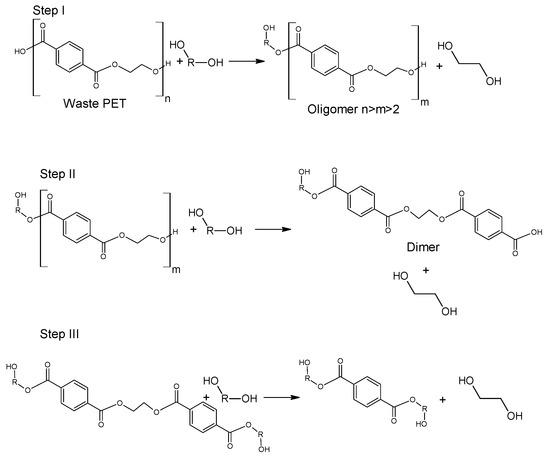
Figure 4. Glycolysis of poly(ethylene terephthalate) [57].
Processed PET in the form of polyhydric alcohols or oligomers with applied glycol on the end-groups have found numerous applications. Depolymerized PET can be used as an additive to poly(vinyl chloride) (PVC) [5]. Another application of oligoesters is in the production of polyurethanes, as polyol groups containing reactant. The depolymerized PET with ethylene glycol can be reused to produce fresh poly(ethylene terephthalate) molecules [57].
2.2.3. Ethanolysis
Ethanolysis of poly(ethylene terephthalate) is widely regarded as an environmentally friendly alternative to methanolysis, which has been well known for many years. This is mainly due to the possibility of replacing harmful methanol with safer ethanol [58]. Diethyl terephthalate formed in the depolymerization (hydrolysis) reaction can be reused for the synthesis of poly(ethylene terephthalate) (Figure 5).
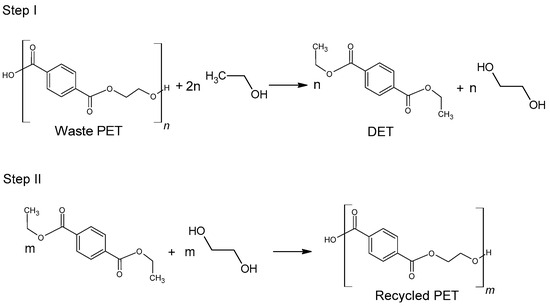
Figure 5. Recycling of waste PET via direct ethanolysis.
The use of ethanol in the process of depolymerization of waste PET should be considered in a much broader aspect than only as a process leading to diethyl terephthalate. Speaking about the process of ethanolysis of poly(ethylene terephthalate), one should also take into account the process whose final product is terephthalic acid and it is a process similar to the process of alkaline PET hydrolysis described earlier. Three reactions take place under alkaline catalysis (NaOH) in a water-ethanol medium: depolymerization of PET to a mixture of diethyl terephthalate (Step I), its hydrolysis to TPA disodium salt (Step II) and precipitation of free TPA (Step III, Figure 6). Ethanol is recycled and can be reused (Closed-loop recycling process), making it very competitive with conventional alkaline hydrolysis processes [24][59][60].
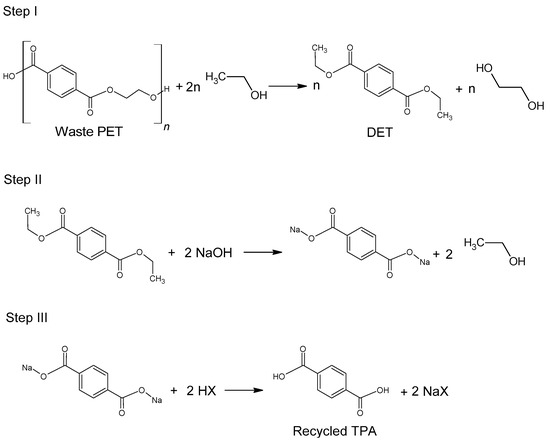
Figure 6. Three-step recycling process of waste PET into terephthalic acid.
The process can be carried out under relatively mild conditions (reaction temperature 50–80 °C, the proportion of ethanol to water 20–100 vol%, the amount of NaOH 5–15%wt) [61]. Under optimal conditions, TPA efficiency reaches 95%. In the method presented in Figure 6, diethyl terephthalate formed in Step I is not separated. Under process conditions, it hydrolyzes to terephthalic acid.
Diethyl terephthalate is obtained by direct ethanolysis of PET (Figure 5). The direct PET ethanolysis process requires temperatures above 200 °C and sometimes the addition of a catalyst. Li et al. [62] described a method of direct PET ethanolysis carried out under pressure and at a temperature of 180–200 °C without the addition of a catalyst. The author reported that under the assumed reaction conditions, nearly 100% PET conversion can be obtained and the DET yield reached 97%. Zinc acetate is an effective catalyst for PET ethanolysis, which allows DET yields of 96–97% to be achieved [63][64]. From a technological point of view, the method is very convenient. Due to differences in solubility, the ethylene glycol formed in the reaction is the lower, immiscible phase, which can be separated from the upper phase containing diethyl terephthalate. Compounds containing acidic sulfonic groups, such as, sulfonic ionic liquids, can also act as catalysts for the PET ethanolysis reaction [65]. By carrying out the process for 14 h at 80 °C in the presence of sulfobutylammonium ionic liquid, diethyl terephthalate can be obtained with a yield of 96%. However, using the techniques of depolymerization of waste PET leading to the production of useful phthalate monomers (terephthalic acid esters), solvolysis processes carried out in supercritical conditions prevail. The basics of the process were presented in the last century in Japan, where a method of PET depolymerization using supercritical water and methanol was developed [58]. Supercritical solvents are very attractive media for conducting many chemical processes mainly because the solvent and transport properties of a single solution can be appreciably and continuously varied with relatively minor changes in either temperature or pressure. Variation in the supercritical fluid density also influences the chemical potential of solutes, reaction rate and equilibrium constant [66]. Depolymerization of waste PET in supercritical methanol is the subject of extensive research [67]. In recent years, however, there have been increasing reports on solvolysis of waste polyester polymers in supercritical ethanol [58][68]. As described by Castro et al. [58], the ethanolysis process was carried out in a supercritical ethanol environment at a temperature of 255 °C, a pressure of 115 or 165 bar and reaction times between 5.0 and 6.5 h without the addition of a catalyst. Under these process conditions, PET was practically completely depolymerized and the main product of the reaction, apart from ethylene glycol and ethanol, was diethyl terephthalate. Later studies included the addition of catalysts, such as metal oxides [69][70] and ionic liquids [71][72]. As the research has shown, the addition of catalysts to the PET ethanolysis process does not have a major impact on the conversion rates, but conclusively shortens the reaction time. Later studies showed that increasing the temperature to 275–350 °C removed the need for the addition of catalysts while maintaining high PET conversion rates and DET efficiency [73][74]. Recent studies have also shown the high efficiency of the PET ethanolysis process in supercritical conditions and the possibility of using this method on a technical scale (Table 4).
Table 4. PET alcoholysis with ethanol.
| DET Yield [%] | Reaction Temperature [°C] | Reaction Time [min] | Pressure [bar] | Catalyst | Solvent | Reference |
|---|---|---|---|---|---|---|
| 97.3 | 200 | 210 | n/a | Zn(OAc)2 | Ethanol | [62] |
| 92 | 220 | 120 | n/a | Zn(OAc)2 | Ethanol | [63] |
| 97 | 180 | 60 | n/a | Zn(OAc)2 + Al(OH)3 | Ethanol | [64] |
| 95.8 | 80 | 20 | 1 | Sulphonic IL | Ethanol | [65] |
| 98.5 | 240 | 300 | 115–165 1 | - | Ethanol | [74] |
| >99 | 255 | 90 | 115 1 | Co3O4 or NiO | Ethanol | [69] |
| 92.2 | 270 | 60 | n/a 1 | ZnO/Al2O3 | Ethanol | [70] |
| 98 | 255 | 45 | 115 | [bmim]BF4 | Ethanol | [71][72] |
| 95 | 275–300 | 90 | n/a 1 | - | Ethanol | [73] |
| 98 | 310 | 60 | n/a 1 | - | Ethanol | [74] |
1 under supercritical conditions, n/a—not applicable.
2.2.4. Alcoholysis with Alcohols > C2
Diethyl terephthalate is used as a safer alternative to dimethyl terephthalate in the synthesis of polyesters. Terephthalates of higher alcohols are no longer used in this specific application. However, they are equally valuable due to their plasticizing properties. This also applies to dibutyl terephthalate, which is produced on an industrial scale. Dibutyl terephthalate has fast melting and low migration properties and provides greater flexibility of finished products. These properties mean that it is used, for example, in the production of flexible floor coverings based on PVC, adhesives and sealants, and in the production of printing inks [75][76].
Dibutyl terephthalate is most often produced by esterification of terephthalic acid, but the use of waste for its synthesis seems to be a more ecological alternative. Depolymerization of waste PET by butanol alcoholysis requires a completely different process than in the case of alcohols such as methanol or ethanol. It also requires the addition of a catalyst. According to the description given in the patent CN102603532, the PET depolymerization process in the presence of 1-butanol and sulfonic ionic liquid allows dibutyl terephthalate to be obtained with a yield of 93.9%. According to the description, the alcoholysis process was carried out at 190 °C for 9 h [65]. In turn, the patent description WO2022112715 provides a general method for the synthesis of terephthalic acid esters, including dibutyl ester [77]. In this method, waste PET is reacted with excess butanol at a temperature between 50–70 °C for 1.5–3 h in the presence of a catalyst being a suitably selected mixture of cyclic guanidine (TBD) or amidine (DBU) derivatives and sodium methoxide. In this method, dibutyl terephthalate can be obtained with a yield between 80–85%. The original method of depolymerization of waste polyesters, including PET, was described in [48]. The essence of the method is the use of specific catalysts based on DBU and various imidazole derivatives (Figure 7).
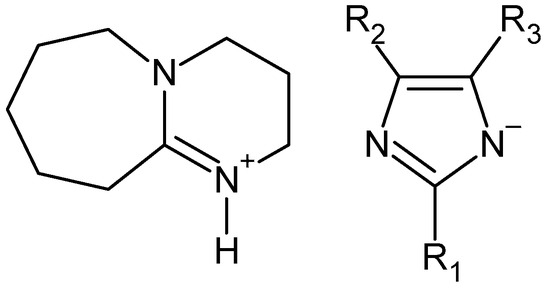
Figure 7. DBU ionic liquid as catalyst for alcoholysis of waste polyesters (PC, PLA, PHB, and PET) [48].
The alcoholysis process was carried out in relatively mild conditions (70 °C) for 2 h. In the case of butanol, a PET conversion of 91% was obtained with a DBT yield of 73%, but for alcohols C5–C6 the yield of the corresponding terephthalic acid ester decreases.
For higher alcohols, clearly higher rates of yield of the appropriate diesters of terephthalic acid can only be obtained in processes carried out in more conventional parameters, i.e., higher temperature and pressure. According to the patent description CN102234227, the PET depolymerization process in the presence of higher alcohols can be carried out at a temperature of 180–200 °C for 4–9 h [78]. Zinc acetate is an effective catalyst for the reaction, but the described method does not provide detailed information on the conversion of PET and the yield of the corresponding terephthalic acid esters. Two other patents CN105503605 and CN106986326 describe the method of PET depolymerization with monohydric alcohols C4-C8 catalyzed by tetrabutyl titanate [79][80]. In addition, this method requires a slightly higher temperature range between 200–230 °C. However, the authors of both solutions also do not provide PET conversion rates and yields of the corresponding esters (Table 5).
Table 5. PET alcoholysis with alcohols C3–C7.
| DET Yield [%] | Alcohol | Reaction Temperature [°C] | Reaction Time [min] | Catalyst | Reference |
|---|---|---|---|---|---|
| 92.3 | 1-Butanol | 190 | 300 | Sulphonic IL | [65] |
| 95.3 | 1-Hexanol | 190 | 600 | Sulphonic IL | [65] |
| 80–85 | 1-Butanol | 50–70 | 90–180 | TBD(DBU) + CH3ONa | [77] |
| 78 | 1-Propanol | 70 | 120 | [HDBU][Im] | [48] |
| 73 | 1-Butanol | 70 | 120 | [HDBU][Im] | [48] |
| n/a | C4–C8 | 180–200 | 240–540 | Zn(OAc)2 | [78] |
| n/a | C4–C8 | 200–230 | 120–600 | TBT | [79] |
| n/a | C4–C8 | 200–230 | 120–600 | TBT | [80] |
2.2.5. Alcoholysis with C8 Alcohols
Depolymerization of poly(ethylene terephthalate) can by conducted using higher alcohols, i.e., 2-ethylhexanole, isononyl and isodecyl alcohol. Products obtained in this manner are valuable chemicals that can be used as PVC plasticizers. This process can be catalyzed by organometallic catalysts, ionic liquids and superbases. One of the earliest works on alcoholysis of PET was presented by Gupta et al. [81]. Authors investigated the application of organotin catalyst in depolymerization of waste PET from different sources. The authors conducted depolymerization of waste PET from beverage and food bottles, packaging film, fabrics, car parts, photographic and X-ray films. Additionally, the catalyst was tested in depolymerization reaction of crystalized PET and glycol modified PET as well as PBT. Obtained DOTP samples were tested with PVC to determine their plasticizing properties. It was concluded that all obtained samples exhibit similar properties to commercial DOTP obtained from terephthalic acid. The most important difference was the difference in color of the product which is heavily influenced by the color of used substrate. Hardness, brittle point, tensile strength and elongation at break were at similar values regardless of the origin of applied plasticizer.
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782.
- Namazi, H. Polymers in our daily life. Bioimpacts 2017, 7, 73–74.
- Smith, R.; Oliver, C.; Williams, D. The enzymatic degradation of polymers in vitro. J. Biomed. Mater. Res. 1987, 21, 991–1003.
- Zhang, J.; Wamg, X.; Gong, J.; Gu, Z. A study on the biodegradability of polyethylene terephthalate fiber and diethylene glycol terephthalate. J. Appl. Polym. Sci. 2004, 93, 1089–1096.
- Geyer, B.; Lorenz, G.; Kandelbauer, A. Recycling of poly(ethylene terephthalate)—A review focusing on chemical methods. EXPRESS Polym. Lett. 2016, 10, 559–586.
- Hong, M.; Chen, E. Chemically recyclable polymers: A circular economy approach to sustainability. Green Chem. 2017, 19, 3692–3706.
- Antonakou, E.V.; Achilias, D.S. Recent Advances in Polycarbonate Recycling: A Review of Degradation Methods and Their Mechanisms. Waste Biomass Valor. 2013, 4, 9–21.
- Zia, K.M.; Bhatti, H.N.; Bhatti, I.A. Methods for polyurethane and polyurethane composites, recycling and recovery: A review. React. Funct. Polym. 2007, 67, 675–692.
- Yoshioka, T.; Motoki, T.; Okuwaki, A. Kinetics of Hydrolysis of Poly(ethylene terephthalate) Powder in Sulfuric Acid by a Modified Shrinking-Core Model. Ind. Eng. Chem. Res. 2001, 40, 75–79.
- Ghaemy, M.; Mossaddegh, K. Depolymerisation of poly(ethylene terephthalate) fibre wastes using ethylene glycol. Polym. Degrad. Stab. 2005, 90, 570–576.
- Liu, S.; Wang, Z.; Li, L.; Yu, S.; Xie, C.; Liu, F. Butanol alcoholysis reaction of polyethylene terephthalate using acidic ionic liquid as catalyst. J. Appl. Polym. Sci. 2013, 130, 1840–1844.
- Zhou, J.; Hsu, T.; Wang, J. Mechanochemical Degradation and Recycling of Synthetic Polymers. Angew. Chem. Int. Ed. 2023, 62, e202300768.
- Cao, F.; Wang, L.; Zheng, R.; Guo, L.; Chena, Y.; Qian, X. Research and progress of chemical depolymerization of waste PET and high-value application of its depolymerization products. RSC Adv. 2022, 12, 31564–31576.
- Karayannidis, G.P.; Achilias, D.S. Chemical recycling of poly(ethylene terephthalate). Macromol. Mater. Eng. 2007, 292, 128–146.
- Stanica-Ezeanu, D.; Matei, D. Natural depolymerization of waste poly(ethylene terephthalate) by neutral hydrolysis in marine water. Sci. Rep. 2021, 11, 4431.
- Valh, J.V.; Voncina, B.; Lobnik, A.; Zemljic, L.F.; Skodic, L.; Vajnhandl, S. Conversion of polyethylene terephthalate to high-quality terephthalic acid by hydrothermal hydrolysis: The study of process parameters. Textile Res. J. 2020, 90, 1446–1461.
- Colnik, M.; Knez, Z.; Skerget, M. Sub- and supercritical water for chemical recycling of polyethylene terephthalate waste. Chem. Eng. Sci. 2021, 233, 116389.
- Kazutoshi, I.; Takahiro, I.; Katsuki, K. Hydrolysis of PET by Combining Direct Microwave Heating with High Pressure. Procedia Eng. 2016, 148, 314–318.
- Siddiqui, M.N.; Achilias, D.S.; Redhwi, H.H.; Bikiaris, D.N.; Katsogiannis, K.A.G.; Karayannidis, G.P. Hydrolytic depolymerization of PET in a microwave reactor. Macromol. Mater. Eng. 2010, 295, 575–584.
- Singh, S.; Sharma, S.; Umar, A.; Kumar Mehta, S.; Bhatti, M.S.; Kansal, S.K. Recycling of waste poly(ethylene terephthalate) bottles by alkaline hydrolysis and recovery of pure nanospindle-shaped terephthalic acid. J. Nano. Nanotechnol. 2018, 18, 5804–5809.
- Štrukil, V. Highly Efficient Solid-State Hydrolysis of Waste Polyethylene Terephthalate by Mechanochemical Milling and Vapor-Assisted Aging. ChemSusChem 2021, 14, 330–338.
- Bhogle, C.S.; Pandit, A.B. Ultrasound-Assisted Alkaline Hydrolysis of Waste Poly(Ethylene Terephthalate) in Aqueous and Nonaqueous Media at Low Temperature. Indian Chem. Eng. 2018, 60, 122–140.
- Zope, V. Hydrolysis of poly-ethylene terephthalate waste using high pressure autoclave: A chemical recycling. Int. J. Basic Appl. Chem. Sci. 2022, 12, 1–5.
- Ugduler, S.; Van Geem, K.M.; Denolf, R.; Roosen, M.; Mys, N.; Ragaert, K.; De Meester, S. Closed-loop recycling of multilayer and colored PET plastic waste by alkaline hydrolysis. Green Chem. 2020, 22, 5376–5394.
- Chen, W.; Yang, Y.; Lan, X.; Zhang, B.; Zhang, X.; Mu, T. Biomass-derived γ-valerolactone: Efficient dissolution and accelerated alkaline hydrolysis of polyethylene terephthalate. Green Chem. 2021, 23, 4065–4073.
- Khalaf, H.I.; Hasan, O.A. Effect of quaternary ammonium salt as a phase transfer catalyst for the microwave depolymerization of polyethylene terephthalate waste bottles. Chem. Eng. J. 2012, 192, 45–48.
- Wang, Y.; Wang, H.; Chen, H.; Liu, H. Towards recycling purpose: Converting PET plastic waste back to terephthalic acid using pH responsive phase transfer catalyst. Chin. J. Chem. Eng. 2022, 51, 53–60.
- Zhang, L.; Gao, J.; Zou, J.; Yi, F. Hydrolysis of poly(ethylene terephthalate) waste bottles in the presence of dual functional phase transfer catalysts. J. Appl. Polym. Sci. 2013, 130, 2790–2795.
- Yang, W.; Wang, J.; Jiao, L.; Song, Y.; Li, C.; Hu, C. Easily recoverable and reusable p-toluenesulfonic acid for faster hydrolysis of waste polyethylene terephthalate. Green Chem. 2022, 24, 1362–1372.
- Li, X.; Lu, H.; Guo, W.; Cao, G.; Liu, H.; Shi, Y. Reaction Kinetics and Mechanism of Catalyzed Hydrolysis of Waste PET Using Solid Acid Catalyst in Supercritical CO2. AIChE J. 2015, 61, 200–214.
- Guo, W.; Lu, H.; Li, X.; Cao, G. Tungsten-promoted titania as solid acid for catalytic hydrolysis of waste bottle PET in supercritical CO2. RSC Adv. 2016, 6, 43171–43184.
- Yang, W.; Liu, C.R.; Chang, L.; Song, Y.; Hu, C. Hydrolysis of waste polyethylene terephthalate catalyzed by easily recyclable terephthalic acid. Waste Manag. 2021, 135, 267–274.
- Scremin, D.M.; Miyazaki, D.Y.; Lunelli, C.E.; Silva, S.A.; Zawadzki, S.F. PET recycling by Alcoholysis using a new heterogeneous catalyst: Study and its use in polyurethane adhesives preparation. Macromol. Symp. 2019, 383, 1800027.
- Shojaei, B.; Abtahi, M.; Najafi, M. Chemical recycling of PET: A stepping-stone toward sustainability. Polym. Adv. Technol. 2020, 31, 2912–2938.
- Brivio, L.; Tollini, F. Chapter Six—PET recycling: Review of the current available technologies and industrial perspectives. Adv. Chem. Eng. 2022, 60, 215–267.
- Kim, B.K.; Hwang, G.C.; Bae, S.Y.; Yi, S.C.; Kumazawa, H. Depolymerization of polyethyleneterephthalate in supercritical methanol. J. Appl. Polym. Sci. 2001, 81, 2102–2108.
- Hofmann, M.; Sundermeier, J.; Alberti, C.; Enthaler, S. Zinc(II) acetate Catalyzed Depolymerization of Poly(ethylene terephthalate). ChemistrySelect 2020, 5, 10010–11001.
- Liu, Y.; Wang, M.; Pan, Z. Catalytic depolymerization of polyethylene terephthalate in hot compressed water. J. Supercrit. Fluids 2012, 62, 226–231.
- Mishra, S.; Goje, A.S. Kinetic and thermodynamic study of methanolysis of poly(ethylene terephthalate)waste powder. Polym. Int. 2003, 52, 337–342.
- Sanchez, A.; Collinson, S. The selective recycling of mixed plastic waste of polylactic acid and polyethylene terephthalate by control of process conditions. Eur. Polym. J. 2011, 47, 1970–1976.
- Siddiqui, M.N.; Redhwi, H.H.; Achilias, D.S. Recycling of poly(ethylene terephthalate) waste through methanolic pyrolysis in a microwave reactor. J. Anal. Appl. Pyrolysis 2012, 98, 214–220.
- Pham, D.D.; Cho, J. Low-energy catalytic methanolysis of poly(ethyleneterephthalate). Green Chem. 2021, 23, 511–525.
- Tang, S.; Li, F.; Liu, J.; Guo, B.; Tian, Z.; Lv, J. Calcined sodium silicate as solid base catalyst for alcoholysis of poly(ethylene terephthalate). J. Chem. Technol. Biotechnol. 2022, 97, 1305–1314.
- Gangotena, P.; Ponce, S.; Gallo-Córdova, A.; Streitwieser, D.; Mora, J. Highly Active MgP Catalyst for Biodiesel Production and Polyethylene Terephthalate Depolymerization. ChemistrySelect 2022, 7, e202103765.
- Laldinpuii, Z.T.; Khiangte, V.; Lalhmangaihzuala, S.; Lalmuanpuia, C.; Pachuau, Z.; Lalhriatpuia, C.; Vanlaldinpuiac, K. Methanolysis of PET Waste Using Heterogeneous Catalyst of Bio-waste Origin. J. Polym. Environ. 2022, 30, 1600–1614.
- Du, J.T.; Sun, Q.; Zeng, X.F.; Wang, D.; Wang, J.X.; Chen, J.F. ZnO nanodispersion as pseudohomogeneous catalyst for alcoholysis of polyethylene terephthalate. Chem. Eng. Sci. 2020, 220, 115642.
- Sharma, V.; Parashar, P.; Srivastava, P.; Kumar, S.; Agarwal, D.; Richharia, N. Recycling of waste PET-bottles using dimethyl sulfoxide and hydrotalcite catalyst. J. Appl. Polym. Sci. 2013, 129, 1513–1519.
- Liu, M.; Guo, J.; Gu, Y.; Gao, J.; Liu, F. Versatile Imidazole-Anion-Derived Ionic Liquids with Unparalleled Activity for Alcoholysis of Polyester Wastes under Mild and Green Conditions. ACS Sustain. Chem. Eng. 2018, 6, 15127–15134.
- Paszun, D.; Spychaj, T. Chemical Recycling of Poly(Ethylene Terephthalate). Ind. Eng. Chem. Res. 1997, 36, 1373–1383.
- Ostrysz, R.; Kłosowska, Z.; Jankowska, F. Method of preparation of thixotropic and chemoresistant unsaturated polyester resins. G.B. Patent 1158561, 16 July 1969.
- Xin, J.; Zhang, Q.; Huang, J.; Huang, R.; Jaffery, Q.Z.; Yan, D.; Zhou, Q.; Xu, J.; Lu, X. Progress in the Catalytic Glycolysis of Polyethylene Terephthalate. J. Environ. Manag. 2021, 296, 113267.
- Sinha, V.; Patel, M.R.; Patel, J.V. Pet Waste Management by Chemical Recycling: A Review. J Polym Environ. 2010, 18, 8–25.
- Chen, C.-H.; Chen, C.-Y.; Lo, Y.-W.; Mao, C.-F.; Liao, W.-T. Studies of Glycolysis of Poly(Ethylene Terephthalate) Recycled from Postconsumer Soft-Drink Bottles. I. Influences of Glycolysis Conditions. J. Appl. Polym. Sci. 2001, 80, 943–948.
- Chen, C.-H.; Chen, C.-Y.; Lo, Y.-W.; Mao, C.-F.; Liao, W.-T. Studies of Glycolysis of Poly(Ethylene Terephthalate) Recycled from Postconsumer Soft-Drink Bottles. II. Factorial Experimental Design. J. Appl. Polym. Sci. 2001, 80, 956–962.
- Ghosal, K.; Nayak, C. Recent Advances in Chemical Recycling of Polyethylene Terephthalate Waste into Value Added Products for Sustainable Coating Solutions—Hope vs. Hype. Mater. Adv. 2022, 3, 1974–1992.
- López-Fonseca, R.; Duque-Ingunza, I.; de Rivas, B.; Arnaiz, S.; Gutiérrez-Ortiz, J.I. Chemical Recycling of Post-Consumer PET Wastes by Glycolysis in the Presence of Metal Salts. Polym. Degrad. Stab. 2010, 95, 1022–1028.
- Nikles, D.E.; Farahat, M.S. New Motivation for the Depolymerization Products Derived from Poly(Ethylene Terephthalate) (PET) Waste: A Review. Macromol. Mater. Eng. 2005, 290, 13–30.
- De Castro, R.E.N.; Vidotti, G.J.; Rubira, A.F.; Muniz, E.C. Depolymerization of Poly(ethylene terephthalate) Wastes Using Ethanol and Ethanol/Water in Supercritical Conditions. J. Appl. Polym. Sci. 2006, 101, 2009–2016.
- Sanda, O.; Taiwo, E.A.; Osinkolu, G.A. Alkaline Solvolysis of Poly(Ethylene Terephthalate) in Butan–1–ol Media: Kinetics and Optimization Studies. British. J. Appl. Sci. Technol. 2017, 21, 1–12.
- Wang, X.; An, W.; Du, R.; Tian, F.; Yang, Y.; Zhao, X.; Xu, S.; Wang, Y. Rapid hydrolysis of PET in high-concentration alcohol aqueous solution by pore formation and spontaneous separation of terephthalate. J. Environ. Chem. Eng. 2023, 11, 109434.
- Fregoso-Infante, A.; Vega-Rangel, R.; Figueroa, G. Chemical Method for Recycling Polyethylene Terephtalate (pet) Wastes. SP Patent WO2005082826, 9 September 2005.
- Tang, X.H.; Li, N.; Li, G.; Wang, A.; Cong, Y.; Xu, G.; Wang, X.; Zhang, T. Synthesis of gasoline and jet fuel range cycloalkanes and aromatics with poly(ethyleneterephthalate) wastes. Green Chem. 2019, 21, 2709–2719.
- Anderson, R.L.; Sikkenga, D.L. Ethanolysis of PET to form DET and Oxidation DET to form Carboxylic Acids. FR Patent WO2007076384, 5 July 2007.
- Azimova, M.; Nubel, P.; Bergeron, R. Improved Catalyst Performance for Polyester Recycling. Patent WO2021257920, 23 December 2021.
- Shiwei, L.; Shitao, Y.; Congxia, X.; Fusheng, L.; Lu, L.; Minfang, Z. Method for Preparing Plasticizer Terephthalate by Alcoholysis of Waste Polyester PET. CN Patent CN102603532, 29 October 2014.
- Hutchenson, K.W. Supercritical Fluid Technology in Material Science and Engineering. Sung, Y.P., Ed.; Marcel Dekker: New York, NY, USA, 2002; p. 87.
- Yang, Y.; Lu, Y.; Xiang, H.; Xu, Y.; Li, Y. Study on methanolytic depolymerization of PET with supercritical methanol for chemical recycling. Polym. Degrad. Stab. 2002, 75, 185–191.
- Fávaro, S.L.; Freitas, A.R.; Ganzerli, T.A.; Pereira, A.G.B.; Cardozo, A.L.; Baron, O.; Muniz, E.C.; Girotto, E.M.; Radovanovic, E. PET and Aluminum Recycling from Multilayer Food Packaging Using Supercritical Ethanol. J. Supercrit. Fluids 2013, 75, 138–143.
- Fernandes, J.R.; Amaro, L.P.; Muniz, E.C.; Favaro, S.L.; Radovanovic, E. PET Depolimerization in Supercritical Ethanol Conditions Catalysed by Nanoparticles of Metal Oxides. J. Supercrit. Fluids 2020, 158, e104715.
- Yang, Y.; Chen, F.; Shen, T.; Pariatamby, A.; Wen, X.; Yan, M.; Kanchanatip, E. Catalytic depolymerization of waste polyethylene terephthalate plastic in supercritical ethanol by ZnO/γ-Al2O3 catalyst. Process Saf. Environ. Protect. 2023, 173, 881–892.
- Nunes, C.; Vieira da Silva, M.J.; Cristina da Silva, D.; dos Reis Freitas, A.; Rosa, F.A.; Rubira, A.F.; Muniz, E.C. PET Depolymerisation in Supercritical Ethanol Catalysed by . RSC Adv. 2014, 4, 20308–20316.
- Nunes, C.; Souza, P.; Freitas, A.; Silva, M.; Rosa, F.; Muniz, E. Poisoning Effects of Water and Dyes on the Catalysis of Poly(Ethylene Terephthalate) (PET) Depolymerization under Supercritical Ethanol. Catalysts 2017, 7, e43.
- Lozano-Martinez, P.; Torres-Zapata, T.; Martin-Sanchez, N. Directing Depolymerization of PET with Subcritical and Supercritical Ethanol to Different Monomers through Changes in Operation Conditions. ACS Sustain. Chem. Eng. 2021, 9, 9846–9853.
- Yan, M.; Yang, Y.; Shen, T.; Grisdanurak, N.; Pariatamby, A.; Khalid, M.; Hantoko, D.; Wibowo, H. Effect of operating parameters on monomer production from depolymerization of waste polyethylene terephthalate in supercritical ethanol. Process Saf. Environ. Protect. 2023, 169, 212–219.
- Hillert, G.; Schäfer, M. Dispersion adhesive and use of a dispersion adhesive. Patent Germany DE102009020497A1, 3 April 2010.
- Castle, L.; Mayo, A.; Gilbert, J. Migration of plasticizers from printing inks into foods. Food Addit. Contam. 1989, 6, 437–444.
- Medimagh, R. Improved Method for Recycling Pet by Alcoholysis. WO2022112715, 2 June 2022.
- Ye, D. Method for Preparing Polyvinyl Chloride (PVC) Plasticizer through Ester Exchange Reaction of Waste Polyethylene Terephthalate (PET) and Monohydric Alcohol. CN Patent CN102234227, 30 April 2010.
- Hu, H.; Wang, L. Method for Preparing Dimethyl Terephthalate from Polybutylene Terephthalate Waste Material. CN Patent CN105503605A, 14 December 2015.
- Fang, C.; Zhou, X.; Yang, R.; Pan, S.; Lei, W. Carbon Nanotube Prepared from Waste PET, Preparation Method and Application in Conductive Material, Super Capacitor and Catalyst Support. CN Patent CN106986326, 8 May 2017.
- Dupont, L.A.; Gupta, V.P. Degradative transesterification of terephthalate polyesters to obtain DOTP plasticizer for flexible PVC. J. Vinyl Addit. Technol. 1993, 15, 100–104.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.9K
Revisions:
2 times
(View History)
Update Date:
12 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




