
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kevin Yang Wu | -- | 2185 | 2023-10-09 20:15:56 | | | |
| 2 | Kevin Yang Wu | -5 word(s) | 2180 | 2023-12-03 17:29:36 | | | | |
| 3 | Catherine Yang | Meta information modification | 2180 | 2023-12-04 02:27:07 | | |
Video Upload Options
Macular edema is characterized by the accumulation of fluid in the macula, leading to vision impairment. Suprachoroidal injections can help with macular edema by delivering medication directly to the affected area, allowing for targeted treatment. Suprachoroidal injections offer several advantages in treating macular edema such as providing a more localized delivery of medication compared to other route and allowing for sustained drug release, which prolongs the duration of the therapeutic effect. This approach therefore minimizes potential side effects and systemic exposure to the medication as well as improving patient comfort. Depending on the exact etiology of macular edema, different substances can be delivered through this method, whether is is triamcinolone acetonide or corticosteroids.
1. Suprachoroidal Injection for Macular Edema Secondary to Non-Infectious Uveitis
Promising preclinical results of suprachoroidal triamcinolone acetonide (SCTA) injection concentrating corticosteroids in the retina, retinal pigment epithelium (RPE), and choroid, while minimizing exposure to anterior chamber structures, led to subsequent clinical trials [1]. The PEACHTREE trial, a randomized, double-masked study led by Yeh et al., demonstrated the effectiveness and safety of SCTA for treating macular edema (ME) in non-infectious uveitis (NIU). SCTA-treated eyes showed significant visual improvement, with 46.9% gaining 15 or more ETDRS letters by week 24, compared to 15.6% in the control group. SCTA also led to ME resolution and reduced rescue therapy need. Treatment-related adverse events occurred in both groups, but cataracts and elevated IOP rates were comparable and no serious treatment-related adverse events were reported. These findings highlight SCTA's potential as a therapeutic approach for ocular diseases [2].
Henry et al. confirmed improved visual and anatomical outcomes in patients receiving SCTA for ME due to NIU, regardless of age, at 24 weeks [3]. Merrill et al.'s post-hoc analysis revealed consistent benefits of SCTA regardless of concurrent systemic corticosteroid use or steroid-sparing therapy. Notably, SCTA had a statistically significant impact on mean best corrected visual acuity (BCVA) change for patients receiving steroid therapy, while the mean CST change was not statistically significant. SCTA outperformed sham treatment in terms of functional and anatomical outcomes, but further studies are needed to assess the suitability of SCTA therapy for patients on different treatment regimens [4].
The MAGNOLIA extension safety study by Khurana et al. found that the significant improvement in BCVA and CST reduction observed in SCTA-treated eyes was maintained for 48 weeks compared to the control group. The SCTA group had a significantly longer median time to rescue therapy, although the need for rescue treatment was similar in both groups. The occurrence of ocular adverse events (AEs) was comparable between the groups, with subcapsular cataract being the most common AE. Elevated IOP readings were observed in 14.3% of SCTA patients, but no surgical management was required [5].
In the AZALEA safety clinical trial conducted by Henry et al., SCTA demonstrated good tolerability and safety over 24 weeks in patients with ME. BCVA, CST, and retinal thickness improved throughout the study period, although statistical significance was not assessed. Treatment-related AEs included pain, intraocular pressure (IOP) rise, and cataract formation, with no surgical interventions required [6].
Yeh et al.'s DOGWOOD study in patients with ME due to NIU showed that a 4.0 mg SCTA injection was efficacious and well-tolerated. Significant improvements in CST and BCVA were observed at 1 and 2 months. The majority of patients with baseline anterior cell grade or vitreous haze score >0 showed improvement. Treatment-related AEs were reported in both SCTA groups, but no significant IOP elevation or serious AEs were reported [7].
Hanif et al. confirmed the safety and efficacy of 4.0 mg SCTA for ME secondary to NIU in a single-arm study. Statistically significant improvements in CMT and BCVA were observed at 1 and 3 months, with no significant changes in IOP [8].
Munir et al. found that 4.0 mg SCTA led to early improvements in BCVA for up to 6 months in patients with ME, including those with NIU. The occurrence of AEs, particularly elevated IOP, varied based on baseline IOP levels [9].
These studies support the efficacy and safety of SCTA for the treatment of ME secondary to NIU, suggesting its potential for broader application and the need for further investigation.
The unique compartmentalization and ocular distribution provided by SC injection resulted in a low incidence of AEs in these studies. MAGNOLIA and AZALEA trials demonstrated low rates of AEs, such as increased IOP and cataract progression, although some cases required surgical or medical management. No serious AEs like retinal detachment were reported. The studies reported varying rates of AEs (18.4% to 64.3%) and different occurrence rates of IOP elevation, cataract formation, and progression. Larger-scale, masked, and randomized studies with more participants are needed for more reliable and generalizable results. Comparisons with other therapies are currently limited as the existing studies focus on SCTA alone or sham injection. Nevertheless, these studies collectively support the efficacy and safety of SCTA, which is the first and only FDA-approved therapy utilizing the SCS for ME secondary to NIU. These findings also open doors for investigating other therapeutics administered via the SCS, such as anti-VEGF and viral gene agents.
2. Suprachoroidal Injection for Diabetic Macular Edema
Diabetic macular edema (DME) is a common complication of diabetic retinopathy and is a leading cause of vision loss. The current first-line treatment for DME involves the use of intravitreal (IV) injections of anti-vascular endothelial growth factor (anti-VEGF) agents such as Ranibizumab, Aflibercept, and Bevacizumab. These agents have demonstrated significant efficacy in improving vision [10].
However, there are limitations associated with these treatments. One limitation is the need for frequent IV injections, which can be burdensome for patients. Additionally, there are potential adverse events (AEs) related to the IV application of these agents. As an alternative, corticosteroids, particularly triamcinolone acetonide (TA), have been used as a second-line treatment for DME due to their anti-inflammatory properties. Intravitreal triamcinolone acetonide (IVTA) has been shown to effectively reduce DME and improve vision. However, it is associated with ocular AEs such as increased intraocular pressure (IOP) and cataract progression [11].
In this landscape, suprachoroidal triamcinolone acetonide (SCTA) has emerged as an alternative approach for DME treatment. SCTA offers the potential advantage of limiting anterior exposure and potentially decreasing ocular AEs. Several clinical trials have evaluated the safety and efficacy of SCTA in DME. For example, the TYBEE trial, conducted by Barakat et al. in 2021, compared SCTA with IV Aflibercept versus IV Aflibercept alone in treatment-naïve DME patients. The results after 24 weeks showed that the difference in visual acuity improvement was not statistically significant between the groups. However, the combination group exhibited a greater reduction in CMT and required fewer as-needed (prn) injections compared to the monotherapy group [12].
Studies have also explored the efficacy and safety of SCTA in combination with IV Bevacizumab versus IV Bevacizumab alone. Fazel et al. conducted a phase II/III randomized controlled pilot trial in 2023, randomly assigning eyes with untreated DME to receive SCTA in combination with IV Bevacizumab or IV Bevacizumab alone. They found that adding a single dose of SCTA prior to IV Bevacizumab led to significant improvements in visual acuity and reductions in CMT without major ocular AEs. The mean intraocular pressure remained stable throughout the study [13]. Another study by Anwar et al. in 2022 randomly assigned participants to receive either SCTA or IV Bevacizumab, and the results showed that a single dose of SCTA resulted in greater improvements in visual acuity and a more significant reduction in CMT compared to IV Bevacizumab [14].
In a prospective interventional study by Zakaria et al. (2022), comparisons were made between intravitreal triamcinolone acetonide (IVTA) and suprachoroidal triamcinolone acetonide (SCTA) administration. The study randomized 45 eyes in 32 patients to receive IVTA alone or with two different doses of SCTA. Both treatment arms showed significant improvements in visual acuity and central macular thickness (CMT) after 1 and 3 months. The 4.0 mg SCTA group demonstrated the most substantial improvement in visual acuity and sustained its effect for a longer duration. Incidence of AEs did not significantly differ between the routes, but considering reinjection before 6 months was recommended [15].
Shaikh et al. (2023) found that SCTA and IVTA were similarly effective at reducing CMT and best-corrected visual acuity (BCVA) at 3 months. Both groups showed significant improvements in BCVA and CMT, but no significant differences were observed between the groups. IOP elevation was significantly higher in the IVTA group, and cataract progression was slower in the SCTA group [16].
In DME post-vitrectomy, Marashi and Zaza (2022) observed significant vision improvement and a reduction in CMT after 8 weeks in eyes treated with SCTA, with no observed IOP elevation or cataract progression [17].
Emerging research is investigating the use of SC gene therapy for DME. RGX-314, an AAV8 vector-based gene therapy, is being evaluated in the ongoing ALTITUDE trial, a phase II study for center-involved DME. Initial data from the trial suggests positive outcomes in terms of diabetic retinopathy severity score improvement [18].
SCTA at a 4.0 mg/mL dose offers advantages over conventional therapies for primary and refractory DME. Combined with intravitreal (IV) anti-VEGF agents, SCTA consistently reduces macular thickness, improves visual acuity, and provides a longer duration of action compared to IV anti-VEGF alone. SCTA benefits patients with refractory DME and reduces the need for multiple injections. Unlike systemic corticosteroid delivery, SCTA targets DME's vascular and inflammatory aspects with reduced adverse events (AEs) and longer efficacy [19].
Further research is needed to explore SCTA's long-term efficacy, optimal dosing strategies, and comparative effectiveness for DME treatment, including its impact on visual outcomes, durability, and AEs. Most studies report significant improvements in visual acuity and central subfield thickness within a 3-month timeframe. IOP elevation and cataract progression can still occur but have a lower incidence compared to IV administration. Comparative studies with other therapies like posterior subtenon TA would be valuable, and larger multicenter studies are needed for longer follow-up [19].
3. Suprachoroidal Injection for Macular Edema Secondary to Retina Vein Occlusion
Industry-sponsored clinical trials have assessed the effectiveness of combining suprachoroidal triamcinolone acetonide (SCTA) with intravitreal (IV) anti-VEGF agents compared to IV anti-VEGF monotherapy for the treatment of macular edema (ME) resulting from retinal vein occlusion (RVO) [20][21][22][23].
The TANZANITE trial, a phase II study, demonstrated that the combination of SCTA (4.0 mg/0.1 mL) with IV aflibercept reduced the need for retreatment and led to greater visual acuity improvement and decreased central subfield thickness (CST) compared to IV aflibercept alone [20]. Extension data from the study showed that a significant percentage of patients in the combination group did not require retreatments over a 9-month period [21].
The phase III SAPPHIRE study compared the combination of SCTA with IV aflibercept to IV aflibercept monotherapy and found a significant improvement in visual acuity in both groups, but no other benefits were observed in the combination arm [22]. Preliminary data indicated that the combination therapy had a favorable safety profile [22].
The TOPAZ trial, a phase III study, aimed to investigate the superiority of SCTA in combination with IV ranibizumab or IV bevacizumab compared to monotherapy, but it was prematurely stopped due to the findings from the SAPPHIRE trial [23].
Independent studies have evaluated the efficacy and safety of combining suprachoroidal triamcinolone acetonide (SCTA) with intravitreal (IV) anti-VEGF agents for retinal vein occlusion (RVO)-associated macular edema (ME) [24][25][26][27][28]. A prospective randomized study on branch retinal vein occlusion (BRVO) patients demonstrated that the combination of IV ranibizumab with SCTA required fewer injections and led to significant improvements in visual acuity and central macular thickness [24].
SCTA as a monotherapy has also shown promising results in improving visual acuity and reducing central retinal thickness in RVO-associated ME [25][26]. A study involving RVO patients with severe subfoveal hard exudates found that a single suprachoroidal infusion of bevacizumab and triamcinolone acetonide resulted in visual acuity improvements, resolution of subretinal exudates, and reduced macular edema [27].
While the combination of SCTA and IV anti-VEGF therapy offers benefits such as fewer retreatments and anatomical improvements, further research is needed to determine optimal combination therapies, long-term efficacy, and safety [28]. Ongoing clinical trials are investigating the effects of suprachoroidal injections in RVO and other retinal diseases [28].
4. Suprachoroidal Injection for Post-Operative/Pseudophakic Cystoid Macular Edema
SCTA shows promise as a treatment for pseudophakic cystoid macular edema (PCME), a common complication after cataract surgery [29][30][31][32][33].
Studies have demonstrated the efficacy of SCTA and IVTA in reducing central foveal thickness (CFT) and improving visual acuity in pseudophakic patients with rDME caused by epiretinal membrane [29]. In one study, SCTA injections (4.0 mg/0.1 mL) and IVTA injections (4.0 mg/0.1 mL) resulted in significant improvements in CFT and BCVA [29] (Figure 1). The IVTA group experienced higher intraocular pressure (IOP) elevations compared to the SCTA group [29].
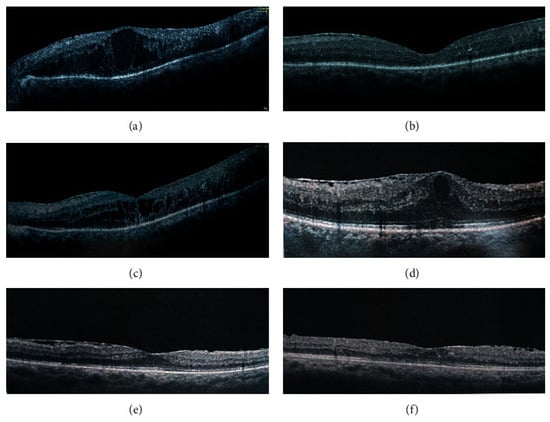
Zhang et al. (2022) demonstrated that SCTA injections (0.2 mL of 40 mg/mL) in patients with various conditions, including CME and PCME, led to significant improvements in BCVA and CST without significant differences in IOP [30]. No complications were observed during the study period [30]. Furthermore, case studies also reported positive outcomes with SCTA for PCME, including improved BCVA and decreased CFT [31][32].
Although these findings seem promising, further research, including randomized studies, is needed to evaluate the long-term efficacy and safety of SCTA for post-operative complications like PCME [33].
References
- Bhattacharyya, S.; Hariprasad, S.M.; Albini, T.A.; Dutta, S.K.; John, D.; Padula, W.V.; Harrison, D.; Joseph, G. Suprachoroidal Injection of Triamcinolone Acetonide Injectable Suspension for the Treatment of Macular Edema Associated with Uveitis in the United States: A Cost-Effectiveness Analysis. Value Health 2022, 25, 1705–1716.
- Yeh, S.; Khurana, R.N.; Shah, M.; Henry, C.R.; Wang, R.C.; Kissner, J.M.; Ciulla, T.A.; Noronha, G. Efficacy and Safety of Suprachoroidal CLS-TA for Macular Edema Secondary to Noninfectious Uveitis: Phase 3 Randomized Trial. Ophthalmology 2020, 127, 948–955.
- Henry, C.R.; Kapik, B.; Ciulla, T.A. Suprachoroidal Triamcinolone Acetonide Injectable Suspension for Macular Edema Associated with Uveitis: Visual and Anatomic Outcomes by Age. Investig. Ophthalmol. Vis. Sci. 2022, 63, 3206-A0432.
- Merrill, P.T.; Henry, C.R.; Nguyen, Q.D.; Reddy, A.; Kapik, B.; Ciulla, T.A. Suprachoroidal CLS-TA with and without Systemic Corticosteroid and/or Steroid-Sparing Therapy: A Post-Hoc Analysis of the Phase 3 PEACHTREE Clinical Trial. Ocul. Immunol. Inflamm. 2021, 1–8.
- Khurana, R.N.; Merrill, P.; Yeh, S.; Suhler, E.; Barakat, M.R.; Uchiyama, E.; Henry, C.R.; Shah, M.; Wang, R.C.; Kapik, B.; et al. Extension Study of the Safety and Efficacy of CLS-TA for Treatment of Macular Oedema Associated with Non-Infectious Uveitis (MAGNOLIA). Br. J. Ophthalmol. 2022, 106, 1139–1144.
- Henry, C.R.; Shah, M.; Barakat, M.R.; Dayani, P.; Wang, R.C.; Khurana, R.N.; Rifkin, L.; Yeh, S.; Hall, C.; Ciulla, T. Suprachoroidal CLS-TA for Non-Infectious Uveitis: An Open-Label, Safety Trial (AZALEA). Br. J. Ophthalmol. 2022, 106, 802–806.
- Yeh, S.; Kurup, S.K.; Wang, R.C.; Foster, C.S.; Noronha, G.; Nguyen, Q.D.; Do, D.V.; DOGWOOD Study Team. Suprachoroidal injection of triamcinolone acetonide, CLS-TA, for macular edema due to noninfectious uveitis: A Randomized, Phase 2 Study (DOGWOOD). Retina 2019, 39, 1880–1888.
- Hanif, J.; Iqbal, K.; Perveen, F.; Arif, A.; Iqbal, R.N.; Jameel, F.; Hanif, K.; Seemab, A.; Khan, A.Y.; Ahmed, M. Safety and Efficacy of Suprachoroidal Injection of Triamcinolone in Treating Macular Edema Secondary to Noninfectious Uveitis. Cureus 2021, 13, e20038.
- Munir, M.S.; Rehman, R.; Nazir, S.; Sharif, N.; Chaudhari, M.Z.; Saleem, S. Visual Outcome after Suprachoroidal Injection of Triamcinolone Acetate in Cystoid Macular Edema of Different Pathology. Pak. J. Med. Health Sci. 2022, 16, 164.
- Chen, J.; Wang, H.; Qiu, W. Intravitreal Anti-Vascular Endothelial Growth Factor, Laser Photocoagulation, or Combined Therapy for Diabetic Macular Edema: A Systematic Review and Network Meta-Analysis. Front. Endocrinol. 2023, 14, 1096105.
- Gao, L.; Zhao, X.; Jiao, L.; Tang, L. Intravitreal Corticosteroids for Diabetic Macular Edema: A Network Meta-Analysis of Randomized Controlled Trials. Eye Vis. 2021, 8, 35.
- Barakat, M.R.; Wykoff, C.C.; Gonzalez, V.; Hu, A.; Marcus, D.; Zavaleta, E.; Ciulla, T.A. Suprachoroidal CLS-TA plus Intravitreal Aflibercept for Diabetic Macular Edema: A Randomized, Double-Masked, Parallel-Design, Controlled Study. Ophthalmol. Retina 2021, 5, 60–70.
- Fazel, F.; Malekahmadi, M.; Feizi, A.; Oliya, B.; Tavakoli, M.; Fazel, M. Suprachoroidal Injection of Triamcinolone Acetonide plus Intravitreal Bevacizumab in Diabetic Macular Edema: A Randomized Pilot Trial. BMC Ophthalmol. 2023, 23, 40.
- Anwar, F.; Khan, A.A.; Majhu, T.M.; Javaid, R.M.M.; Ghaffar, M.T.; Bokhari, M.H. Comparison of Suprachoroidal Injection of Triamcinolone Acetonide Versus Intravitreal Bevacizumab in Primary Diabetic Macular Odema. Pak. J. Med. Health Sci. 2022, 16, 304.
- Zakaria, Y.G.; Salman, A.G.; Said, A.M.A.; Abdelatif, M.K. Suprachoroidal versus Intravitreal Triamcinolone Acetonide for the Treatment of Diabetic Macular Edema. Clin. Ophthalmol. 2022, 16, 733–746.
- Shaikh, K.; Ahmed, N.; Kazi, U.; Zia, A.; Aziz, M.Z. Comparison between Suprachoroidal Triamcinolone and Intravitreal Triamcinolone Acetonide in Patients of Resistant Diabetic Macular Edema. Pak. J. Ophthalmol. 2023, 39.
- Marashi, A.; Zazo, A. Suprachoroidal Injection of Triamcinolone Acetonide Using a Custom-Made Needle to Treat Diabetic Macular Edema Post Pars Plana Vitrectomy: A Case Series. J. Int. Med. Res. 2022, 50, 03000605221089807.
- Dhoot, D.S. Suprachoroidal Delivery of RGX-314 for Diabetic Retinopathy: The Phase II ALTITUDE™ Study. Investig. Ophthalmol. Vis. Sci. 2022, 63, 1152.
- Veiga Reis, F.; Dalgalarrondo, P.; da Silva Tavares Neto, J.E.; Wendeborn Rodrigues, M.; Scott, I.U.; Jorge, R. Combined Intravitreal Dexamethasone and Bevacizumab Injection for the Treatment of Persistent Diabetic Macular Edema (DexaBe Study): A Phase I Clinical Study. Int. J. Retina Vitr. 2023, 9, 13.
- Campochiaro, P.A.; Wykoff, C.C.; Brown, D.M.; Boyer, D.S.; Barakat, M.; Taraborelli, D.; Noronha, G.; Tanzanite Study Group. Suprachoroidal Triamcinolone Acetonide for Retinal Vein Occlusion: Results of the Tanzanite Study. Ophthalmol. Retina 2018, 2, 320–328.
- Clearside Biomedical’s TANZANITE Extension Study in Patients with Macular Edema Associated with Retinal Vein Occlusion Presented at the 40th Annual Macula Society Meeting|Clearside Biomedical, Inc.-IR Site. Available online: https://ir.clearsidebio.com/news-releases/news-release-details/clearside-biomedicals-tanzanite-extension-study-patients-macular (accessed on 15 July 2023).
- Clearside Biomedical, Inc. SAPPHIRE: A Randomized, Masked, Controlled Trial to Study the Safety and Efficacy of Suprachoroidal CLS-TA in Conjunction with Intravitreal Aflibercept in Subjects with Retinal Vein Occlusion; Clearside Biomedical, Inc.: Alpharetta, GA, USA, 2021.
- Clearside Biomedical, Inc. A Randomized, Masked, Controlled Trial to Study the Safety and Efficacy of Suprachoroidal CLS-TA in Combination with an Intravitreal Anti-VEGF Agent in Subjects with Retinal Vein Occlusion; Clearside Biomedical, Inc.: Alpharetta, GA, USA, 2021.
- Nawar, A.E. Modified Microneedle for Suprachoroidal Injection of Triamcinolone Acetonide Combined with Intravitreal Injection of Ranibizumab in Branch Retinal Vein Occlusion Patients. Clin. Ophthalmol. 2022, 16, 1139–1151.
- Ali, B.M.; Azmeh, A.M.; Alhalabi, N.M. Suprachoroidal Triamcinolone Acetonide for the Treatment of Macular Edema Associated with Retinal Vein Occlusion: A Pilot Study. BMC Ophthalmol. 2023, 23, 60.
- Muslim, I.; Chaudhry, N.; Javed, R.M.M. Effect of Supra-Choroidal Triamcinolone Injection on Best-Corrected Visual Acuity and Central Retinal Thickness in Patients with Macular Edema Secondary to Retinal Vein Occlusion. Pak. J. Ophthalmol. 2022, 38.
- Rizzo, S.; Ebert, F.G.; Bartolo, E.D.; Barca, F.; Cresti, F.; Augustin, C.; Augustin, A. Suprachoroidal drug Infusion for the Treatment of Severe Subfoveal Hard Exudates. Retina 2012, 32, 776.
- Abdelshafy, A. One Year Results for Suprachoroidal Triamcinolone Acetonide Injection in Various Retinal Diseases; Benha University: Benha, Egypt, 2022.
- Abdelshafy Tabl, A.; Tawfik Soliman, T.; Anany Elsayed, M.; Abdelshafy Tabl, M. A Randomized Trial Comparing Suprachoroidal and Intravitreal Injection of Triamcinolone Acetonide in Refractory Diabetic Macular Edema Due to Epiretinal Membrane. J. Ophthalmol. 2022, 2022, 7947710.
- Zhang, D.-D.; Che, D.-Y.; Zhu, D.-Q. A Simple Technique for Suprachoroidal Space Injection of Triamcinolone Acetonide in Treatment of Macular Edema. Int. J. Ophthalmol. 2022, 15, 2017–2021.
- Oli, A.; Waikar, S. Modified Inexpensive Needle for Suprachoroidal Triamcinolone Acetonide Injections in Pseudophakic Cystoid Macular Edema. Indian J. Ophthalmol. 2021, 69, 765–767.
- Marashi, A.; Zazo, A. A Manually Made Needle for Treating Pseudophakic Cystoid Macular Edema by Injecting Triamcinolone Acetonide in the Suprachoroidal Space: A Case Report. Am. J. Ophthalmol. Case Rep. 2022, 25, 101254.
- Abdelshafy, A. Suprachoroidal Triamcinolone Acetonide Injection in Two Chorioretinal Diseases: One Year Results; Benha University: Benha, Egypt, 2022.





