Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bangxi Zhang | -- | 2510 | 2023-10-09 05:29:52 | | | |
| 2 | Rita Xu | Meta information modification | 2510 | 2023-10-09 05:47:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, X.; Fu, T.; Li, H.; Zhang, B.; Li, W.; Zhang, B.; Wang, X.; Wang, J.; Chen, Q.; He, X.; et al. Morel in Heavy Metal-Contaminated Soils. Encyclopedia. Available online: https://encyclopedia.pub/entry/49944 (accessed on 07 February 2026).
Li X, Fu T, Li H, Zhang B, Li W, Zhang B, et al. Morel in Heavy Metal-Contaminated Soils. Encyclopedia. Available at: https://encyclopedia.pub/entry/49944. Accessed February 07, 2026.
Li, Xue, Tianhong Fu, Hongzhao Li, Bangxi Zhang, Wendi Li, Baige Zhang, Xiaomin Wang, Jie Wang, Qing Chen, Xuehan He, et al. "Morel in Heavy Metal-Contaminated Soils" Encyclopedia, https://encyclopedia.pub/entry/49944 (accessed February 07, 2026).
Li, X., Fu, T., Li, H., Zhang, B., Li, W., Zhang, B., Wang, X., Wang, J., Chen, Q., He, X., Chen, H., Zhang, Q., Zhang, Y., Yang, R., & Peng, Y. (2023, October 09). Morel in Heavy Metal-Contaminated Soils. In Encyclopedia. https://encyclopedia.pub/entry/49944
Li, Xue, et al. "Morel in Heavy Metal-Contaminated Soils." Encyclopedia. Web. 09 October, 2023.
Copy Citation
Morel is a popular edible mushroom with considerable medicinal and economic value which has garnered global popularity. Given the susceptibility of morels to HM accumulation, the quality and output of morels are at risk, posing a serious food safety concern that hinders the development of the morel industry.
heavy metals
mitigation strategies
morel
1. Introduction
Morel (Morchella spp., Pezizales, Ascomycota) is a macro fungus with a distinctive fruiting body full of stomata [1]. Known for its high nutritional and medicinal value, morel is one of the most valuable medicinal mushrooms worldwide [2][3]. It is a rich source of high-quality protein, various amino acids, unsaturated fatty acids, polysaccharides, and multiple mineral elements [4][5]. Morel has been found to have important effects on the kidneys and liver, as well as antibacterial, anti-inflammatory, antioxidant, and anti-diabetic properties [6][7][8][9][10]. In addition to its medicinal value, morel has significant economic importance due to its worldwide distribution, with prominent populations found in China, the United States, France, Spain, and Turkey, as well as in Peru, Ecuador, Venezuela, and India [4][11][12][13].
Morel was once a soil-saprotrophic ascomycete mushroom that can now be cultivated routinely in farmland soils [14]. The artificial cultivation of morels started in the United States and was cultivated on a large scale in China. In 2021, the global export value of morel reached USD 9.6 billion, with China being the largest exporter. The artificial cultivation area of morel in China has grown from less than 10,000 acres to more than 200,000 acres, with cultivation areas in more than 20 provinces across the country [15]. Cultivation morel is an attractive and profitable industry due to its low cultivation costs, high production value, and better economic benefits compared to other edible mushrooms that require costly and complex cultivation systems. In addition, morels have unique and delicate flavors and textures, which makes them a popular ingredient in high-end cuisine worldwide.
Morels always use the soil as the substrate for forming fruiting bodies. Therefore, the entire lifecycle of morel, including mycelial growth, primordia formation, and fruiting body development, takes place within the soil environment. Meanwhile, morels have a positive impact on soil fertility and soil microbial community structure. Soil microorganisms such as Arthrobacter, Bradyhizobium, Devosia, Pseudarthrobacter, Pseudolabrys, and Nitrospira that grow around morels have nitrogen fixation and nitrification abilities [16]. In addition, morels improve soil microbial community structure, which contributes to soil remediation and improves soil use efficiency.
The proliferation of human activities and accelerated industrialization has engendered the proliferation of heavy metal (HM) contamination in soil, as corroborated by Adnan, et al. [17]. The contamination of soil with HMs is a potential menace to the production and quality of morels, food safety, and human health, as HMs are not biodegradable and persistently accumulate in the soil [18]. The 2014 National Soil Pollution Status Survey Bulletin posits that the total exceedance rate of soil in China was 16.1%, with arable, forest, and grassland soil sites having exceedance rates of 19.4%, 10.0%, and 10.4%, respectively. Cadmium (Cd), arsenic (As), and copper (Cu) have been identified as the most widespread HM contaminants in the region. The Cd limit of morel was 0.6 mg kg−1, and As limit was 0.5 mg kg−1 according to GB 2762-2022 in China [19]. The escalation of soil HM contamination has led to HM contamination of morels in some areas, as exemplified by HM content testing of 59 batches of morels in Qianxinan, Guizhou, where Cd exceeded the limit by 33.9% and chromium by 1.7% [20]. Similarly, morels collected from apple orchards contaminated with lead (Pb) arsenate in the eastern United States exceeded the standard for both Pb and As [21]. The HM content of morel is higher compared to some plants and other edible mushrooms [22][23][24][25]. Morel is highly susceptible to soil HM content, and the HM content in the fruiting bodies is positively correlated with the HM content in the soil [26]. The uptake and biosorption of HMs in morel lead to their enrichment in various forms, such as compartmentalization, exclusion, complexity rendering, and so on [25][27][28].
In the pursuit of reducing HM accumulation in mushrooms, many research studies have focused on optimizing cultivation substrates and methods, as well as the incorporation of beneficial metal elements. For example, Weng, et al. [29] utilized forage as an alternative to wood chips in the cultivation process, which resulted in a significant decrease in HM levels including Cd, Pb, and Cr, while also improving the overall quality of the mushrooms. Jiang, et al. [30] introduced selenium and lanthanum complex reagents (Na2SeO3 87.619 mg kg−1, LaCl3 70.670 mg kg−1) to the growth medium of A. brasiliensis, leading to a reduction of HMs such as Pb, Cd, Hg, and As in the fruiting bodies by 11.58%, 55.24%, 46.43%, and 52.38%, respectively. Some HM binding sites on the cell wall of morel are non-specific, and Cd2+, Zn2+, and Mg2+ are usually taken up by the same transporter [31][32]. Se, Zn, or Mg can form complexes with Cd in soil, which exhibit relatively stable structures and ultimately reduce substrate uptake and cellular uptake of Cd [33][34]. However, the sources of HMs in morels also include soil, atmosphere, and water (Figure 1). The most direct and effective solution to reduce HM accumulation in morels is to minimize soil contamination. Various techniques for soil HM remediation, including physical, chemical, and bioremediation approaches, have been well-established and widely implemented.
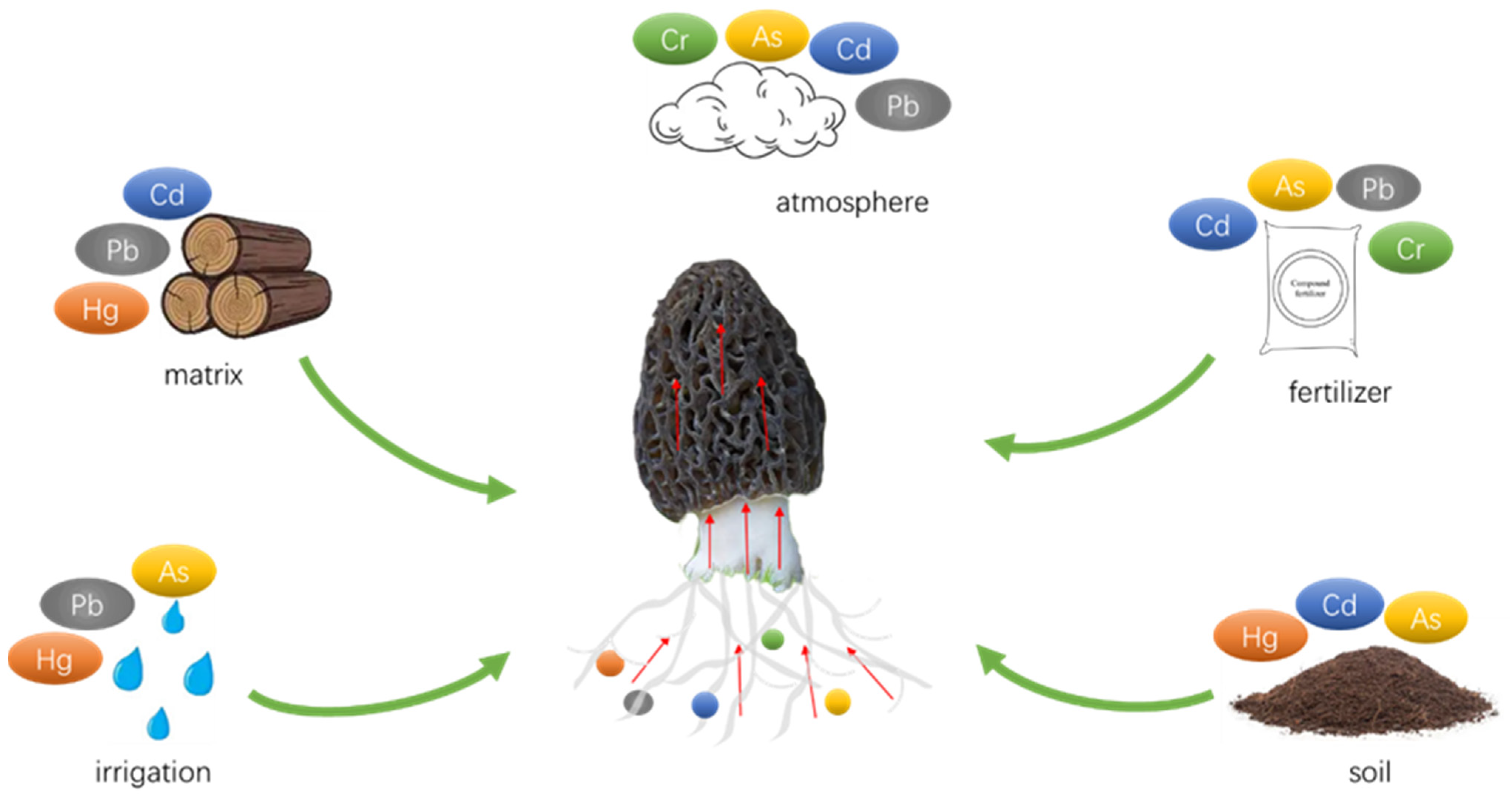
Figure 1. Sources of heavy metals in morel.
Up to now, there are 59 reviews of morels on Google Scholar, including 54 articles and 5 books, mainly covering the classification of morels, the progress of artificial cultivation, fungal diseases, genetics and systematics, chemical composition, and pharmacological effects, but there are no reports on HM control strategies of morels, as shown in Figure 2. However, there are 237 articles related to “morel” and “HMs” in PubMed, and HM pollution control technology in morels has received wide interest. Therefore, the research summarizes the mechanisms of HMs that are easily enriched during the cultivation of morels and evaluates soil passivation technology, microbial remediation, strain screening and cultivation, and agronomic measures as potential ways to prevent HM pollution. The importance of establishing a comprehensive system to prevent HM pollution in morel was emphasized.
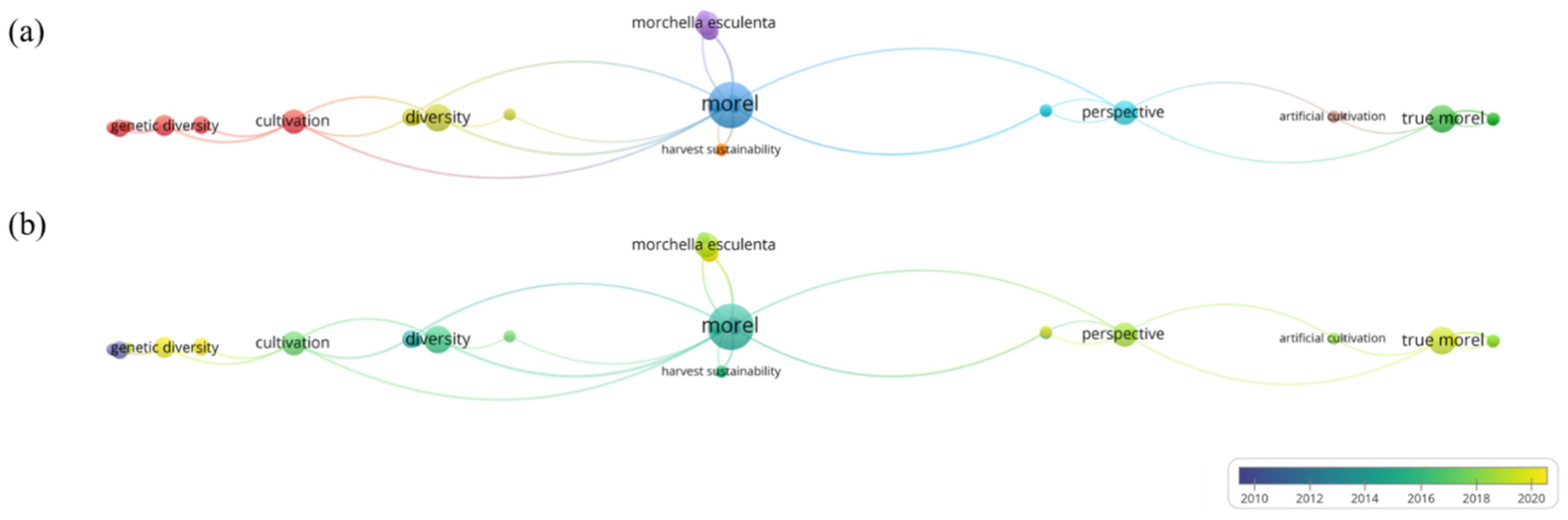
Figure 2. Bibliometric analysis of the theme. (a) Topic distribution. The map shows three clusters. Orange clusters represent harvest sustainability. Red and brown clusters involve artificial cultivation, growth characteristics, and gene diversity. Blue and green clusters represent the variety and perspective of morel. (b) Trend topic network diagram based on keywords used from January 2000 to December 2020. Indicators show the current publication from blue to green. The size of the circle represents the frequency of keywords. The distance between the two circles indicates their correlation.
2. Morel Varieties and Cultivation Methods
2.1. Main Morel Varieties
In Europe, Asia, and North America, a diverse range of morel species can be found, with 34, 32, and 21 species, respectively [35]. China, particularly Yunnan, Sichuan, Guizhou, Chongqing, and Tibet, is a center of species diversity of morels, with several species widely distributed in the region [36][37][38][39][40]. Notable species include M. esculenta, M. crassipes, M. spongiola, M. conica, M. elata, and other species [41][42]. However, due to seasonal and quantity limitations, it is challenging to meet the demand for wild morels, which fruit primarily in the spring season, with a few species fruit in summer or autumn [43]. Morels have been observed to emerge in the autumn in Israel and the Southwest Himalayas; the species and timing of their emergence are erratic and may be influenced by local environmental factors such as precipitation, temperature, or the life cycle of morels [44][45][46]. Consequently, the artificial cultivation of morels has become a popular research topic. Artificially grown morels, which are similar to wild morels in nutritional value but contain lower levels of HMs, are safer and more cost-effective [47]. Currently, there are nine successfully domesticated cultivars of morels, including Esculenta Clade (M. conica, M. angusticeps, M. importuna, M. sextelata, M. eximia), Esculenta Clade (M. cassipes, M. esculenta, M. deliciosa), and Rufobrunnea Clade (M. rufobrunnea) [48]. Of these, only M. importuna, M. sextelata, M. eximia, and M. rufobrunnea have been cultivated on a large scale for commercial purposes, with M. importuna being the most widely cultivated species globally and accounting for over 95% of the total cultivated area [49].
2.2. Cultivation Methods
Since 2012, the cultivation of morels has been making steady progress, with a new emphasis on field and forest cultivation through exogenous nutrient bag technology [48]. Field cultivation is a smart and cost-effective option, but it comes with its fair share of challenges, as it can be vulnerable to the whims of nature—think sudden temperature changes, drought, and gale-force winds. However, understory planting in evergreen forests with crown densities above 80%, such as fir forests, viburnum forests, and citrus forests, can mitigate these risks. More importantly, we should stay away from industrially developed farmland soil and avoid surrounding mines. Reducing HM pollution sources is one of the effective means to reduce the content of HMs in morels.
Sowing methods and soil conditions are also crucial for successful cultivation. Common sowing methods include furrow, spreading, and hole sowing, as shown in Figure 3, and nutrient bags are typically spaced 20–30 cm apart after 7–15 days of sowing [50][51][52]. In Chongqing, Li, et al. [53] found that the content of HMs in edible fungi collected in different seasons was also different. The content of HMs in Volvariella volvacea, Pleurotus ostreatus, Lentinula edodes, and Flammulina velutipes was high in winter and lowest in spring. But the best time to sow a wide range of M. importuna is in October, with a harvest period from February to March, because the fungi are known for their love of water and low-temperature tolerance, with an optimal growth temperature of 25 °C [54][55]. Fortunately, with the development of science and technology, intelligent mushroom houses can make the cultivation of morel without a time limit. Traditional field cultivation requires a soft, flat terrain position with favorable water and clay or sandy soil mixed with humus soil, which provides the necessary nutrients and space for morel mycelial growth [35][48].
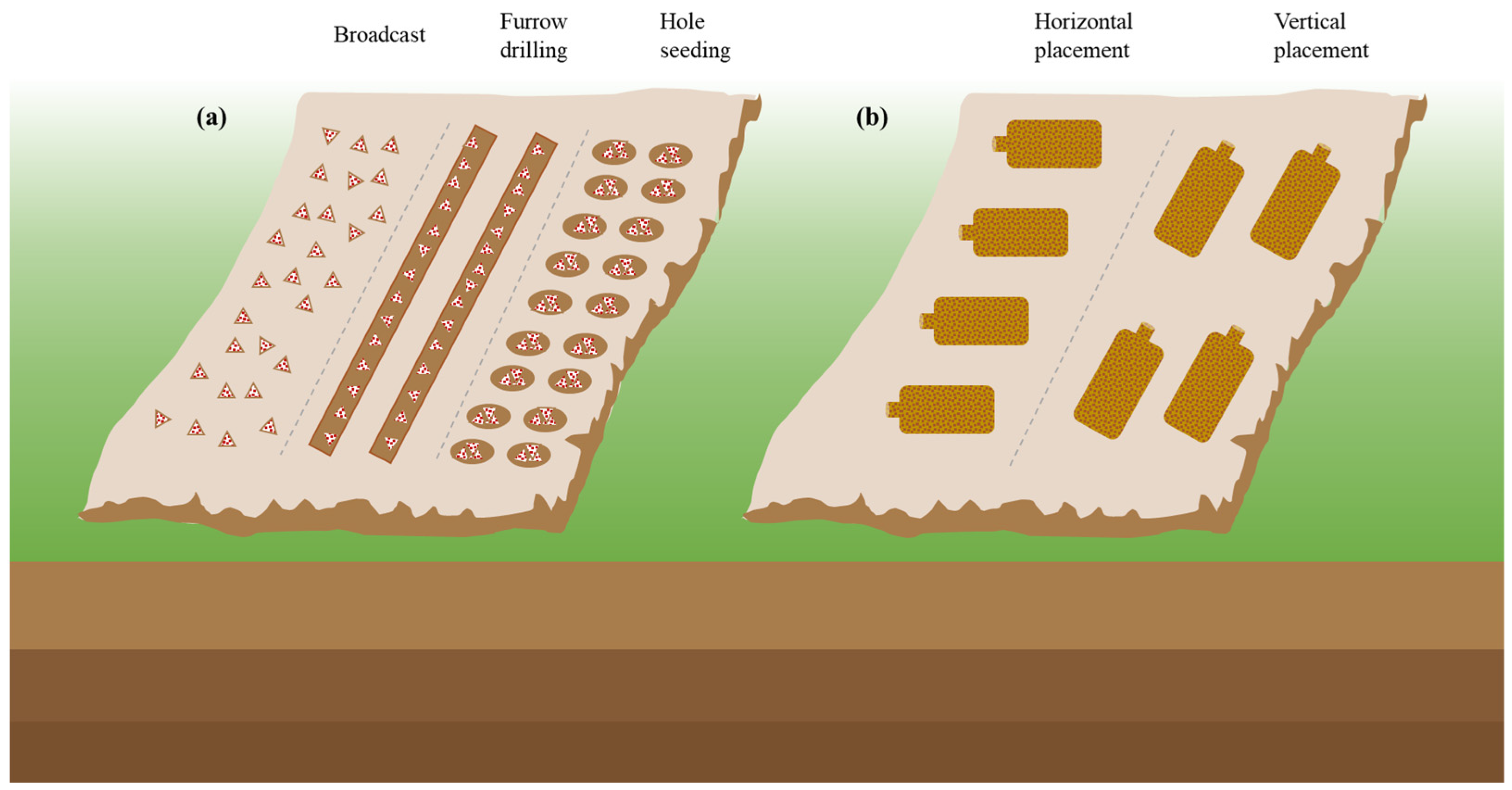
Figure 3. (a) The sowing methods and (b) nutrition bag placement.
Industrial cultivation of morels can be achieved through indoor or outdoor cultivation, each with its respective advantages and disadvantages [56]. The HM content of mushrooms grown under different cultivation methods is also different. For example, soilless cultivation of Grifola frondosa can effectively block the enrichment of Pb by its fungi [57]. The growth of Cordyceps militaris fruit bodies exhibited a proportional inhibition in response to the presence of Pb, Hg, and Cd in the growth medium, displaying a dose-dependent relationship [58]. For morels, the Cd content under different cultivation modes can be ordered from high to low as a soil covering cultivation > layer frame cultivation > oblique insertion cultivation [59]. At the same time, we should avoid the occurrence of HMs in the cultivation matrix. As the initial site of mycelium development, mushrooms showed a higher tendency to absorb cadmium from the matrix [60]. Equally important is the raw material of the nutrition bag, which must ensure that there is no HM pollution source; otherwise, it will directly lead to the pollution of morel.
3. Mechanisms of HMs Uptake by Morels
Several studies have highlighted the presence of HM enrichment in morel, including Cd, Cr, As, Pb, and Hg [61][62][63]. For instance, Mohammad, et al. [24] observed bioaccumulation factors (BF) of HMs in M. esculenta to be Cd (0.84), Cu (0.8), Co (0.69), Pb (0.61), Ni (0.6), Mn (0.51), and Cr (0.3). Despite these findings, the intricate process of HM uptake by morel remains unknown. This may be attributed to the fact that morel requires specific elements to promote its growth or initiate a series of self-defense mechanisms to mitigate the harm caused by HMs [62]. For instance, Fe has been found to promote the growth of M. conica mycelium and the formation of fruiting bodies, while Zn application increases the content of amino acids in fruiting bodies. Additionally, HMs may exist in various forms within the cell. In a recent study, Xiong, et al. [64] employed HPLC-ICP-MS to examine various mercury forms in rambutan, indicating that morel’s uptake of Hg involves four forms: methylmercury, ethylmercury, inorganic mercury, and phenylmercury. Nevertheless, no comprehensive research has been conducted to explicate the underlying mechanisms of HM enrichment in this fungus. Existing studies suggest that morel’s HM enrichment involves two processes: (i) cellular active uptake, which is dependent on cell metabolism, and (ii) biosorption, which is not dependent on cellular metabolism and includes extracellular accumulation, cell surface adsorption, and intracellular accumulation. These findings are consistent with previous observations on HM enrichment by mushrooms.
3.1. Cellular Active Absorption
Morels have various protection mechanisms against HMs stress like compartmentalization, exclusion, complexity rendering, and the synthesis of binding proteins, including phytochelatins (PCs), metallothioneins (MTs), Cd-binding peptides (Cd-BPs), cysteines (Cys), and histidines (HIs) [65]. They play crucial roles in the signaling, uptake, detoxification, and accumulation of metal [66]. HMs that enter the cell can be sequestered in vesicles through the action of various PCs, MTs, glutathione (GSH), or specific metal-binding ligands, as shown in Figure 4 [67][68][69]. This mechanism reduces HM toxicity within the cell, which is a major contributor to HM enrichment in the mycelium. PCs and MTs are classes of Cys-rich proteins that bind HMs with high affinity and form complexes segregated into vesicles in plants and fungi [70][71][72].
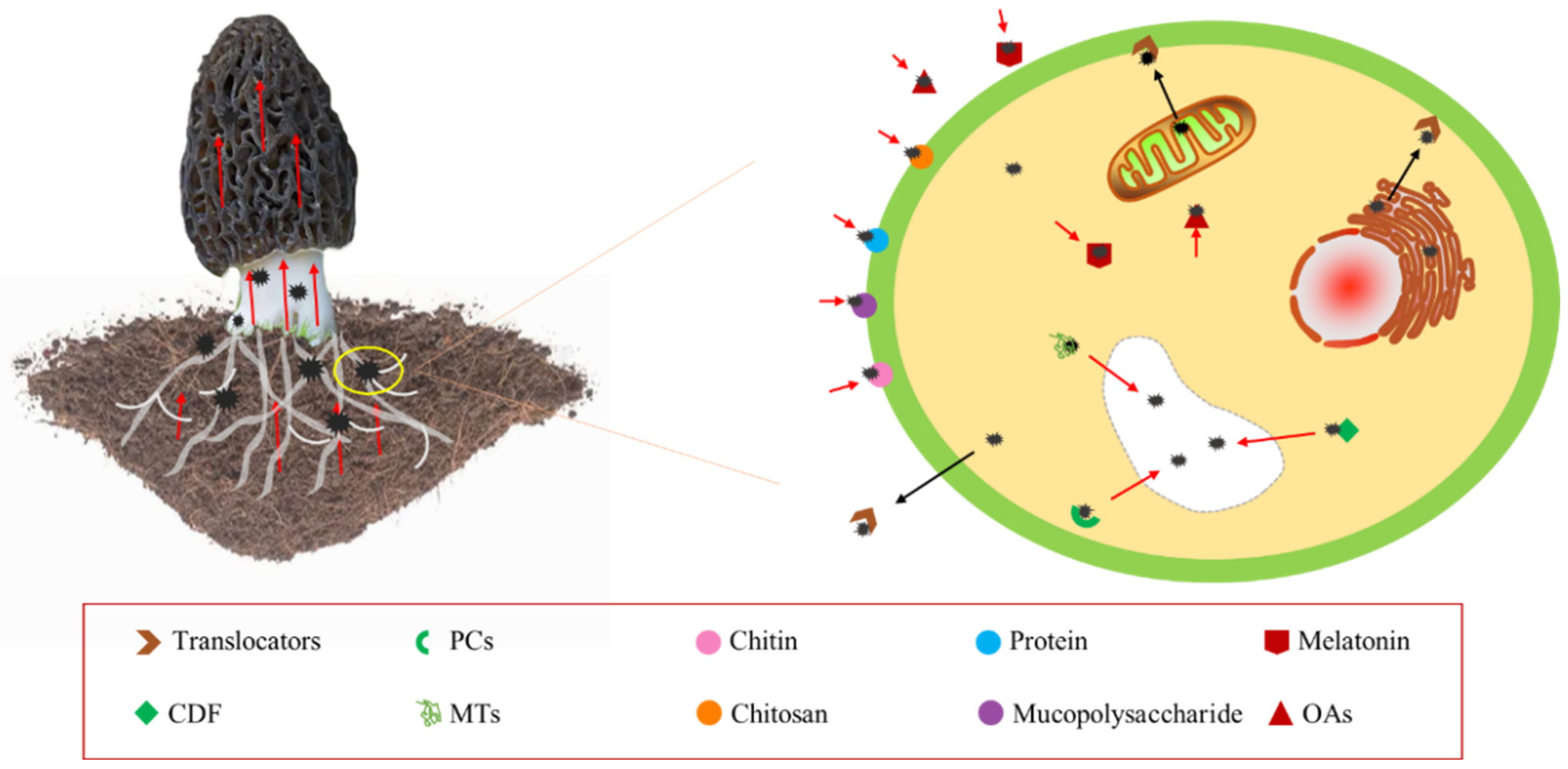
Figure 4. Enrichment mechanism of heavy metals by morel.
Intracellular signaling molecules, such as hydrogen sulfide (H2S), also play a role in HM uptake. H2S has been found to activate Cd2+/H reverse transporter proteins on the vesicle membrane, resulting in increased Cd sequestration in the vesicle [73]. Moreover, cation diffusion facilitators (CDFs) have been identified as a recently discovered family of proteins responsible for HM ion transport. CDFs are capable of transporting a single cation or multiple divalent cations in many cases [74][75]. In the yeast S. cerevisiae, CDFs transport metals from the cytoplasm to the vesicles [76].+
3.2. Biosorption
The uptake of HMs by morel involves physicochemical interactions of functional groups present on the cell surface, which allow for electrostatic adsorption, ion exchange, precipitation, and complexation without requiring cell metabolism, as illustrated in Figure 4 [77][78][79]. In Cd stress, 50% of Cd is bound to the cell wall, 30% remains in the cytoplasm, and 20% is translocated to the vesicles [80]. The cell wall’s major components, such as titin, chitosan, mucopolysaccharides, and proteins, form chelates with HMs by providing adsorption, ion exchange, and covalent binding sites, including carboxyl, hydroxyl, sulfhydryl, amino, and phosphate groups [81]. HMs are immobilized on the cell surface through ion exchange, complexation, and precipitation [82][83][84]. Chitin, a biopolymer found in the shells of marine crustaceans and fungal cell walls, has been used to extract HMs such as Cu, Zn, Cd, Ni, and Pb from water, owing to its ability to bind HMs through amino and hydroxyl groups [85][86][87][88]. The chitin content of morel has been reported to be around 16% [89].
In addition to these mechanisms, Liu, et al. [90] observed that a cysteine-rich hydrophobic protein on the morel cell wall can form chelates with HM ions due to the presence of sulfhydryl groups in cysteine. Under HM stress, the cell wall secretes melatonin and organic acids that bind to HMs, thereby reducing stress [91]. Laccase, widely present in morel cells, is an enzyme that catalyzes melanin synthesis [92]. Wang, et al. [93] found that laccase activity presented downtrends as the concentration of Cd increased, which might be the promotion of more laccase synthesis of melatonin under Cd stress. Higher laccase levels catalyze the synthesis of melanin, which deposits HMs outside the cells.
References
- Hibbett, D.S.; Binder, M.; Bischoff, J.F.; Blackwell, M.; Cannon, P.F.; Eriksson, O.E.; Huhndorf, S.; James, T.; Kirk, P.M.; Lücking, R.; et al. A higher-level phylogenetic classification of the Fungi. Mycol. Res. 2007, 111, 509–547.
- Sayeed, R.; Kausar, S.; Thakur, M. Morchella esculenta Fr.: Biodiversity, sustainable conservation, marketing and ethno-mycological studies on medicinal fungus from Kashmir Himalayas, India. Mushroom Res. 2018, 27, 77–86.
- Ajmal, M.; Akram, A.; Ara, A.; Akhund, S.; Nayyar, B.G. Morchella esculenta: An edible and health beneficial mushroom. Pak. J. Food Sci. 2015, 25, 71–78.
- Sambyal, K.; Singh, R.V. A comprehensive review on Morchella importuna: Cultivation aspects, phytochemistry, and other significant applications. Folia Microbiol. 2021, 66, 147–157.
- Cheung, P.C.-K. Mushrooms as Functional Foods; Wiley Online Books: Hoboken, NJ, USA, 2008.
- Pan, X.; Meng, J.; Xu, L.; Chang, M.; Feng, C.; Geng, X.; Cheng, Y.; Guo, D.; Liu, R.; Wang, Z.; et al. In-depth investigation of the hypoglycemic mechanism of Morchella importuna polysaccharide via metabonomics combined with 16S rRNA sequencing. Int. J. Biol. Macromol. 2022, 220, 659–670.
- Begum, N.; Nasir, A.; Parveen, Z.; Muhammad, T.; Ahmed, A.; Farman, S.; Jamila, N.; Shah, M.; Bibi, N.S.; Khurshid, A. Evaluation of the hypoglycemic activity of Morchella conica by targeting protein tyrosine phosphatase 1B. Front. Pharmacol. 2021, 12, 661803.
- Wang, Z.; Wang, H.; Kang, Z.; Wu, Y.; Xing, Y.; Yang, Y. Antioxidant and anti-tumour activity of triterpenoid compounds isolated from Morchella mycelium. Arch. Microbiol. 2020, 202, 1677–1685.
- Thakur, M.; Sharma, I.; Tripathi, A. Ethnomedicinal aspects of morels with special reference to Morchella esculenta (Guchhi) in Himachal Pradesh (India): A Review. Curr. Res. Environ. Appl. Mycol. 2021, 11, 284–293.
- Sunil, C.; Xu, B. Mycochemical profile and health-promoting effects of morel mushroom Morchella esculenta (L.)—A review. Food Res. Int. 2022, 159, 111571.
- Richard, F.; Bellanger, J.-M.; Clowez, P.; Hansen, K.; O’Donnell, K.; Urban, A.; Sauve, M.; Courtecuisse, R.; Moreau, P.-A. True morels (Morchella, Pezizales) of Europe and North America: Evolutionary relationships inferred from multilocus data and a unified taxonomy. Mycologia 2015, 107, 359–382.
- Baroni, T.J.; Beug, M.W.; Cantrell, S.A.; Clements, T.A.; Iturriaga, T.; Læssøe, T.; Holgado Rojas, M.E.; Aguilar, F.M.; Quispe, M.O.; Lodge, D.J. Four new species of Morchella from the Americas. Mycologia 2018, 110, 1205–1221.
- Pilz, D.; McLain, R.; Alexander, S.; Villarreal-Ruiz, L.; Berch, S.; Wurtz, T.L.; Parks, C.G.; McFarlane, E.; Baker, B.; Molina, R.; et al. Ecology and Management of Morels Harvested from the Forests of Western North America; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Corvallis, OR, USA, 2007.
- Tan, H.; Yu, Y.; Tang, J.; Liu, T.; Miao, R.; Huang, Z.; Martin, F.M.; Peng, W. Build your own mushroom soil: Microbiota succession and nutritional accumulation in semi-synthetic substratum drive the fructification of a soil-saprotrophic morel. Front. Microbiol. 2021, 12, 656656.
- Xu, Y.; Tang, J.; Wang, Y.; He, X.; Tan, H.; Yu, Y.; Chen, Y.; Peng, W. Large-scale commercial cultivation of morels: Current state and perspectives. Appl. Microbiol. Biotechnol. 2022, 106, 4401–4412.
- Yu, F.-M.; Jayawardena, R.S.; Thongklang, N.; Lv, M.-L.; Zhu, X.-T.; Zhao, Q. Morel Production Associated with Soil Nitrogen-Fixing and Nitrifying Microorganisms. J. Fungi 2022, 8, 299.
- Adnan, M.; Xiao, B.; Xiao, P.; Zhao, P.; Li, R.; Bibi, S. Research Progress on Heavy Metals Pollution in the Soil of Smelting Sites in China. Toxics 2022, 10, 231.
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205.
- Li, J.; Yang, W.; Ren, J.; Cao, B.; Zhu, X.; Lin, L.; Ye, W.; Zhao, R. A New Species Agrocybe striatipes, also a Newly Commercially Cultivated Mushroom with Highly Nutritional and Healthy Values. J. Fungi 2023, 9, 383.
- Rao, S.; Qiu, S.; Xie, F. Determination of heavy metal content and health risk assessment of cultivated edible fungus in Guizhou province. Food Ferment. Ind. 2021, 47, 54–58.
- Shavit, E.; Efrat, S. Lead and arsenic in Morchella esculenta fruitbodies collected in lead arsenate contaminated apple orchards in the northeastern United States: A preliminary study. Fungi Mag. Spring 2010, 3, 11–18.
- Wang, Y.; Tan, R.; Zhou, L.; Lian, J.; Wu, X.; He, R.; Yang, F.; He, X.; Zhu, W. Heavy metal fixation of lead-contaminated soil using Morchella mycelium. Environ. Pollut. 2021, 289, 117829.
- Alaimo, M.G.; Saitta, A.; Ambrosio, E. Bedrock and soil geochemistry influence the content of chemical elements in wild edible mushrooms (Morchella group) from South Italy (Sicily). Acta Mycol. 2019, 54, 1–36.
- Mohammad, J.; Khan, S.; Shah, M.T.; Islam-ud-din, A.A. Essential and nonessential metal concentrations in morel mushroom (Morchella esculenta) in Dir-Kohistan, Pakistan. Pak. J. Bot. 2015, 47, 133–138.
- Strapáč, I.; Bedlovičová, Z.; Baranová, M. Edible spruce (Morchella esculenta), accumulator of toxic elements in the environment. Folia Vet. 2019, 63, 55–59.
- García, M.A.; Alonso, J.; Melgar, M.J. Bioconcentration of chromium in edible mushrooms: Influence of environmental and genetic factors. Food Chem. Toxicol. 2013, 58, 249–254.
- Gursoy, N.; Sarikurkcu, C.; Cengiz, M.; Solak, M.H. Antioxidant activities, metal contents, total phenolics and flavonoids of seven Morchella species. Food Chem. Toxicol. 2009, 47, 2381–2388.
- Zhang, N.; Zhao, M.; Xie, J.; Wang, Y.; Wen, X.; He, X. Tolerance of Morchella importuna towards heavy metals. Mycosystema 2017, 36, 367–375.
- Weng, B.-Q.; Zheng, H.; Wang, Y.-X.; Jiang, Z.-H. Advance in nutrient transformation in edible fungus cultivated with herbage and the main regulation technology. J. Agric. Sci. Technol. 2006, 8, 40.
- Jiang, Z.-H.; Lu, C.-X.; Xiao, S.-X.; Wang, Y.; Lei, J.; Wang, B. Effects of Se and La on the yield of Agaricus brasiliensis and its heavy metal and amino acid contents. Chin. J. Appl. Environ. Biol. 2014, 20, 1011–1015.
- Baldrian, P. Interactions of heavy metals with white-rot fungi. Enzym. Microb. Technol. 2003, 32, 78–91.
- Drewnowska, M.; Sąpór, A.; Jarzyńska, G.; Nnorom, I.C.; Sajwan, K.S.; Falandysz, J. Mercury in Russula mushrooms: Bioconcentration by yellow-ocher Brittle Gills Russula ochroleuca. J. Environ. Sci. Health Part A 2012, 47, 1577–1591.
- Yu, Y.; Yuan, S.; Zhuang, J.; Wan, Y.; Wang, Q.; Zhang, J.; Li, H. Effect of selenium on the uptake kinetics and accumulation of and oxidative stress induced by cadmium in Brassica chinensis. Ecotoxicol. Environ. Saf. 2018, 162, 571–580.
- Xu, H.; Yan, J.; Qin, Y.; Xu, J.; Shohag, M.; Wei, Y.; Gu, M. Effect of different forms of selenium on the physiological response and the cadmium uptake by rice under cadmium stress. Int. J. Environ. Res. Public Health 2020, 17, 6991.
- Loizides, M. Morels: The story so far. Field Mycol. 2017, 18, 42–53.
- Liu, H.; Zhang, J.; Li, T.; Shi, Y.; Wang, Y. Mineral element levels in wild edible mushrooms from Yunnan, China. Biol. Trace Elem. Res. 2012, 147, 341–345.
- Du, X.-H.; Wu, D.-M.; He, G.-Q.; Wei, W.; Xu, N.; Li, T.-L. Six new species and two new records of Morchella in China using phylogenetic and morphological analyses. Mycologia 2019, 111, 857–870.
- Yang, C.; Meng, Q.; Zhou, X.; Cui, Y.; Fu, S. Separation and identification of chemical constituents of Morchella conica isolated from Guizhou Province China. Biochem. Syst. Ecol. 2019, 86, 103919.
- Liu, D.; Cheng, H.; Bussmann, R.W.; Guo, Z.; Liu, B.; Long, C. An ethnobotanical survey of edible fungi in Chuxiong City, Yunnan, China. J. Ethnobiol. Ethnomed. 2018, 14, 42.
- Wang, L.; Zhou, Q.; Liu, J.; Shi, G. Genetic Diversity of Natural Morchella spp. from the Southern Gansu Province Based on rDNA-ITS, China. Agric. Sci. Dig. A Res. J. 2021, 41, 560–565.
- Zhao, Q.; Kang, P.; Qi, S.; Cheng, Y.; Xu, Z. Current statues of morels resources and sustainable development strategies. Southwest China J. Agric. Sci. 2010, 23, 266–269.
- Mortimer, P.E.; Karunarathna, S.C.; Li, Q.; Gui, H.; Yang, X.; Yang, X.; He, J.; Ye, L.; Guo, J.; Li, H. Prized edible Asian mushrooms: Ecology, conservation and sustainability. Fungal Divers. 2012, 56, 31–47.
- Du, X.-H.; Zhao, Q.; Yang, Z.L. A review on research advances, issues, and perspectives of morels. Mycology 2015, 6, 78–85.
- Goldway, M.; Rachel, A.; Goldberg, D.; Hadar, Y.; Levanon, D. Morchella conica exhibiting a long fruiting season. Mycol. Res. 2000, 104, 1000–1004.
- Tiwari, M.; Kamal, S.; Singh, S.; Upadhyay, R.; Rai, R. Myco-ecological studies of natural morel bearing sites in Shivalik hills of Himachal Pradesh, India. Micol. Apl. Int. 2004, 16, 1–6.
- Matočec, N.; Kušan, I.; Mrvoš, D.; Raguzin, E. The autumnal occurrence of the vernal genus Morchella (Ascomycota, Fungi). Nat. Croat. 2014, 23, 163–177.
- Malone, T.; Swinton, S.M.; Pudasainee, A.; Bonito, G. Economic Assessment of Morel (Morchella spp.) Foraging Activities in Michigan, USA. Econ. Bot. 2022, 76, 1–15.
- Liu, Q.; Ma, H.; Zhang, Y.; Dong, C. Artificial cultivation of true morels: Current state, issues and perspectives. Crit. Rev. Biotechnol. 2018, 38, 259–271.
- He, P.; Wang, K.; Cai, Y.; Liu, W. Live cell confocal laser imaging studies on the nuclear behavior during meiosis and ascosporogenesis in Morchella importuna under artificial cultivation. Micron 2017, 101, 108–113.
- Liu, F.; Wang, A.; Wu, R.; Wang, Y.; Zhao, J.; Deng, W. High-yield Cultivation Techniques of Morchella spp. in South China. Chin. J. Trop. Crops 2021, 42, 3199.
- Wenjun, S.; Haiping, D.; Yan, S.; Dan, L.; Lli, X.; Kaiguo, L. Efficient Technique of Rapeseed and Morchella spp. Interplanting in Mountainous Areas. Med. Plant 2022, 13, 7–10.
- Tan, H.; Kohler, A.; Miao, R.; Liu, T.; Zhang, Q.; Zhang, B.; Jiang, L.; Wang, Y.; Xie, L.; Tang, J. Multi-omic analyses of exogenous nutrient bag decomposition by the black morel Morchella importuna reveal sustained carbon acquisition and transferring. Environ. Microbiol. 2019, 21, 3909–3926.
- Li, Y.; Zhang, X.; Yuan, L. Investigation and Analysis of Heavy Metals in Edible Fungi Collected from Chongqing Beibei. J. Chongqing Norm. Univ. Nat. Sci. Ed. 2007, 24, 81.
- Cao, Y.-T.; Lu, Z.-P.; Gao, X.-Y.; Liu, M.-L.; Sa, W.; Liang, J.; Wang, L.; Yin, W.; Shang, Q.-H.; Li, Z.-H. Maximum Entropy Modeling the Distribution Area of Morchella Dill. ex Pers. Species in China under Changing Climate. Biology 2022, 11, 1027.
- Thakur, M.; Sharma, I.; Tripathi, A. Optimization of growth conditions for large scale production of mycelium from different Morchella spp. Of Himalayan region. Plant Arch. 2020, 20, 5847–5853.
- Dissanayake, A.A.; Mills, G.L.; Bonito, G.; Rennick, B.; Nair, M. Chemical composition and anti-inflammatory and antioxidant activities of extracts from cultivated morel mushrooms, species of genus Morchella (Ascomycota). Int. J. Med. Mushrooms 2021, 23, 73–83.
- Xu, L.; Wu, Y.; Cai, Z.; Zheng, W.; Ye, C.; Wu, Y. Concentrations of heavy metals, pesticide residues and Se in Grifola frondosa with different cultivation methods. Acta Agric. Zhejiangensis 2016, 28, 79–83.
- Chen, Y.-S.; Liu, B.-L.; Chang, Y.-N. Effects of light and heavy metals on Cordyceps militaris fruit body growth in rice grain-based cultivation. Korean J. Chem. Eng. 2011, 28, 875–879.
- Yu, H.; Shen, X.; Chen, H.; Dong, H.; Zhang, L.; Yuan, T.; Zhang, D.; Shang, X.; Tan, Q.; Liu, J.; et al. Analysis of heavy metal content in Lentinula edodes and the main influencing factors. Food Control 2021, 130, 108198.
- Rácz, L.; Oldal, V. Investigation of uptake processes in a soil/mushroom system by AES and AAS methods. Microchem. J. 2000, 67, 115–118.
- Obodai, M.; Ferreira, I.C.; Fernandes, Â.; Barros, L.; Mensah, D.L.N.; Dzomeku, M.; Urben, A.F.; Prempeh, J.; Takli, R.K. Evaluation of the chemical and antioxidant properties of wild and cultivated mushrooms of Ghana. Molecules 2014, 19, 19532–19548.
- Liu, H.; Xu, J.; Li, X.; Zhang, Y.; Yin, A.; Wang, J.; Long, Z. Effects of microelemental fertilizers on yields, mineral element levels and nutritional compositions of the artificially cultivated Morchella conica. Sci. Hortic. 2015, 189, 86–93.
- Zhang, F.; Long, L.; Hu, Z.; Yu, X.; Liu, Q.; Bao, J.; Long, Z. Analyses of artificial morel soil bacterial community structure and mineral element contents in ascocarp and the cultivated soil. Can. J. Microbiol. 2019, 65, 738–749.
- Xiong, S.; Xiao, Q.; He, J.; Wang, J.; He, H.; Lan, H. Determination of mercury speciation in dried edible fungi by microwave extraction combined with HPLC-ICP-MS. Food Ferment. Ind. 2020, 46, 252–256.
- Sharma, P.; Pandey, A.K.; Udayan, A.; Kumar, S. Role of microbial community and metal-binding proteins in phytoremediation of heavy metals from industrial wastewater. Bioresour. Technol. 2021, 326, 124750.
- Chaudhary, K.; Agarwal, S.; Khan, S. Role of phytochelatins (PCs), metallothioneins (MTs), and heavy metal ATPase (HMA) genes in heavy metal tolerance. Mycoremediation Environ. Sustain. 2018, 2, 39–60.
- Motaharpoor, Z.; Taheri, H.; Nadian, H. Rhizophagus irregularis modulates cadmium uptake, metal transporter, and chelator gene expression in Medicago sativa. Mycorrhiza 2019, 29, 389–395.
- Sytar, O.; Ghosh, S.; Malinska, H.; Zivcak, M.; Brestic, M. Physiological and molecular mechanisms of metal accumulation in hyperaccumulator plants. Physiol. Plant. 2021, 173, 148–166.
- Dhalaria, R.; Kumar, D.; Kumar, H.; Nepovimova, E.; Kuča, K.; Torequl Islam, M.; Verma, R. Arbuscular mycorrhizal fungi as potential agents in ameliorating heavy metal stress in plants. Agronomy 2020, 10, 815.
- Zhu, B.; Chen, Y.; Wei, N. Engineering biocatalytic and biosorptive materials for environmental applications. Trends Biotechnol. 2019, 37, 661–676.
- Srivastava, N. Remediation of heavy metals through genetically engineered microorganism. In Environmental Pollution and Remediation; Springer: Singapore, 2021; pp. 315–366.
- Sultan, I.; Haq, Q.M.R. Bacterial Mechanisms for Metal (loid) s Remediation; CRC Press: Boca Raton, FL, USA, 2022; p. 115.
- Sun, J.; Wang, R.; Zhang, X.; Yu, Y.; Zhao, R.; Li, Z.; Chen, S. Hydrogen sulfide alleviates cadmium toxicity through regulations of cadmium transport across the plasma and vacuolar membranes in Populus euphratica cells. Plant Physiol. Biochem. 2013, 65, 67–74.
- Cotrim, C.A.; Jarrott, R.J.; Martin, J.L.; Drew, D. A structural overview of the zinc transporters in the cation diffusion facilitator family. Acta Crystallogr. Sect. D Struct. Biol. 2019, 75, 357–367.
- Vera-Bernal, M.; Martínez-Espinosa, R.M. Insights on cadmium removal by bioremediation: The case of Haloarchaea. Microbiol. Res. 2021, 12, 354–375.
- Yang, Z.; Yang, F.; Liu, J.-L.; Wu, H.-T.; Yang, H.; Shi, Y.; Liu, J.; Zhang, Y.-F.; Luo, Y.-R.; Chen, K.-M. Heavy metal transporters: Functional mechanisms, regulation, and application in phytoremediation. Sci. Total Environ. 2022, 809, 151099.
- Priya, A.; Gnanasekaran, L.; Dutta, K.; Rajendran, S.; Balakrishnan, D.; Soto-Moscoso, M. Biosorption of heavy metals by microorganisms: Evaluation of different underlying mechanisms. Chemosphere 2022, 307, 135957.
- Ali Redha, A. Removal of heavy metals from aqueous media by biosorption. Arab J. Basic Appl. Sci. 2020, 27, 183–193.
- Ayele, A.; Haile, S.; Alemu, D.; Kamaraj, M. Comparative Utilization of Dead and Live Fungal Biomass for the Removal of Heavy Metal: A Concise Review. Sci. World J. 2021, 2021, 5588111.
- Blaudez, D.; Botton, B.; Chalot, M. Cadmium uptake and subcellular compartmentation in the ectomycorrhizal fungus Paxillus involutus. Microbiology 2000, 146, 1109–1117.
- Sag, Y.; Kutsal, T. Recent trends in the biosorption of heavy metals: A review. Biotechnol. Bioprocess Eng. 2001, 6, 376–385.
- Kumar, A.; Subrahmanyam, G.; Mondal, R.; Cabral-Pinto, M.; Shabnam, A.A.; Jigyasu, D.K.; Malyan, S.K.; Fagodiya, R.K.; Khan, S.A.; Yu, Z.-G. Bio-remediation approaches for alleviation of cadmium contamination in natural resources. Chemosphere 2021, 268, 128855.
- Chen, M.-M.; Zheng, X.; Li, X.-F. Capability and Mechanisms of Macrofungi in Heavy Metal Accumulation: A Review. J. Agric. Resour. Environ. 2017, 34, 499.
- Rana, A.; Sindhu, M.; Kumar, A.; Dhaka, R.K.; Chahar, M.; Singh, S.; Nain, L. Restoration of heavy metal-contaminated soil and water through biosorbents: A review of current understanding and future challenges. Physiol. Plant. 2021, 173, 394–417.
- Yousefi, N.; Jones, M.; Bismarck, A.; Mautner, A. Fungal chitin-glucan nanopapers with heavy metal adsorption properties for ultrafiltration of organic solvents and water. Carbohydr. Polym. 2021, 253, 117273.
- Jaafarzadeh, N.; Mengelizadeh, N.; Takdastan, A.; Farsani, M.H.; Niknam, N.; Aalipour, M.; Hadei, M.; Bahrami, P. Biosorption of heavy metals from aqueous solutions onto chitin. Int. J. Environ. Health Eng. 2015, 4, 7.
- Araújo, D.; Ferreira, I.C.; Torres, C.A.; Neves, L.; Freitas, F. Chitinous polymers: Extraction from fungal sources, characterization and processing towards value-added applications. J. Chem. Technol. Biotechnol. 2020, 95, 1277–1289.
- Boulaiche, W.; Hamdi, B.; Trari, M. Removal of heavy metals by chitin: Equilibrium, kinetic and thermodynamic studies. Appl. Water Sci. 2019, 9, 39.
- Papadaki, A.; Diamantopoulou, P.; Papanikolaou, S.; Philippoussis, A. Evaluation of biomass and chitin production of Morchella mushrooms grown on starch-based substrates. Foods 2019, 8, 239.
- Liu, P.-H.; Huang, Z.-X.; Luo, X.-H.; Chen, H.; Weng, B.-Q.; Wang, Y.-X.; Chen, L.-S. Comparative transcriptome analysis reveals candidate genes related to cadmium accumulation and tolerance in two almond mushroom (Agaricus brasiliensis) strains with contrasting cadmium tolerance. PLoS ONE 2020, 15, e0239617.
- Yang, Y.; Yang, J.; Wang, H.; Jin, Y.; Liu, J.; Jia, R.; Wang, Z.; Kang, Z. Analysis of primary metabolites of Morchella fruit bodies and mycelium based on widely targeted metabolomics. Arch. Microbiol. 2022, 204, 98.
- Zhang, Q.; Miao, R.; Liu, T.; Huang, Z.; Peng, W.; Gan, B.; Zhang, X.; Tan, H. Biochemical characterization of a key laccase-like multicopper oxidase of artificially cultivable Morchella importuna provides insights into plant-litter decomposition. 3 Biotech 2019, 9, 171.
- Wang, Y.; Zhang, B.; Chen, N.; Wang, C.; Feng, S.; Xu, H. Combined bioremediation of soil co-contaminated with cadmium and endosulfan by Pleurotus eryngii and Coprinus comatus. J. Soils Sediments 2018, 18, 2136–2147.
More
Information
Subjects:
Mycology; Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
09 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




