
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohammad Ashfaq | -- | 2457 | 2023-10-10 12:26:45 | | | |
| 2 | Lindsay Dong | Meta information modification | 2457 | 2023-10-16 03:31:15 | | |
Video Upload Options
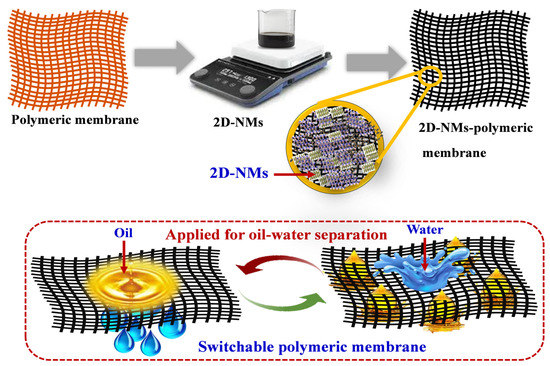
Oil leakage and organic solvent industrial accidents harm the ecosystem, especially aquatic and marine life. Oil–water separation is required to combat this issue, which substantially enhances the ecosystem and recovery of oils from water bodies. The advent of two-dimensional nanomaterials (2D-NMs) gives newer insight in developing membranes due to their exceptional characteristics like hydrophobicity/hydrophilicity, selectivity, antifouling ability, flexibility, and stability. Incorporating 2D-NMs within the polymeric membranes makes them exceptional candidates for removing oil from water. Moreover, 2D-NMs offer rapid sorption/desorption rates and boost water transportation. Additionally, 2D-NMs provide roughness that significantly enhances the fouling resistance in the polymeric membrane.
1. Introduction
2. Improvement of Polymeric Membranes Properties by Incorporating 2D-NMs
3. 2D-NM-Incorporated Polymeric Membranes for Oil–Water Separation
3.1. Graphene and Its Derivative
3.2. Molybdenum Disulfide (MoS2)
3.3. Boron Nitride (BN)
3.4. Transitional Metal Carbide/Nitride/Carbonitrides (MXene)
3.5. Tungsten Disulfide (WS2)
4. Recyclability of the 2D-NM-Incorporated Polymeric Membranes
5. Strategies to Improve Oil–Water Separation Efficiency
References
- Huang, S.; Ras, R.H.A.; Tian, X. Antifouling membranes for oily wastewater treatment: Interplay between wetting and membrane fouling. Curr. Opin. Colloid Interface Sci. 2018, 36, 90–109.
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595.
- Sathya, K.; Nagarajan, K.; Carlin Geor Malar, G.; Rajalakshmi, S.; Raja Lakshmi, P. A comprehensive review on comparison among effluent treatment methods and modern methods of treatment of industrial wastewater effluent from different sources. Appl. Water Sci. 2022, 12, 70.
- Zulkefli, N.F.; Alias, N.H.; Jamaluddin, N.S.; Abdullah, N.; Abdul Manaf, S.F.; Othman, N.H.; Marpani, F.; Mat-Shayuti, M.S.; Kusworo, T.D. Recent Mitigation Strategies on Membrane Fouling for Oily Wastewater Treatment. Membranes 2021, 12, 26.
- Cordes, E.E.; Jones, D.O.B.; Schlacher, T.A.; Amon, D.J.; Bernardino, A.F.; Brooke, S.; Carney, R.; DeLeo, D.M.; Dunlop, K.M.; Escobar-Briones, E.G.; et al. Environmental Impacts of the Deep-Water Oil and Gas Industry: A Review to Guide Management Strategies. Front. Environ. Sci. 2016, 4, 58.
- Singh, H.; Bhardwaj, N.; Arya, S.K.; Khatri, M. Environmental impacts of oil spills and their remediation by magnetic nanomaterials. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100305.
- Asif, Z.; Chen, Z.; An, C.; Dong, J. Environmental Impacts and Challenges Associated with Oil Spills on Shorelines. J. Mar. Sci. Eng. 2022, 10, 762.
- Gandharapu, P.; Shende, R.C.; Jangid, M.K.; Mukhopadhyay, A. Stable and fast oil recovery by environmentally friendly and easy to synthesize pristine graphitic carbon nitride. Clean Technol. Environ. Policy 2023, 1–7.
- Bhushan, B. Bioinspired oil–water separation approaches for oil spill clean-up and water purification. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2019, 377, 20190120.
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/water separation techniques: A review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058.
- Varjani, S.; Joshi, R.; Srivastava, V.K.; Ngo, H.H.; Guo, W. Treatment of wastewater from petroleum industry: Current practices and perspectives. Environ. Sci. Pollut. Res. Int. 2020, 27, 27172–27180.
- Wen, C.; Wang, H.; Wang, L.; Lou, Z.; Sun, Z.; Zhou, Z. The reduction of waste lubricant oil distillate through the enhancement of organics degradation by ozonation with elevated temperature and stable pH for the zero discharge. J. Clean. Prod. 2019, 240, 118194.
- Kung, C.H.; Zahiri, B.; Sow, P.K.; Mérida, W. On-demand oil-water separation via low-voltage wettability switching of core-shell structures on copper substrates. Appl. Surf. Sci. 2018, 444, 15–27.
- Yan, S.; Li, Y.; Xie, F.; Wu, J.; Jia, X.; Yang, J.; Song, H.; Zhang, Z. Environmentally Safe and Porous MS@TiO2@PPy Monoliths with Superior Visible-Light Photocatalytic Properties for Rapid Oil–Water Separation and Water Purification. ACS Sustain. Chem. Eng. 2020, 8, 5347–5359.
- Xu, H.; Jia, W.; Ren, S.; Wang, J. Magnetically responsive multi-wall carbon nanotubes as recyclable demulsifier for oil removal from crude oil-in-water emulsion with different pH levels. Carbon 2019, 145, 229–239.
- Hu, G.; Li, J.; Zeng, G. Recent development in the treatment of oily sludge from petroleum industry: A review. J. Hazard. Mater. 2013, 261, 470–490.
- Kriipsalu, M.; Marques, M.; Nammari, D.R.; Hogland, W. Bio-treatment of oily sludge: The contribution of amendment material to the content of target contaminants, and the biodegradation dynamics. J. Hazard. Mater. 2007, 148, 616–622.
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33.
- Xiang, B.; Sun, Q.; Zhong, Q.; Mu, P.; Li, J. Current research situation and future prospect of superwetting smart oil/water separation materials. J. Mater. Chem. A 2022, 10, 20190–20217.
- Sutrisna, P.D.; Kurnia, K.A.; Siagian, U.W.R.; Ismadji, S.; Wenten, I.G. Membrane fouling and fouling mitigation in oil–water separation: A review. J. Environ. Chem. Eng. 2022, 10, 107532.
- Al-anzi, B.S.; Siang, O.C. Recent developments of carbon based nanomaterials and membranes for oily wastewater treatment. RSC Adv. 2017, 7, 20981–20994.
- Ismail, N.H.; Salleh, W.N.W.; Ismail, A.F.; Hasbullah, H.; Yusof, N.; Aziz, F.; Jaafar, J. Hydrophilic polymer-based membrane for oily wastewater treatment: A review. Sep. Purif. Technol. 2020, 233, 116007.
- Sengupta, P.; Prasad, B.L.V. Surface Modification of Polymers for Tissue Engineering Applications: Arginine Acts as a Sticky Protein Equivalent for Viable Cell Accommodation. ACS Omega 2018, 3, 4242–4251.
- Tanasă, F.; Zănoagă, M.; Teacă, C.-A.; Nechifor, M.; Shahzad, A. Modified hemp fibers intended for fiber-reinforced polymer composites used in structural applications—A review. I. Methods of modification. Polym. Compos. 2020, 41, 5–31.
- Drobota, M.; Ursache, S.; Aflori, M. Surface Functionalities of Polymers for Biomaterial Applications. Polymers 2022, 14, 2307.
- Chauhan, D.; Singh, N.; Afreen, S.; Talreja, N.; Ashfaq, M.; Sankararamakrishnan, N.; Chaudhary, G.R. A thermoresponsive CA-PNIPAM-based electrospun nanofibrous membrane for oil/water separation. New J. Chem. 2022, 46, 18984–18989.
- Tsai, H.-S.; Wang, Y.; Liu, C.; Wang, T.; Huo, M. The elemental 2D materials beyond graphene potentially used as hazardous gas sensors for environmental protection. J. Hazard. Mater. 2022, 423, 127148.
- Talreja, N.; Chauhan, D.; Ashfaq, M. Photo-Antibacterial Activity of Two-Dimensional (2D)-Based Hybrid Materials: Effective Treatment Strategy for Controlling Bacterial Infection. Antibiotics 2023, 12, 398.
- Talreja, N.; Ashfaq, M.; Chauhan, D.; Mangalaraja, R.V. Cu-MXene: A potential biocide for the next-generation biomedical application. Mater. Chem. Phys. 2023, 294, 127029.
- Ashfaq, M.; Talreja, N.; Singh, N.; Chauhan, D. 2D-Nanolayer (2D-NL)-Based Hybrid Materials: A Next-Generation Material for Dye-Sensitized Solar Cells. Electronics 2023, 12, 570.
- Chauhan, D.; Ashfaq, M.; Mangalaraja, R.V.; Talreja, N. 2D-Nanosheets Based Hybrid Nanomaterials Interaction with Plants. In Nanomaterial Interactions with Plant Cellular Mechanisms and Macromolecules and Agricultural Implications; Al-Khayri, J.M., Alnaddaf, L.M., Jain, S.M., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 299–316.
- Ashfaq, M.; Talreja, N.; Chauhan, D.; Afreen, S.; Sultana, A.; Srituravanich, W. Two-dimensional (2D) hybrid nanomaterials for diagnosis and treatment of cancer. J. Drug Deliv. Sci. Technol. 2022, 70, 103268.
- Ashfaq, M.; Talreja, N.; Chauhan, D.; Viswanathan, M.R. Synthesis of Cu-doped 2D-WS2 nanosheet-based nano-antibiotic materials for inhibiting E. Coli and S. aureus bacterial strains. New J. Chem. 2022, 46, 5581–5587.
- Saini, H.; Kallem, P.; Otyepková, E.; Geyer, F.; Schneemann, A.; Ranc, V.; Banat, F.; Zbořil, R.; Otyepka, M.; Fischer, R.A.; et al. Two-dimensional MOF-based liquid marbles: Surface energy calculations and efficient oil–water separation using a ZIF-9-III@PVDF membrane. J. Mater. Chem. A 2021, 9, 23651–23659.
- Li, J.; Li, Y.; Lu, Y.; Wang, Y.; Guo, Y.; Shi, W. Preparation of 2D Materials and Their Application in Oil-Water Separation. Biomimetics 2023, 8, 35.
- Rasul, M.G.; Kiziltas, A.; Arfaei, B.; Shahbazian-Yassar, R. 2D boron nitride nanosheets for polymer composite materials. NPJ 2D Mater. Appl. 2021, 5, 56.
- Zeng, G.; Wei, K.; Zhang, H.; Zhang, J.; Lin, Q.; Cheng, X.; Sengupta, A.; Chiao, Y.-H. Ultra-high oil-water separation membrane based on two-dimensional MXene(Ti3C2Tx) by co-incorporation of halloysite nanotubes and polydopamine. Appl. Clay Sci. 2021, 211, 106177.
- Luo, X.; He, Z.; Gong, H.; He, L. Recent advances in oil-water separation materials with special wettability modified by graphene and its derivatives: A review. Chem. Eng. Process. Process Intensif. 2022, 170, 108678.
- Zhang, L.; Liu, Y.; Zeng, G.; Yang, Z.; Lin, Q.; Wang, Y.; Wang, X.; Pu, S. Two-dimensional Na-Bentonite@MXene composite membrane with switchable wettability for selective oil/water separation. Sep. Purif. Technol. 2023, 306, 122677.
- Yang, Y.; Guo, Z.; Liu, W. Special Superwetting Materials from Bioinspired to Intelligent Surface for On-Demand Oil/Water Separation: A Comprehensive Review. Small 2022, 18, 2204624.
- Dansawad, P.; Yang, Y.; Li, X.; Shang, X.; Li, Y.; Guo, Z.; Qing, Y.; Zhao, S.; You, S.; Li, W. Smart membranes for oil/water emulsions separation: A review. Adv. Membr. 2022, 2, 100039.
- Hou, X.; Zhang, R.; Fang, D. Flexible and robust polyimide membranes with adjustable surface structure and hierarchical pore distribution for oil/water emulsion and heavy oil separation. J. Membr. Sci. 2021, 640, 119769.
- Shen, X.; Zheng, Q.; Kim, J.-K. Rational design of two-dimensional nanofillers for polymer nanocomposites toward multifunctional applications. Prog. Mater. Sci. 2021, 115, 100708.
- Cao, J.; Li, J.; Majdi, H.S.; Le, B.N.; Amine Khadimallah, M.; Elhosiny Ali, H.; Assilzadeh, H. Assessment of graphene-based polymers for sustainable wastewater treatment: Development of a soft computing approach. Chemosphere 2023, 313, 137189.
- Liang, J.; Huang, Y.; Zhang, L.; Wang, Y.; Ma, Y.; Guo, T.; Chen, Y. Molecular-Level Dispersion of Graphene into Poly(vinyl alcohol) and Effective Reinforcement of their Nanocomposites. Adv. Funct. Mater. 2009, 19, 2297–2302.
- Park, J.; Bazylewski, P.; Fanchini, G. Porous graphene-based membranes for water purification from metal ions at low differential pressures. Nanoscale 2016, 8, 9563–9571.
- Han, Z.-y.; Huang, L.-j.; Qu, H.-j.; Wang, Y.-x.; Zhang, Z.-j.; Rong, Q.-l.; Sang, Z.-q.; Wang, Y.; Kipper, M.J.; Tang, J.-g. A review of performance improvement strategies for graphene oxide-based and graphene-based membranes in water treatment. J. Mater. Sci. 2021, 56, 9545–9574.
- Afreen, S.; Omar, R.A.; Talreja, N.; Chauhan, D.; Ashfaq, M. Carbon-Based Nanostructured Materials for Energy and Environmental Remediation Applications. In Approaches in Bioremediation: The New Era of Environmental Microbiology and Nanobiotechnology; Prasad, R., Aranda, E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 369–392.
- Sasidharan, V.; Sachan, D.; Chauhan, D.; Talreja, N.; Ashfaq, M. Three-dimensional (3D) polymer—Metal–carbon framework for efficient removal of chemical and biological contaminants. Sci. Rep. 2021, 11, 7708.
- Liu, G.; Jin, W.; Xu, N. Graphene-based membranes. Chem. Soc. Rev. 2015, 44, 5016–5030.
- Prince, J.A.; Bhuvana, S.; Anbharasi, V.; Ayyanar, N.; Boodhoo, K.V.K.; Singh, G. Ultra-wetting graphene-based PES ultrafiltration membrane—A novel approach for successful oil-water separation. Water Res. 2016, 103, 311–318.
- Liu, Y.; Zhao, Y.; Zhang, X.; Huang, X.; Liao, W.; Zhao, Y. MoS2-based membranes in water treatment and purification. Chem. Eng. J. 2021, 422, 130082.
- Krasian, T.; Punyodom, W.; Worajittiphon, P. A hybrid of 2D materials (MoS2 and WS2) as an effective performance enhancer for poly(lactic acid) fibrous mats in oil adsorption and oil/water separation. Chem. Eng. J. 2019, 369, 563–575.
- Joy, J.; George, E.; Haritha, P.; Thomas, S.; Anas, S. An overview of boron nitride based polymer nanocomposites. J. Polym. Sci. 2020, 58, 3115–3141.
- Minju, N.; Ananthakumar, S.; Savithri, S. Superswelling Hybrid Sponge from Water Glass for Selective Absorption of Crude Oil and Organic Solvents. ACS Omega 2019, 4, 17990–18001.
- Ensoylu, M.; Deliormanlı, A.M.; Atmaca, H. Hexagonal Boron Nitride/PCL/PLG Coatings on Borate Bioactive Glass Scaffolds for Bone Regeneration. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1551–1566.
- García Doménech, N.; Purcell-Milton, F.; Sanz Arjona, A.; Casasín García, M.-L.; Ward, M.; Cabré, M.B.; Rafferty, A.; McKelvey, K.; Dunne, P.; Gun’ko, Y.K. High-Performance Boron Nitride-Based Membranes for Water Purification. Nanomaterials 2022, 12, 473.
- Nam, S.-N.; Park, C.M.; Jang, M.; Huang, Y.; Jang, A.; Son, A.; Yoon, Y. Review of boron nitride-based membranes in liquid purification/separation applications. Chem. Eng. J. 2023, 453, 139740.
- Doménech, N.G.; Coogan, Á.; Purcell-Milton, F.; Casasín García, M.L.; Arjona, A.S.; Cabré, M.B.; Rafferty, A.; McKelvey, K.; Dunne, P.; Gun’ko, Y.K. Partially oxidised boron nitride as a 2D nanomaterial for nanofiltration applications. Nanoscale Adv. 2022, 4, 4895–4904.
- Lei, W.; Portehault, D.; Liu, D.; Qin, S.; Chen, Y. Porous boron nitride nanosheets for effective water cleaning. Nat. Commun. 2013, 4, 1777.
- Ren, C.E.; Hatzell, K.B.; Alhabeb, M.; Ling, Z.; Mahmoud, K.A.; Gogotsi, Y. Charge- and Size-Selective Ion Sieving Through Ti3C2Tx MXene Membranes. J. Phys. Chem. Lett. 2015, 6, 4026–4031.
- Ding, L.; Li, L.; Liu, Y.; Wu, Y.; Lu, Z.; Deng, J.; Wei, Y.; Caro, J.; Wang, H. Effective ion sieving with Ti3C2Tx MXene membranes for production of drinking water from seawater. Nat. Sustain. 2020, 3, 296–302.
- Ihsanullah, I.; Bilal, M. Recent advances in the development of MXene-based membranes for oil/water separation: A critical review. Appl. Mater. Today 2022, 29, 101674.
- Sun, Y.; Fujisawa, K.; Lin, Z.; Lei, Y.; Mondschein, J.S.; Terrones, M.; Schaak, R.E. Low-Temperature Solution Synthesis of Transition Metal Dichalcogenide Alloys with Tunable Optical Properties. J. Am. Chem. Soc. 2017, 139, 11096–11105.
- Mohl, M.; Rautio, A.-R.; Asres, G.A.; Wasala, M.; Patil, P.D.; Talapatra, S.; Kordas, K. 2D Tungsten Chalcogenides: Synthesis, Properties and Applications. Adv. Mater. Interfaces 2020, 7, 2000002.
- Rong, J.; Qiu, F.; Zhang, T.; Zhang, X.; Zhu, Y.; Xu, J.; Yang, D.; Dai, Y. A facile strategy toward 3D hydrophobic composite resin network decorated with biological ellipsoidal structure rapeseed flower carbon for enhanced oils and organic solvents selective absorption. Chem. Eng. J. 2017, 322, 397–407.
- Dmitrieva, E.S.; Anokhina, T.S.; Novitsky, E.G.; Volkov, V.V.; Borisov, I.L.; Volkov, A.V. Polymeric Membranes for Oil-Water Separation: A Review. Polymers 2022, 14, 980.
- Hussain, A.; Al-Yaari, M. Development of Polymeric Membranes for Oil/Water Separation. Membranes 2021, 11, 42.
- Bakly, S.; Ibrar, I.; Saleem, H.; Yadav, S.; Al-Juboori, R.; Naji, O.; Altaee, A.; Zaidi, S.J. Polymer-based nano-enhanced forward osmosis membranes. In Advancement in Polymer-Based Membranes for Water Remediation; Nayak, S.K., Dutta, K., Gohil, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 13; pp. 471–501.
- Shahzad, A.; Oh, J.M.; Azam, M.; Iqbal, J.; Hussain, S.; Miran, W.; Rasool, K. Advances in the Synthesis and Application of Anti-Fouling Membranes Using Two-Dimensional Nanomaterials. Membranes 2021, 11, 605.
- Nain, A.; Sangili, A.; Hu, S.-R.; Chen, C.-H.; Chen, Y.-L.; Chang, H.-T. Recent progress in nanomaterial-functionalized membranes for removal of pollutants. iScience 2022, 25, 104616.
- Jawad, J.; Zaidi, S.J. Fabrication of sustainable membranes with functionalized nanomaterials (FNMs). In Membranes with Functionalized Nanomaterials; Dutta, S., Hussain, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 4; pp. 129–158.




