
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | HUANG Wenwu | -- | 1830 | 2023-10-10 09:08:27 | | | |
| 2 | Fanny Huang | -1 word(s) | 1829 | 2023-10-13 07:34:35 | | |
Video Upload Options
Non-alcoholic fatty liver disease, commonly abbreviated to NAFLD, is a pervasive ailment within the digestive system, exhibiting a rising prevalence, and impacting individuals at increasingly younger ages. Studies have shown that synbiotics, which regulate intestinal microecology, can positively impact glucolipid metabolism, and improve NAFLD-related indicators. Sonchus brachyotus DC., a Chinese herb, exhibits hepatoprotective and potent antioxidant properties, suggesting its potential therapeutic use in NAFLD. Our preclinical animal model investigation suggests that the synergy between Sonchus brachyotus DC. extracts and synbiotics is significantly more effective in preventing and treating NAFLD, compared to the isolated use of either component. As a result, this combination holds the potential to introduce a fresh and encouraging therapeutic approach to addressing NAFLD.
1. Introduction
2. Nonalcoholic Fatty Liver Disease and Its Treatment
2.1. Nonalcoholic Fatty Liver Disease (NAFLD)
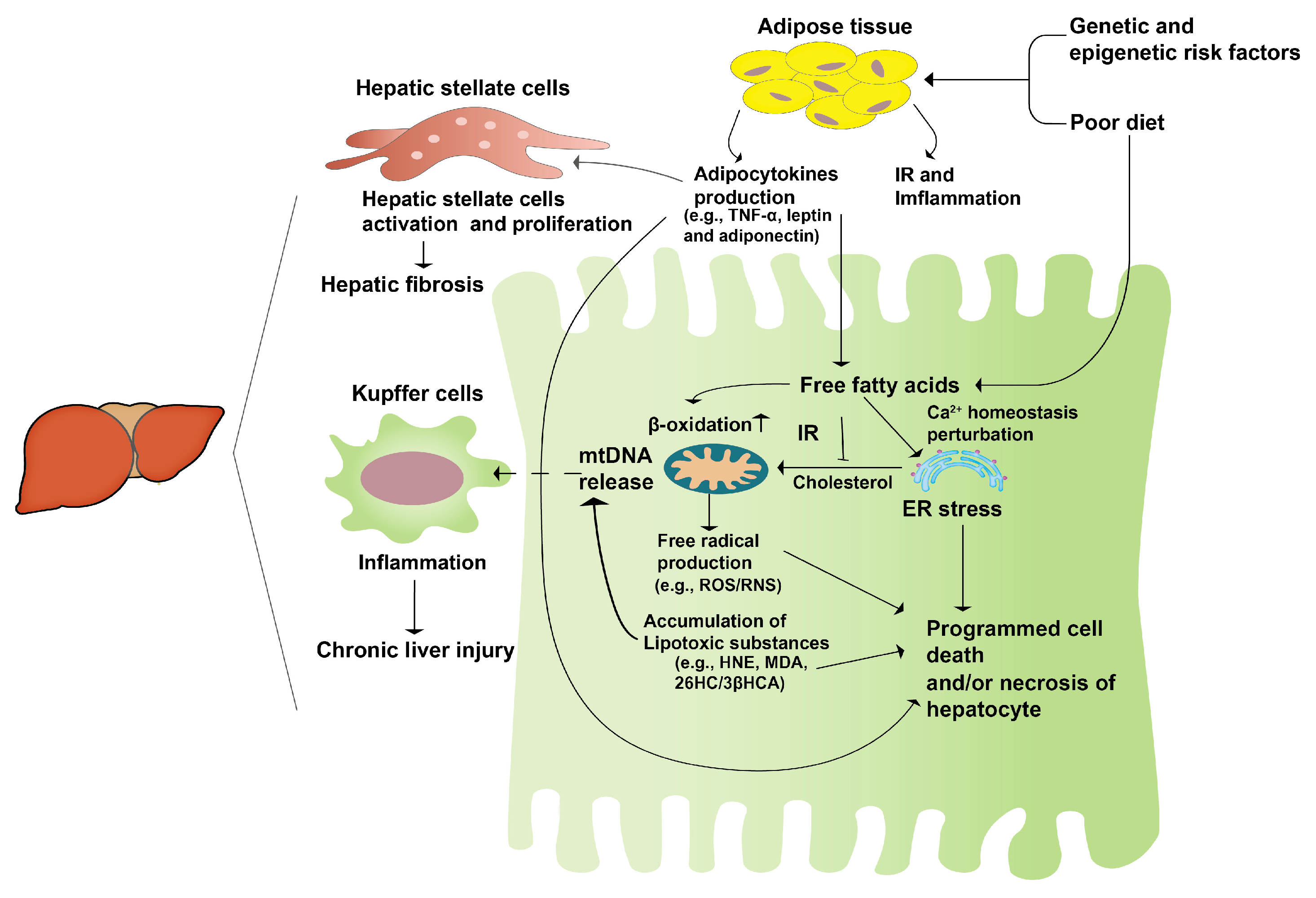
2.2. The Pathogenesis of NAFLD
2.3. The Treatment for NAFLD
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84.
- Fernando, D.H.; Forbes, J.M.; Angus, P.W.; Herath, C.B. Development and Progression of Non-Alcoholic Fatty Liver Disease: The Role of Advanced Glycation End Products. Int. J. Mol. Sci. 2019, 20, 5037.
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904.
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682.
- Wu, T.; Liao, X.; Zhong, B. Epidemiology of nonalcoholic fatty liver disease in some regions of China. J. Clin. Hepatol. 2020, 36, 1370–1373.
- Ganesh, S.; Rustgi, V.K. Current Pharmacologic Therapy for Nonalcoholic Fatty Liver Disease. Clin. Liver Dis. 2016, 20, 351–364.
- Ruan, Y.; Sun, J.; He, J.; Chen, F.; Chen, R.; Chen, H. Effect of Probiotics on Glycemic Control: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. PLoS ONE 2015, 10, e0132121.
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357.
- Marengo, A.; Rosso, C.; Bugianesi, E. Liver Cancer: Connections with Obesity, Fatty Liver, and Cirrhosis. Annu. Rev. Med. 2016, 67, 103–117.
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209.
- Eslam, M.; Sanyal, A.J.; George, J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1.
- Petersen, K.F.; Oral, E.A.; Dufour, S.; Befroy, D.; Ariyan, C.; Yu, C.; Cline, G.W.; DePaoli, A.M.; Taylor, S.I.; Gorden, P.; et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J. Clin. Investig. 2002, 109, 1345–1350.
- Irimia, J.M.; Meyer, C.M.; Segvich, D.M.; Surendran, S.; DePaoli-Roach, A.A.; Morral, N.; Roach, P.J. Lack of liver glycogen causes hepatic insulin resistance and steatosis in mice. J. Biol. Chem. 2017, 292, 10455–10464.
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845.
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141.
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metab. Clin. Exp. 2016, 65, 1038–1048.
- Almeda-Valdés, P.; Cuevas-Ramos, D.; Alberto Aguilar-Salinas, C. Metabolic syndrome and non-alcoholic fatty liver disease. Ann. Hepatol. 2009, 8, S18–S24.
- Rector, R.S.; Thyfault, J.P.; Wei, Y.; Ibdah, J.A. Non-alcoholic fatty liver disease and the metabolic syndrome: An update. World J. Gastroenterol. 2008, 14, 185–192.
- Capanni, M.; Calella, F.; Biagini, M.R.; Genise, S.; Raimondi, L.; Bedogni, G.; Svegliati-Baroni, G.; Sofi, F.; Milani, S.; Abbate, R.; et al. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: A pilot study. Aliment. Pharmacol. Ther. 2006, 23, 1143–1151.
- Masterton, G.S.; Plevris, J.N.; Hayes, P.C. Review article: Omega-3 fatty acids—A promising novel therapy for non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2010, 31, 679–692.
- Dasarathy, S.; Dasarathy, J.; Khiyami, A.; Yerian, L.; Hawkins, C.; Sargent, R.; McCullough, A.J. Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2015, 49, 137–144.
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 363, 1185–1186.
- Pearlman, M.; Loomba, R. State of the art: Treatment of nonalcoholic steatohepatitis. Curr. Opin. Gastroenterol. 2014, 30, 223–237.
- Hajiaghamohammadi, A.A.; Ziaee, A.; Oveisi, S.; Masroor, H. Effects of metformin, pioglitazone, and silymarin treatment on non-alcoholic Fatty liver disease: A randomized controlled pilot study. Hepat. Mon. 2012, 12, e6099.
- Bell, L.N.; Wang, J.; Muralidharan, S.; Chalasani, S.; Fullenkamp, A.M.; Wilson, L.A.; Sanyal, A.J.; Kowdley, K.V.; Neuschwander-Tetri, B.A.; Brunt, E.M.; et al. Relationship between adipose tissue insulin resistance and liver histology in nonalcoholic steatohepatitis: A pioglitazone versus vitamin E versus placebo for the treatment of nondiabetic patients with nonalcoholic steatohepatitis trial follow-up study. Hepatology 2012, 56, 1311–1318.
- Takahashi, Y.; Sugimoto, K.; Inui, H.; Fukusato, T. Current pharmacological therapies for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2015, 21, 3777–3785.
- Gastaldelli, A.; Harrison, S.A.; Belfort-Aguilar, R.; Hardies, L.J.; Balas, B.; Schenker, S.; Cusi, K. Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology 2009, 50, 1087–1093.
- Promrat, K.; Lutchman, G.; Uwaifo, G.I.; Freedman, R.J.; Soza, A.; Heller, T.; Doo, E.; Ghany, M.; Premkumar, A.; Park, Y.; et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology 2004, 39, 188–196.
- Cusi, K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: Pathophysiology and clinical implications. Gastroenterology 2012, 142, 711–725.e6.
- Lavine, J.E.; Schwimmer, J.B.; Van Natta, M.L.; Molleston, J.P.; Murray, K.F.; Rosenthal, P.; Abrams, S.H.; Scheimann, A.O.; Sanyal, A.J.; Chalasani, N.; et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: The TONIC randomized controlled trial. JAMA 2011, 305, 1659–1668.
- Sato, K.; Gosho, M.; Yamamoto, T.; Kobayashi, Y.; Ishii, N.; Ohashi, T.; Nakade, Y.; Ito, K.; Fukuzawa, Y.; Yoneda, M. Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: A meta-analysis of randomized controlled trials. Nutrition 2015, 31, 923–930.
- Yao, M.; Qv, L.; Lu, Y.; Wang, B.; Berglund, B.; Li, L. An Update on the Efficacy and Functionality of Probiotics for the Treatment of Non-Alcoholic Fatty Liver Disease. Engineering 2021, 7, 679–686.
- Li, B.; Chen, Y.; Pan, J.J.G.T.C.M. The hepatoprotective effect of semen cassiae extract by antagonizing insulin resistance and inhibiting oxidative-glycation in rats with nonalcoholic fatty liver disease. Glob. Tradit. Chin. Med. 2015, 8, 1171–1175.
- Meng, L.-C.; Zheng, J.-Y.; Qiu, Y.-H.; Zheng, L.; Zheng, J.-Y.; Liu, Y.-Q.; Miao, X.-L.; Lu, X.-Y. Salvianolic acid B ameliorates non-alcoholic fatty liver disease by inhibiting hepatic lipid accumulation and NLRP3 inflammasome in ob/ob mice. Int. Immunopharmacol. 2022, 111, 109099.
- Zhang, Y.; Zhou, F. Effects of geniposide on inflammation and oxidative stress of ApoE knockout mice with atherosclerosis and none-alcoholic fatty liver disease. Tradit. Chin. Drug Res. Clin. Pharmacol. 2015, 26, 581–586.




