
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lisa Zeußel | -- | 1657 | 2023-10-09 09:24:22 | | | |
| 2 | Wendy Huang | Meta information modification | 1657 | 2023-10-09 12:00:45 | | | | |
| 3 | Wendy Huang | + 1 word(s) | 1658 | 2023-10-12 05:06:54 | | |
Video Upload Options
Bioactive amines are highly relevant for clinical and industrial application to ensure the metabolic status of a biological process. Apart from this, generally, amine identification is a key step in various bioorganic processes ranging from protein chemistry to biomaterial fabrication. However, many amines have a negative impact on the environment and the excess intake of amines can have tremendous adverse health effects. Thus, easy, fast, sensitive, and reliable sensing methods for amine identification are strongly searched for. A newly emerging activated furan, Meldrum’s acid furfural conjugate (MAFC), or otherwise called MAF, can be used as a colorimetric detecting agent for primary and secondary amines, which can be easily synthesized via the Knoevenagel condensation of simple and inexpensive precursors, 2-furfural and Meldrum’s acid.
1. Introduction
2. Furan-Containing Sensors
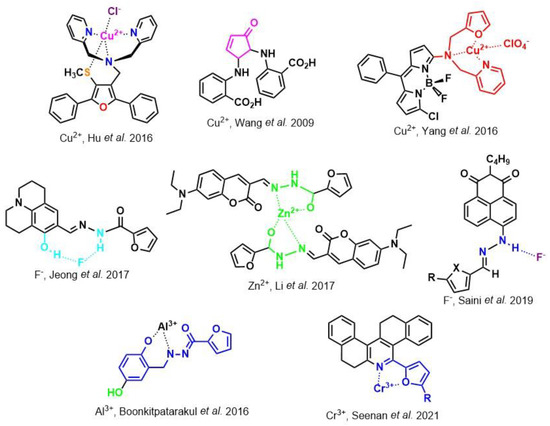
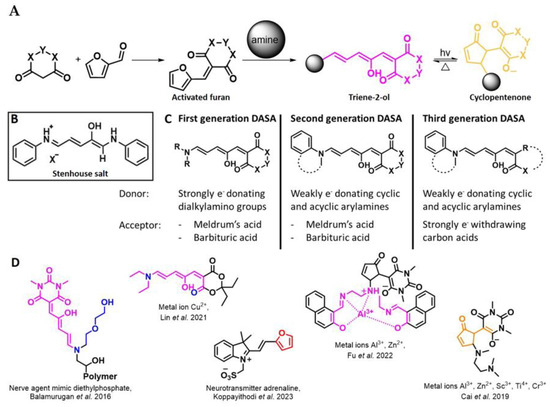
References
- Wang, L.; Ran, X.; Tang, H.; Cao, D. Recent advances on reaction-based amine fluorescent probes. Dye. Pigment. 2021, 194, 109634.
- Andre, R.S.; Mercante, L.A.; Facure, M.H.M.; Sanfelice, R.C.; Fugikawa-Santos, L.; Swager, T.M.; Correa, D.S. Recent Progress in Amine Gas Sensors for Food Quality Monitoring: Novel Architectures for Sensing Materials and Systems. ACS Sens. 2022, 7, 2104–2131.
- Danchuk, A.I.; Komova, N.S.; Mobarez, S.N.; Doronin, S.Y.; Burmistrova, N.A.; Markin, A.V.; Duerkop, A. Optical sensors for determination of biogenic amines in food. Anal. Bioanal. Chem. 2020, 412, 4023–4036.
- Grant, M.J.; Wolfe, K.M.; Harding, C.R.; Welch, G.C. Biogenic amine sensors using organic π-conjugated materials as active sensing components and their commercialization potential. J. Mater. Chem. C 2023, 11, 9749–9767.
- Vasconcelos, H.; Coelho, L.C.C.; Matias, A.; Saraiva, C.; Jorge, P.A.S.; de Almeida, J.M.M.M. Biosensors for Biogenic Amines: A Review. Biosensors 2021, 11, 82.
- Gomes Müller, D.; Quadro Oreste, E.; Grazielle Heinemann, M.; Dias, D.; Kessler, F. Biogenic amine sensors and its building materials: A review. Eur. Polym. J. 2022, 175, 111221.
- Joullié, M.M.; Thompson, T.R.; Nemeroff, N.H. Ninhydrin and ninhydrin analogs. Syntheses and applications. Tetrahedron 1991, 47, 8791–8830.
- Brunelle, E.; Huynh, C.; Le, A.M.; Halámková, L.; Agudelo, J.; Halámek, J. New Horizons for Ninhydrin: Colorimetric Determination of Gender from Fingerprints. Anal. Chem. 2016, 88, 2413–2420.
- Oden, S.; Von Hofsten, B. Detection of fingerprints by the ninhydrin reaction. Nature 1954, 173, 449–450.
- Beckwith, A.C.; Paulis, J.W.; Wall, J.S. Direct estimation of lysine in corn meals by the ninhydrin color reaction. J. Agric. Food Chem. 1975, 23, 194–196.
- Leaf, G. Identification and estimation of ninhydrin–positive substances in physio-logical fluids. Proc. Nutr. Soc. 1970, 29, 105–106.
- Li, Z.; Chang, S.; Lin, L.; Li, Y.; An, Q. A colorimetric assay of 1-aminocyclopropane-1-carboxylate (ACC) based on ninhydrin reaction for rapid screening of bacteria containing ACC deaminase. Lett. Appl. Microbiol. 2011, 53, 178–185.
- Abdellatef, H.E.; Khalil, H.M. Colorimetric determination of gabapentin in pharmaceutical formulation. J. Pharm. Biomed. Anal. 2003, 31, 209–214.
- Picton, S.F.; McCluskey, J.T.; Flatt, P.R.; McClenaghan, N.H. Effects of cytotoxic agents on functional integrity and antioxidant enzymes in clonal beta-cells. Diabetes Metab. 2002, 28, 3S70–3S77; discussion 3S108–3S112.
- Friedman, M. Applications of the ninhydrin reaction for analysis of amino acids, peptides, and proteins to agricultural and biomedical sciences. J. Agric. Food Chem. 2004, 52, 385–406.
- Dietzen, D.J.; Weindel, A.L.; Carayannopoulos, M.O.; Landt, M.; Normansell, E.T.; Reimschisel, T.E.; Smith, C.H. Rapid comprehensive amino acid analysis by liquid chromatography/tandem mass spectrometry: Comparison to cation exchange with post-column ninhydrin detection. Rapid Commun. Mass Spectrom. 2008, 22, 3481–3488.
- Ajayan, K.; Sainath, S.; Sadik, A.; Nair, M.M.; Nair, A.M.; Karthika, K.S.; Vijayakumar, A.; Nair, S.S.; Nair, B.; Chandran R, P.; et al. Bioconjugation of Meldrum’s acid activated furan: A detergent compatible assay for protein quantitation. Anal. Biochem. 2023, 662, 114998.
- Fu, P.; Yan, Q.; Wang, S.; Wu, H.; Cao, D. A visible-light-gated donor–acceptor Stenhouse adduct chemosensor: Synthesis, photochromism and naked-eye colorimetric/fluorometric sensing of Al3+ and Zn2+. New J. Chem. 2022, 46, 12600–12608.
- Kumpf, J.; Freudenberg, J.; Fletcher, K.; Dreuw, A.; Bunz, U.H.F. Detection of amines with extended distyrylbenzenes by strip assays. J. Org. Chem. 2014, 79, 6634–6645.
- Mani, P.; Ojha, A.A.; Reddy, V.S.; Mandal, S. “Turn-on” Fluorescence Sensing and Discriminative Detection of Aliphatic Amines Using a 5-Fold-Interpenetrated Coordination Polymer. Inorg. Chem. 2017, 56, 6772–6775.
- Hu, R.; Lin, S.; Hu, D.; Huang, H.; Wang, M.; Li, R.; Tian, M.; Shuai, Z.; Wei, Y. “Turn-on” fluorescence sensor for organic amines fabricated via sustainable processing. Mater. Chem. Front. 2022, 7, 153–159.
- Verbitskiy, E.V.; Kvashnin, Y.A.; Baranova, A.A.; Khokhlov, K.O.; Chuvashov, R.D.; Schapov, I.E.; Yakovleva, Y.A.; Zhilina, E.F.; Shchepochkin, A.V.; Makarova, N.I.; et al. Synthesis and characterization of linear 1,4-diazine-triphenylamine-based selective chemosensors for recognition of nitroaromatic compounds and aliphatic amines. Dye. Pigment. 2020, 178, 108344.
- Givanoudi, S.; Heyndrickx, M.; Depuydt, T.; Khorshid, M.; Robbens, J.; Wagner, P. A Review on Bio- and Chemosensors for the Detection of Biogenic Amines in Food Safety Applications: The Status in 2022. Sensors 2023, 23, 613.
- Gomes, R.F.A.; Coelho, J.A.S.; Afonso, C.A.M. Synthesis and Applications of Stenhouse Salts and Derivatives. Chemistry 2018, 24, 9170–9186.
- Saini, N.; Wannasiri, C.; Chanmungkalakul, S.; Prigyai, N.; Ervithayasuporn, V.; Kiatkamjornwong, S. Furan/thiophene-based fluorescent hydrazones as fluoride and cyanide sensors. J. Photochem. Photobiol. A Chem. 2019, 385, 112038.
- Hu, Y.; Ke, Q.; Yan, C.; Xu, C.-H.; Huang, X.-H.; Hu, S. A new fluorescence chemosensor for selective detection of copper ion in aqueous solution. Tetrahedron Lett. 2016, 57, 2239–2243.
- Wang, Q.-L.; Zhang, H.; Jiang, Y.-B. A highly selective and sensitive turn-on catalytic chemodosimeter for Cu2+ in aqueous solution. Tetrahedron Lett. 2009, 50, 29–31.
- Yang, C.; Gong, D.; Wang, X.; Iqbal, A.; Deng, M.; Guo, Y.; Tang, X.; Liu, W.; Qin, W. A new highly copper-selective fluorescence enhancement chemosensor based on BODIPY excitable with visible light and its imaging in living cells. Sens. Actuators B Chem. 2016, 224, 110–117.
- Jeong, H.Y.; Lee, S.Y.; Kim, C. Furan and Julolidine-Based “Turn-on” Fluorescence Chemosensor for Detection of F- in a Near-Perfect Aqueous Solution. J. Fluoresc. 2017, 27, 1457–1466.
- Li, C.-R.; Li, S.-L.; Yang, Z.-Y. Development of a coumarin-furan conjugate as Zn2+ ratiometric fluorescent probe in ethanol-water system. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 174, 214–222.
- Boonkitpatarakul, K.; Wang, J.; Niamnont, N.; Liu, B.; Mcdonald, L.; Pang, Y.; Sukwattanasinitt, M. Novel Turn-On Fluorescent Sensors with Mega Stokes Shifts for Dual Detection of Al3+ and Zn2+. ACS Sens. 2016, 1, 144–150.
- Seenan, S.; Manickam, S.; Kulathu Iyer, S. A new furan based fluorescent chemosensor for the recognition of Cr3+ ion and its application in real sample analysis. J. Photochem. Photobiol. A Chem. 2021, 418, 113441.
- Stenhouse, J. Ueber Furfuranilin und Furfurtoluidin. Ann. Chem. Pharm. 1870, 156, 197–205.
- Henschke, J.P.; Liu, Y.; Huang, X.; Chen, Y.; Meng, D.; Xia, L.; Wei, X.; Xie, A.; Li, D.; Huang, Q.; et al. The Manufacture of a Homochiral 4-Silyloxycyclopentenone Intermediate for the Synthesis of Prostaglandin Analogues. Org. Process Res. Dev. 2012, 16, 1905–1916.
- Ulbrich, K.; Kreitmeier, P.; Reiser, O. Microwave- or Microreactor-Assisted Conversion of Furfuryl Alcohols into 4-Hydroxy-2-cyclopentenones. Synlett 2010, 2010, 2037–2040.
- Michalak, K.; Wicha, J. An Enantioselective Total Synthetic Approach to (+)-Heptemerone G and (+)-Guanacastepene A from 2-Furyl Methyl Carbinol. Synlett 2013, 24, 1387–1390.
- Li, S.-W.; Batey, R.A. Mild lanthanide(III) catalyzed formation of 4,5-diaminocyclopent-2-enones from 2-furaldehyde and secondary amines: A domino condensation/ring-opening/electrocyclization process. Chem. Commun. 2007, 3759–3761.
- Helmy, S.; Leibfarth, F.A.; Oh, S.; Poelma, J.E.; Hawker, C.J.; Read de Alaniz, J. Photoswitching using visible light: A new class of organic photochromic molecules. J. Am. Chem. Soc. 2014, 136, 8169–8172.
- Sroda, M.M.; Stricker, F.; Peterson, J.A.; Bernal, A.; Read de Alaniz, J. Donor-Acceptor Stenhouse Adducts: Exploring the Effects of Ionic Character. Chemistry 2021, 27, 4183–4190.
- Huang, Y.; Du, Y.; Yuan, L.; Chu, Z.; He, L. Donor-acceptor Stenhouse adducts as new emerging photoswitches: Synthesis, light-responsive properties, and applications in polymers science. J. Macromol. Sci. Part A 2021, 58, 717–724.
- Balamurugan, A.; Lee, H. A Visible Light Responsive On-Off Polymeric Photoswitch for the Colorimetric Detection of Nerve Agent Mimics in Solution and in the Vapor Phase. Macromolecules 2016, 49, 2568–2574.
- Koppayithodi, S.; Jana, P.; Bandyopadhyay, S. Highly Selective and Quantitative Point-of-Care Diagnostic Method for Adrenaline. Chemistry 2023, 29, e202300327.
- Cai, Y.-D.; Chen, T.-Y.; Chen, X.Q.; Bao, X. Multiresponsive Donor-Acceptor Stenhouse Adduct: Opportunities Arise from a Diamine Donor. Org. Lett. 2019, 21, 7445–7449.
- Lin, Z.; Wang, S.; Yan, Q.; Yan, Q.; Cao, D. Visible light/Cu2+ dual triggered photochromic donor-acceptor stenhouse adducts: Synthesis, properties and applications. Dye. Pigment. 2021, 191, 109384.




