Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | CONSTANTINOS GIAGINIS | -- | 2613 | 2023-10-10 08:43:15 | | | |
| 2 | Sirius Huang | Meta information modification | 2613 | 2023-10-11 03:25:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Spanoudaki, M.; Stoumpou, S.; Papadopoulou, S.K.; Karafyllaki, D.; Solovos, E.; Papadopoulos, K.; Giannakoula, A.; Giaginis, C. Amygdalin as a Promising Anticancer Agent. Encyclopedia. Available online: https://encyclopedia.pub/entry/50029 (accessed on 08 February 2026).
Spanoudaki M, Stoumpou S, Papadopoulou SK, Karafyllaki D, Solovos E, Papadopoulos K, et al. Amygdalin as a Promising Anticancer Agent. Encyclopedia. Available at: https://encyclopedia.pub/entry/50029. Accessed February 08, 2026.
Spanoudaki, Maria, Sofia Stoumpou, Sousana K. Papadopoulou, Dimitra Karafyllaki, Evangelos Solovos, Konstantinos Papadopoulos, Anastasia Giannakoula, Constantinos Giaginis. "Amygdalin as a Promising Anticancer Agent" Encyclopedia, https://encyclopedia.pub/entry/50029 (accessed February 08, 2026).
Spanoudaki, M., Stoumpou, S., Papadopoulou, S.K., Karafyllaki, D., Solovos, E., Papadopoulos, K., Giannakoula, A., & Giaginis, C. (2023, October 10). Amygdalin as a Promising Anticancer Agent. In Encyclopedia. https://encyclopedia.pub/entry/50029
Spanoudaki, Maria, et al. "Amygdalin as a Promising Anticancer Agent." Encyclopedia. Web. 10 October, 2023.
Copy Citation
Amygdalin, also known as vitamin B17 (and laetrile, a synthetic compound), is a cyanogenic glycoside compound that is mainly found in the kernels and pulps of fruits. This compound has been proposed for decades as a promising naturally occurring substance which may provide anticancer effects.
amygdalin
laetrile
vitamin B17
anticancer effects
anticancer molecular mechanisms
cancer
1. Amygdalin: Basic Information and Properties
Amygdalin was discovered in 1803 by Schrader in a study of bitter almond ingredients, and was first isolated in 1830 by two French chemists, Pierre-Jean Robiquet and Antoine François Boutron-Charlard [1][2]. Chemists Haworth and Wylam finally determined its exact chemical structure in 1923 (Figure 1) [3]. Amygdalin (d-mandelonitrile-β-d-glucoside-6-β-glucoside) is a cyanogenic glycoside compound which consists of dibenzaldehyde, hydrocyanic acid, and two glucose molecules (D-mandelonitrile-β-D-glucoside-6-β-glucoside) [4]. Its bioactive form (D-mandelonitrile-β-glucose) was used for a United States Patent (USP). Laetrile is a partly manmade, synthetic form of the natural substance amygdalin (Figure 1) [4]. In Mexico, the structure was differentiated and defined as D-mandelonitrile-β-gentiobioside [5]. The US National Center Institute (NCI) demonstrated that the Mexican form of amygdalin (oral and intravenous) did not comply with US drug standards and the substance was banned for human consumption [5]. Amygdalin is mainly found in the kernels and pulps of fruits such as plums, apricot pits, black cherries, peaches, and bitter almonds, and has been widely used in alternative medicine [6][7][8]. Amygdalin’s molecular formula is C20H27NO11, and its molecular weight is 457.42 g·mol−1 [9].
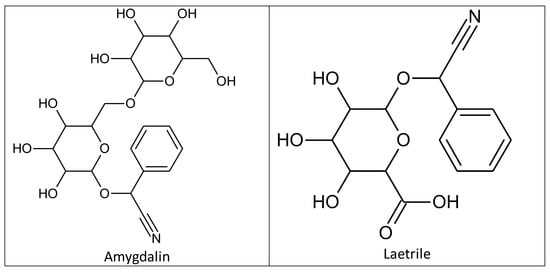
Figure 1. Chemical structures of amygdalin and laetrile.
Amygdalin can produce mandelonitrile and pranazine through hydrolysis under the action of glucosidase, and is eventually broken down into hydrocyanic acid and benzaldehyde [2]. The cytotoxic effect of amygdalin on cancer cells in vitro and the distribution of amygdalin in plants that are commonly consumed in the human diet are two of the most compelling topics in recent research. However, it is not a new compound, and it has been used in traditional and alternative medicine for centuries due to its anticancer and anti-inflammatory properties and, in general, its numerous medical benefits [7][8][10]. It has been helpful in relieving pain and fever; suppressing coughs, thirst, and nausea; and as a cancer prevention and co-treatment agent in recent years [11][12].
2. Anticancer Effects and Molecular Mechanisms of Amygdalin: In Vitro and In Vivo Evidence
To date, several studies have explored the potential anticancer effects of amygdalin, highlighting its anticancer molecular mechanisms especially in lung, breast, prostate, colorectal, cervical, and gastrointestinal cancers. The potential anticancer effects and molecular mechanisms of amygdalin are depicted in Figure 2.
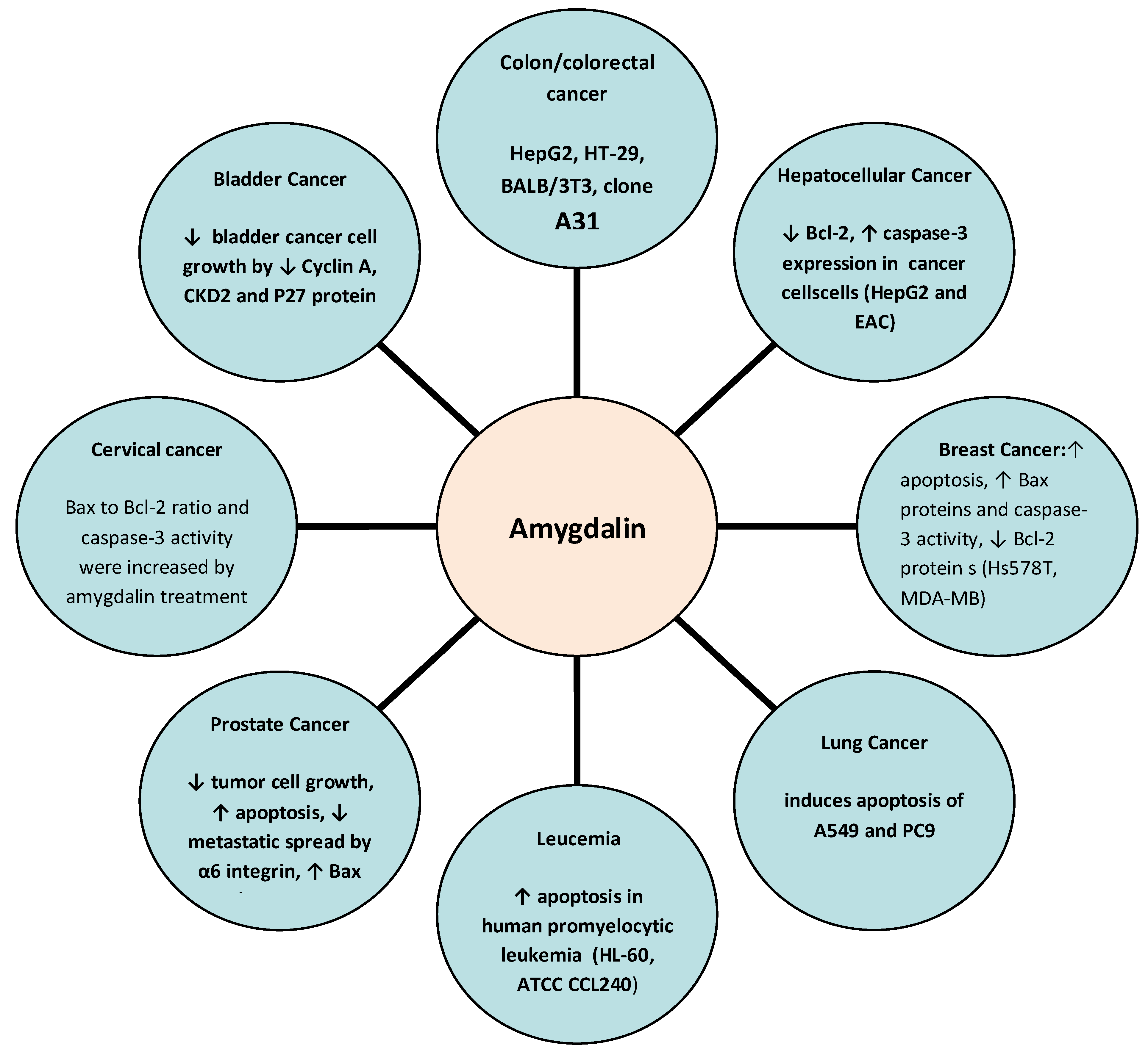
Figure 2. Anticancer molecular mechanisms of amygdalin. ↑: increase, ↓: decrease.
2.1. Lung Cancer
Amygdalin could be beneficial as a co-therapeutic agent in lung tumors. This compound significantly induced the apoptosis of A549 and PC9 lung cancer cells in a dose-dependent manner via the mitochondrion-mediated and caspase-dependent apoptotic pathway [13]. Simultaneously, an increase in cytochrome C and an enhancement of caspase-9 and caspase-3 activities were observed in A549 and PC9 lung cancer cells. In vitro inhibition of proliferation of lung cancer cell lines H1299/M and PA/M required a high concentration of amygdalin [14]. However, at a lower concentration of amygdalin, it was observed that the invasion and migration abilities of H1299/M PA/M cancer cells were significantly inhibited [14]. Thus, it was suggested that amygdalin is likely to have antimetastatic activity, inducing apoptosis and inhibiting the proliferation of lung cancer cells [14].
2.2. Breast Cancer
Amygdalin has been shown to induce apoptosis and inhibit the adhesion of breast cancer cells by increasing the level of pro-apoptotic Bax proteins and caspase-3 activity and decreasing the level of the anti-apoptotic Bcl-2 protein [15]. Significantly, in both MCF-7 and MDA-MB-231 breast cancer cells, amygdalin significantly increased apoptosis by suppressing cell proliferation and improving radiotherapy efficiency through induction of cell cycle arrest (in G1 and sub-G1 cell cycle stages) [16]. Amygdalin was also found to decrease the migration of MDA-MB-231 cells more than MCF-7 cells [17]. Furthermore, the inhibition of proteolytic enzymes was suggested to promote the activation of apoptotic events in MCF-7 breast cancer cells [18]. In addition, amygdalin was shown to increase Bax and decrease Bcl-2 expression in SK-BR-3 and MCF-7 breast cancer cells. However, compared with the amygdalin–ZHER2 affibody conjugate, the effect on Bax and Bcl-2 expression in SK-BR-3 cells was stronger than that in MCF-7 cells [19]. Τhe ability of amygdalin to reduce MCF-7 and T47D human breast cancer cell growth in a concentration-dependent manner by stimulating malondialdehyde (MDA) and oxidized glutathione production was also demonstrated. Moreover, considerable reductions of total glutathione levels and glutathione reductase activity in breast cancer cells were observed [20].
2.3. Prostate Cancer
Amygdalin dose-dependently inhibited tumor growth and reduced tumor clones in prostate cancer cell lines by inhibiting the G0/G1 phase [21]. Moreover, inhibition of prostate cancer cell growth and tumor growth by amygdalin were evident, revealing a function of the metabolic enzymes betaglucosidase (β-glucosidase) and rhodanese in regulating the anticancer activity of amygdalin in vivo [22]. The activation of amygdalin by β-glucosidase could be considered an enzyme/drug therapy strategy that may be a promising new approach for the targeted treatment of prostate cancer [23]. The exposure of certain prostate cancer cells, such as DU-145, to amygdalin was also found to inhibit metastatic spread promoted by α6 integrin [24].
2.4. Colorectal Cancer
In alternative and traditional medicine, amygdalin is commonly used for the prevention and treatment of colorectal tumor malignancies [25]. The anticancer effect of amygdalin on colorectal cancer cells, for instance in human SNU-C4 colorectal cancer cells, has been found to be promoted by reducing the expression of cell-cycle-related genes [26]. Colon cancer cells were reported to be more sensitive to the effect of amygdalin compared to normal cells due to their higher concentration of β-glucosidase and lower levels of the liver enzyme rhodanese, which can convert cyanide to the relatively harmless compound thiocyanate [27].
2.5. Cervical Cancer
Amygdalin has been documented to significantly inhibit the proliferative activity of HeLa cervical cancer cells [28]. The anti-apoptotic protein Bcl-2 was downregulated and the pro-apoptotic Bax was upregulated in HeLa cells treated with amygdalin [29]. Moreover, the Bax-to-Bcl-2 ratio and caspase-3 activity were increased by amygdalin treatment in HeLa cells, reinforcing the apoptotic effect of amygdalin on cervical cancer cells [29][30].
2.6. Gastrointestinal Cancer
Amygdalin has been demonstrated to stimulate the apoptotic process by upregulating caspase-3 expression and downregulating Bcl-2 expression, as well as inhibiting HepG2 and EAC hepatocellular cancer cell proliferation and upregulating Beclin-1 expression [31]. It is noteworthy that the combination of amygdalin with metformin exerted a promising effect when compared to amygdalin alone; their combination was more cytotoxic, showing a greater ability to induce apoptosis and arrest the cell cycle in hepatocellular cancer cells [32]. In addition to this combination, the activity of amygdalin with zinc has also been shown to produce an enhanced apoptotic effect in the treatment of HepG2 compared to the effect of amygdalin without zinc [33].
2.7. Other Tumor Malignancies
The inhibitory effect of amygdalin on the growth and differentiation markers E- and N-cadherin in renal cell carcinoma (RCC) cells was also demonstrated via the application of 10 g/mL amygdalin to the RCC cell lines A498, Caki-1, and KTC-26 for a period of 24 h or 2 weeks in vitro [7]. The investigation of amygdalin’s (1.25–10 mg/mL) impact on several bladder cancer cell lines (UMUC-3, RT112, and TCCSUP) also showed positive results [34]. The most remarkable impacts of amygdalin have been attributed to the cdk2–cyclin A axis. Studies on siRNA knockdown have shown a positive relationship with cdk2/cyclin. Amygdalin has also been found to inhibit tumor development via downregulation of cdk2 and cyclin [34]. In contrast, colony-forming cells from leukemic cell lines and normal bone marrow were relatively tolerant to amygdalin and its metabolites in vitro. Although an increasing rate of apoptosis was observed, there was no selective destruction between human leukemic cell lines and normal bone marrow cells [35].
3. Toxicity of Amygdalin
Excessive consumption of amygdalin can lead to poisonous effects (more than 1 mg/L cyanide in the blood). Amygdalin is converted into glucose, benzaldehyde, and hydrogen cyanide by an endogenous enzyme (β-glucosidase) when fruit pits are crushed. More analytically, when HCN is released, cytochrome oxidase C can react with the iron ion. This can induce the formation of metal-ion complexes which lyse cells and inhibit ATP synthesis [36].
Amygdalin has been reported to exert toxic effects when ingested with supplements. Oral intake of 500 mg amygdalin might release 30 mg of cyanide [37]. Cyanide toxicity can be life-threatening due to the decrease of mitochondrial oxygen utilization, leading to cell death. Cancer cells lack rodhanase, an enzyme which acts as a detoxifying agent by binding iron sulfur centers on cell membranes and converts HCN into a less toxic metabolite, thiocyanate. However, following parenteral administration of amygdalin/laetrile by injection, more than 80% of thiocyanate was detected in rats’ and rabbits’ urine [37]. The adverse side effects of cyanide toxicity include tachycardia, confusion, nausea, headache, and, more severely, neuromyopathy, cyanosis, coma, convulsions, and death [38].
Over recent decades, several in vitro and in vivo studies have been performed, using single or multiple doses and different forms of amygdalin administration (intravenous and intramuscular), that showed no HCN formation, highlighting the crucial role of the gut in human body physiology after substance consumption. The anaerobic bacterial phyla existing in the gut present a high β-glucosidase activity, which is needed for amygdalin to hydrolyze HCN. Nevertheless, HCN toxicity has been found to exist under certain circumstances. In some cases, toxicity derived from the ingestion of variable doses of amygdalin and there were no HCN side effects associated with high doses. Several factors, including probiotic or prebiotic consumption, diet, and age, may alter the gut consortium, which is responsible for the conditions under which toxicity occurs. Notably, serious reactions were not reported for a dose of 3 g orally administrated amygdalin in patients with cancer who were seeking alternative therapies. The minimum lethal dose of amygdalin for an adult is 50 mg or 0.5–3.5 mg/kg of body mass. However, the interaction with concomitant consumption of vitamin C seems to activate its adverse side effects, while vitamin B12 and sodium diosulfate solution have been used as antidotes without adverse side effects [36].
4. Amygdalin/Laetrile Clinical Studies in Human Tumor Malignancies in the 20th Century
Several studies have demonstrated the anticancer activity of amygdalin and its therapeutic use for cancer treatment and pain relief [2][39]. Although the scientific evidence for the anticancer effect of amygdalin based on clinical trials is limited, there have been some trials examining the effect of amygdalin against tumor malignancies in humans. Several clinical trials of amygdalin/laetrile have been considered over the years [4]. In 1980, the Research Drug Branch of the National Cancer Institute (NCI) announced that approximately 200 cancer patients “for whom no other treatment has been effective” were planned to receive the chemical, a special diet, and supplemental vitamins (see “National Cancer Institute begins laetrile clinical trial”, 1980) [40]. Two clinical trials in the field of laetrile in human cancer were conducted over the next two years. The first of these two clinical trials was performed in 1981 and it was conducted on six patients with advanced cancer [4]. Amygdalin was administered both intravenously and orally for a duration of 21 days, with no evidence of toxic reactions. These findings were in accordance with previous observations of a patient after self-administration of laetrile [41]. In 1982, another clinical trial was performed with a total number of 178 patients with cancer who received an amygdalin treatment plus a “metabolic therapy” [42]. No substantial benefits were observed in terms of cure, improvement or stabilization of cancer, improvement of symptoms related to cancer, or extension of life span. The hazards of amygdalin therapy were evidenced in several patients by symptoms of cyanide toxicity or by blood cyanide levels approaching the lethal range [40][41][42]. However, it should be noted that these clinical trials were performed over 40 years ago and should now be considered outdated, highlighting the need for novel clinical trials carried out involving the administration of amygdalin with diverse pharmaceutical formulations that could be more tolerable to and acceptable by the human body, presenting more bioactive efficiency and nontoxic effects.
5. Nanoparticles and Amygdalin in the 21st Century
Nanoparticles are considered a promising method in biotechnology for drug delivery and the treatment of human tumor malignancies while avoiding toxicity. Several studies in human cancer cell lines have demonstrated positive results concerning amygdalin metabolism without side effects. As already mentioned, amygdalin, despite its anticancer actions, has been faced controversy because of the cyanide release. Sohail and Abbas investigated alginate–chitosan nanoparticles (ACNPs) as a mode of drug administration via amygdalin’s encapsulation and delivery to tumor cells (H1299) [43]. The nanoparticles showed stable drug release over a ten-hour period and significant swelling rates in slightly acidic and neutral environments. ACNPs were shown to have a greater antitumor effect on H1299 cell lines than free amygdalin, suggesting greater cellular uptake of the compound encapsulated in nanoparticles. In this regard, biomimetic and biocompatible balginate–chitosan nanoparticles could be applied as an advantageous drug delivery system for prolonged and controlled delivery of amygdalin with enhanced cytotoxic activity against tumor cells, while simultaneously protecting normal human tissues and healthy cells [43].
Silver nanoparticles encapsulating amygdalin and cross-linking to microcapsules charged with chitosan have also been explored in breast cancer cell lines. An anticancer response was also observed in line with the controlled release of amygdalin due to chitosan conjunction, overcoming low cytotoxic effects at high doses [44].
Additionally, nanoparticles demonstrated sustained amygdalin–folic acid release properties and apparent selectivity to cells by suppressing tumor growth. At the same time, they were found to improve radiotherapy efficiency by enhancing apoptosis, blocking the cell cycle, and reducing the proliferation of breast cancer cells by downregulating iron levels and mitogen-activated protein kinases (MAPK/P38). Amygdalin–folic acid was also shown to inhibit the differentiation of CD4 and CD80 complex expression, inducing suppression of transformation of growth factor beta (TGF-beta)/interleukin-6, (IL-2)/interferon-gamma, (INF-g)/interleukin-2, and (IL-2)/vascular endothelial growth factor (VEGF) expression at the signaling pathway, while simultaneously modulating the gene expression of CD8 and the natural killer group 2D [16].
Mosayyebi and colleagues nanoformulated amygdalin with β-cyclodextrin in order to investigate the increase of its action against MCF-7 cell line migration, apoptosis, and migration of genes. The nanoformulated amygdalin showed a greater effect on tumor cells than amygdalin alone [45].
6. Nutritional Supplements with Amygdalin for Cancer Treatment
Amygdalin, laetrile, or vitamin B17 has been claimed as a treatment for various diseases, especially tumor malignancies, since 1845 [5]. However, in 1982, there was a perception that amygdalin might be a toxic drug and not effective in cancer treatment [42]. Recent theoretical and practical developments have revealed that amygdalin may produce beneficial effects for cancer patients [44][45][46]. Amygdalin has been used to treat cancer both as a single agent and in combination with metabolic therapy. Therefore, it is worth mentioning that toxicity from vitamin supplements is not a rare occurrence and amygdalin is recommended to patients as a dietary supplement for cancer, wherein high doses are proposed [47]. Amygdalin tablets and capsules are currently marketed as a natural dietary supplement under the misnomer laetrile or the questionable name “vitamin B17” [48].
Certain case report studies have demonstrated rebound metabolic acidosis following massive amygdalin overdose and life-threatening cyanide toxicity, including nephrogenic diabetes insipidus [49][50][51][52]. Amygdalin toxicity can be caused by the poisonous composite product of benzaldehyde and cyanide after oral ingestion [7]. Both toxicologists and nephrologists should be aware of the potential of this “vitamin” to cause cyanide poisoning [53]. Furthermore, there is currently a serious concern that natural dietary supplements are not subjected to rigorous analytical and clinical trials. Based on the European Union Regulation ((EC) No. 178/2002) concerning general food legislation, dietary supplements are considered foods and not drugs [54]. According to the above data suggesting that the clinical use of amygdalin dietary supplements may be accompanied by adverse side effects, the risk–benefit balance is not therefore favorable for amygdalin in this respect [54][55]. Moreover, amygdalin is incorrectly referred to as vitamin B17; the compound is not a vitamin [8].
References
- Jaszczak-Wilke, E.; Polkowska, Z.; Koprowski, M.; Owsianik, K.; Mitchell, A.E.; Bałczewski, P. Amygdalin: Toxicity, Anticancer Activity and Analytical Procedures for Its Determination in Plant Seeds. Molecules 2021, 26, 2253.
- Song, Z.; Xu, X. Advanced research on anti-tumor effects of amygdalin. J. Cancer Res. Ther. 2014, 10 (Suppl. S1), 3–7.
- Rauws, A.G.; Olling, M.; Timmerman, A. The pharmacokinetics of amygdalin. Arch. Toxicol. 1982, 49, 311–319.
- Moertel, C.G.; Ames, M.M.; Kovach, J.S.; Moyer, T.P.; Rubin, J.R.; Tinker, J.H. A pharmacologic and toxicological study of amygdalin. JAMA 1981, 245, 591–594.
- Davignon, J.P.; Trissel, L.A.; Kleinman, L.M. Pharmaceutical assessment of amygdalin (Laetrile) products. Cancer Treat Rep. 1978, 62, 99–104.
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Amygdalin content of seeds, kernels and food products commercially available in the UK. Food Chem. 2014, 152, 133–139.
- Juengel, E.; Thomas, A.; Rutz, J.; Makarevic, J.; Tsaur, I.; Nelson, K.; Haferkamp, A.; Blaheta, R.A. Amygdalin inhibits the growth of renal cell carcinoma cells in vitro. Int. J. Mol. Med. 2016, 37, 526–532.
- Salama, R.H.; El Rahman Ramadan, A.G.; Alsanory, T.A.; Herdan, M.O.; Fathallah, O.M.; Alsanory, A.A. Experimental and Therapeutic Trials of Amygdalin. Int. J. Biochem. Pharmacol. 2019, 1, 21–26.
- Figurová, D.; Tokárová, K.; Greifová, H.; Knížatová, N.; Kolesárová, A.; Lukáč, N. Inflammation, It’s Regulation and Antiphlogistic Effect of the Cyanogenic Glycoside Amygdalin. Molecules 2021, 26, 5972.
- He, X.-Y.; Wu, L.-J.; Wang, W.-X.; Xie, P.-J.; Chen, Y.-H.; Wang, F. Amygdalin—A pharmacological and toxicological review. J. Ethnopharmacol. 2020, 254, 112717.
- Li, Y.-L.; Li, Q.-X.; Liu, R.-J.; Shen, X.-Q. Chinese Medicine Amygdalin and β-Glucosidase Combined with Antibody Enzymatic Prodrug System as A Feasible Antitumor Therapy. Chin. J. Integr. Med. 2018, 24, 237–240.
- Luo, D.; Wang, Q.; Meng, Y.; Cao, L.; Feng, N.; Du, W.; Li, H.; Dong, X.; Xiumin Ma, X.; Luo, L. Effects of amygdalin on type II collagen-induced arthritis in rats. Biologia 2020, 75, 423–430.
- Lin, S.; Wen, J.; Xu, X.; Shi, J.; Zhang, W.; Zheng, T.; Hou, Y.; Zhang, Y.; Li, Z.; Wang, K.; et al. Amygdalin Induced Mitochondria-Mediated Apoptosis of Lung Cancer Cells via Regulating NFκB-1/NFκB Signaling Cascade in Vitro and in Vivo. Am. J. Chin. Med. 2022, 50, 1361–1386.
- Qian, L.; Xie, B.; Wang, Y.; Qian, J. Amygdalin-mediated inhibition of non-small cell lung cancer cell invasion in vitro. Int. J. Clin. Exp. Pathol. 2015, 8, 5363–5370.
- Lee, H.M.; Moon, A. Amygdalin Regulates Apoptosis and Adhesion in Hs578T Triple-Negative Breast Cancer Cells. Biomol. Ther. 2016, 24, 62–66.
- Askar, M.A.; El-Sayyad, G.S.; Guida, M.S.; Khalifa, E.; Shabana, E.S.; Abdelrahman, I.Y. Amygdalin-folic acid-nanoparticles inhibit the proliferation of breast cancer and enhance the effect of radiotherapy through the modulation of tumor-promoting factors/immunosuppressive modulators in vitro. BMC Complement Med. Ther. 2023, 23, 162.
- Mosayyebi, B.; Mohammadi, L.; Kalantary-Charvadeh, A.; Rahmati, M. Amygdalin Decreases Adhesion and Migration of MDA-MB-231 and MCF-7 Breast Cancer Cell Lines. Curr. Mol. Pharmacol. 2021, 14, 667–675.
- Cecarini, V.; Salima Selmi, S.; Cuccioloni, M.; Gong, C.; Bonfili, L.; Zheng, Y.; Cortese, M.; Angeletti, M.; Kilani, S.; Eleuteri, A.M. Targeting Proteolysis with Cyanogenic Glycoside Amygdalin Induces Apoptosis in Breast Cancer Cells. Molecules 2022, 27, 7591.
- Moradipoodeh, B.; Jamalan, M.; Zeinali, M.; Fereidoonnezhad, M.; Mohammadzadeh, G. Specific targeting of HER2-positive human breast carcinoma SK-BR-3 cells by amygdaline-Z(HER2) affibody conjugate. Mol. Biol. Rep. 2020, 47, 7139–7151.
- Abboud, M.M.; Al Awaida, W.; Alkhateeb, H.H.; Abu-Ayyad, A.N. Antitumor Action of Amygdalin on Human Breast Cancer Cells by Selective Sensitization to Oxidative Stress. Nutr. Cancer 2019, 71, 483–490.
- Tsaur, I.; Thomas, A.; Monecke, M.M.; Zugelder, M.; Rutz, J.; Grein, T.; Maxeiner, S.; Xie, H.; Chun, F.K.-H.; Florian Rothweiler, F.; et al. Amygdalin Exerts Antitumor Activity in Taxane-Resistant Prostate Cancer Cells. Cancers 2022, 14, 3111.
- Alwan, A.M.; Afshari, J.T. In Vivo Growth Inhibition of Human Caucasian Prostate Adenocarcinoma in Nude Mice Induced by Amygdalin with Metabolic Enzyme Combinations. Biomed. Res. Int. 2022, 2022, 4767621.
- Zhou, J.; Hou, J.; Rao, J.; Zhou, C.; Liu, Y.; Gao, W. Magnetically Directed Enzyme/Prodrug Prostate Cancer Therapy Based on β-Glucosidase/Amygdalin. Int. J. Nanomed. 2020, 15, 4639–4657.
- Mani, J.; Neuschäfer, J.; Resch, C.; Rutz, J.; Maxeiner, S.; Roos, F.; Chun, F.K.; Juengel, E.; Blaheta, R.A. Amygdalin Modulates Prostate Cancer Cell Adhesion and Migration In Vitro. Nutr. Cancer 2020, 72, 528–537.
- Dimitrov, M.; Iliev, I.; Bardarov, K.; Georgieva, D.; Todorova, T. Phytochemical characterization and biological activity of apricot kernels’ extract in yeast-cell based tests and hepatocellular and colorectal carcinoma cell lines. J. Ethnopharmacol. 2021, 279, 114333.
- Park, H.J.; Yoon, S.H.; Han, L.S.; Zheng, L.T.; Jung, K.H.; Uhm, Y.K.; Lee, J.H.; Jeong, J.S.; Joo, W.S.; Yim, S.V.; et al. Amygdalin inhibits genes related to cell cycle in SNU-C4 human colon cancer cells. World J. Gastroenterol. 2005, 11, 5156–5161.
- Cassiem, W.; de Kock, M. The anti-proliferative effect of apricot and peach kernel extracts on human colon cancer cells in vitro. BMC Complement Altern Med. 2019, 19, 32.
- Shi, J.; Qianqian Chen, Q.; Xu, M.; Xia, Q.; Tiansheng Zheng, T.; Teng, J.; Li, M.; Fan, L. Recent updates and future perspectives about amygdalin as a potential anticancer agent: A review. Cancer Med. 2019, 8, 3004–3011.
- Chen, Y.; Ma, J.; Wang, F.; Hu, J.; Cui, A.; Wei, C.; Yang, Q.; Li, F. Amygdalin induces apoptosis in human cervical cancer cell line HeLa cells. Immunopharmacol. Immunotoxicol. 2013, 35, 43–51.
- Asselin, E.; Mills, G.B.; Tsang, B.K. XIAP regulates Akt activity and caspase-3-dependent cleavage during cisplatin-induced apoptosis in human ovarian epithelial cancer cells. Cancer Res. 2001, 61, 1862–1868.
- Hosny, S.; Sahyon, H.; Youssef, M.; Negm, A.; Prunus Armeniaca, L. Seed Extract and Its Amygdalin Containing Fraction Induced Mitochondrial-Mediated Apoptosis and Autophagy in Liver Carcinogenesis. Anticancer Agents Med. Chem. 2021, 21, 621–629.
- Mamdouh, A.M.; Khodeer, D.M.; Tantawy, M.A.; Moustafa, Y.M. In-vitro and in-vivo investigation of amygdalin, metformin, and combination of both against doxorubicin on hepatocellular carcinoma. Life Sci. 2021, 285, 119961.
- El-Desouky, M.A.; Fahmi, A.A.; Abdelkader, I.Y.; Nasraldin, K.M. Anticancer Effect of Amygdalin (Vitamin B-17) on Hepatocellular Carcinoma Cell Line (HepG2) in the Presence and Absence of Zinc. Anticancer. Agents Med. Chem. 2020, 20, 486–494.
- Makarević, J.; Rutz, J.; Juengel, E.; Kaulfuss, S.; Reiter, M.; Tsaur, I.; Bartsch, G.; Haferkamp, A.; Blaheta, R.A. Amygdalin blocks bladder cancer cell growth in vitro by diminishing cyclin A and cdk2. PLoS ONE 2014, 9, e105590.
- Koeffler, H.P.; Lowe, L.; Golde, D.W. Amygdalin (Laetrile): Effect on clonogenic cells from human myeloid leukemia cell lines and normal human marrow. Cancer Treat. Rep. 1980, 64, 105–109.
- Jaswal, V.; Palanivelu, J.; Ramalingam, C. Effects of the Gut microbiota on Amygdalin and its use as an anti-cancer therapy: Substantial review on the key components involved in altering dose efficacy and toxicity. Biochem. Biophys. Rep. 2018, 14, 125–132.
- Newton, G.W.; Schmidt, E.S.; Lewis, J.P.; Conn, E.; Lawrence, R. Amygdalin toxicity studies in rats predict chronic cyanide poisoning in humans. West. J. Med. 1981, 134, 97–103.
- Meredith, T.J. Epidemiology of poisoning. Pharmacol. Ther. 1993, 59, 251–256.
- Arshi, A.; Hosseini, S.M.; Hosseini, F.S.K.; Amiri, Z.Y.; Hosseini, F.S.; Lavasani, M.L.; Kerdarian, H.; Dehkordi, M.S. The anti-cancer effect of amygdalin on human cancer cell lines. Mol. Biol. Rep. 2019, 46, 2059–2066.
- National Cancer Institute begins laetril clinical trial. JAMA 1980, 244, 538.
- Ames, M.M.; Kovach, J.S.; Flora, K.P. Initial pharmacologic studies of amygdalin (laetrile) in man. Res. Commun. Chem. Pathol. Pharmacol. 1978, 22, 175–185.
- Moertel, C.G.; Fleming, T.R.; Rubin, J.; Kvols, L.K.; Sarna, G.; Koch, R.; Currie, V.E.; Young, C.W.; Jones, S.E.; Davignon, J.P. A clinical trial of amygdalin (Laetrile) in the treatment of human cancer. N. Engl. J. Med. 1982, 306, 201–206.
- Sohail, R.; Abbas, S.R. Evaluation of amygdalin-loaded alginate-chitosan nanoparticles as biocompatible drug delivery carriers for anticancerous efficacy. Int. J. Biol. Macromol. 2020, 153, 36–45.
- Pandey, A.; Ali, S.A.; Negi, Y. Synthesis of polygonal chitosan microcapsules for the delivery of amygdalin loaded silver nanoparticles in breast cancer therapy. Mater. Today Proc. 2021, 43, 3744–3748.
- Mosayyebi, B.; Mahsa Imani, M.; Rahmati, M.; Akbarzadeh, A.; Zarghami, N.; Alizadeh, E.; Rahmati, M. Comparison Between β-Cyclodextrin-Amygdalin Nanoparticle and Amygdalin Effects on Migration and Apoptosis of MCF-7 Breast Cancer Cell Line. J. Clust. Sci. 2022, 33, 935–947.
- Milazzo, S.; Horneber, M.; Ernst, E. Laetrile treatment for cancer. Cochrane Database Syst. Rev. 2015, 2015, CD005476.
- Ronis, M.J.J.; Pedersen, K.B.; Watt, J. Adverse Effects of Nutraceuticals and Dietary Supplements. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 583–601.
- El-Ela, F.I.A.; Gamal, A.; Elbanna, H.A.; ElBanna, A.H.; Salem, H.F.; Tulbah, A.S. In Vitro and In Vivo Evaluation of the Effectiveness and Safety of Amygdalin as a Cancer Therapy. Pharmaceuticals 2022, 15, 1306.
- Dang, T.; Nguyen, C.; Tran, P.N. Physician Beware: Severe Cyanide Toxicity from Amygdalin Tablets Ingestion. Case Rep. Emerg. Med. 2017, 2017, 4289527.
- Wahab, M.F.; Breitbach, Z.S.; Armstrong, D.W.; Strattan, R.; Berthod, A. Problems and Pitfalls in the Analysis of Amygdalin and Its Epimer. J. Agric. Food Chem. 2015, 63, 8966–8973.
- Cmorej, P.; Bruthans, P.; Halamka, J.; Voriskova, I.; Peran, D. Life-Threatening Cyanide Intoxication after Ingestion of Amygdalin in Prehospital Care. Prehosp. Emerg. Care 2022, 26, 455–458.
- O’Brien, B.; Quigg, C.; Leong, T. Severe cyanide toxicity from “vitamin supplements. Eur. J. Emerg. Med. 2005, 12, 257–258.
- Sauer, H.; Wollny, C.; Oster, I.; Tutdibi, E.; Gortnerm, L.; Gottschling, S.; Meyer, S. Severe cyanide poisoning from an alternative medicine treatment with amygdalin and apricot kernels in a 4-year-old child. Wien Med. Wochenschr. 2015, 165, 185–188.
- Shively, R.M.; Harding, S.A.; Hoffman, R.S.; Hill, A.D.; Astua, A.J.; Manini, A.F. Rebound metabolic acidosis following intentional amygdalin supplement overdose. Clin. Toxicol. 2020, 58, 290–293.
- Charen, E.; Harbord, N. Toxicity of Herbs, Vitamins, and Supplements. Adv. Chronic. Kidney Dis. 2020, 27, 67–71.
More
Information
Subjects:
Nutrition & Dietetics; Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
11 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




