Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | NARENDRA THAPA | -- | 2164 | 2023-10-09 01:56:43 | | | |
| 2 | Rita Xu | Meta information modification | 2164 | 2023-10-09 03:51:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Thapa, N.; Wen, T.; Cryns, V.L.; Anderson, R.A. Microtubule Cytoskeleton Organization, Cell Polarity, and Phosphoinositide Signaling. Encyclopedia. Available online: https://encyclopedia.pub/entry/49939 (accessed on 01 March 2026).
Thapa N, Wen T, Cryns VL, Anderson RA. Microtubule Cytoskeleton Organization, Cell Polarity, and Phosphoinositide Signaling. Encyclopedia. Available at: https://encyclopedia.pub/entry/49939. Accessed March 01, 2026.
Thapa, Narendra, Tianmu Wen, Vincent L. Cryns, Richard A. Anderson. "Microtubule Cytoskeleton Organization, Cell Polarity, and Phosphoinositide Signaling" Encyclopedia, https://encyclopedia.pub/entry/49939 (accessed March 01, 2026).
Thapa, N., Wen, T., Cryns, V.L., & Anderson, R.A. (2023, October 09). Microtubule Cytoskeleton Organization, Cell Polarity, and Phosphoinositide Signaling. In Encyclopedia. https://encyclopedia.pub/entry/49939
Thapa, Narendra, et al. "Microtubule Cytoskeleton Organization, Cell Polarity, and Phosphoinositide Signaling." Encyclopedia. Web. 09 October, 2023.
Copy Citation
The capacity for cancer cells to metastasize to distant organs depends on their ability to execute the carefully choreographed processes of cell adhesion and migration. As most human cancers are of epithelial origin (carcinoma), the transcriptional downregulation of adherent/tight junction proteins (e.g., E-cadherin, Claudin and Occludin) with the concomitant gain of adhesive and migratory phenotypes has been extensively studied.
phosphoinositide
microtubules
cell polarity
1. Introduction
Cell adhesion and cell migration are integral cellular processes for vertebrates that are crucial for embryonic development and normal cellular functioning [1]. Dysregulated cell adhesion and migration play key roles in tumor metastasis and have remained a prominent focus in cancer research for decades, with numerous excellent reviews on these cancer topics [1][2][3]. From the perspectives of cancer progression and metastasis, most human cancers (85%) are of epithelial origin (carcinoma), in which indolent epithelial cells gain mesenchymal traits via the epithelial–mesenchymal transition (EMT) that imparts adhesive and migratory phenotypes to transformed epithelial cells, enabling distant metastasis [4][5]. Although the loss of E-cadherin-mediated adherent junctions and cell polarity is a well-portrayed hallmark of carcinoma progression, metastasizing cancer cells undergo extensive reorganization of their cytoskeletal system, which encompasses actin, microtubules and intermediate filaments [6]. The dramatic change in spatial orientation and organization, as well as the originating sites of the microtubule cytoskeleton, are some of the most distinguishing features observed in epithelial cells that undergo conversion to mesenchymal cells [7]. Specifically, the non-centrosomal orientation of microtubules at apical membranes in epithelial cells is gradually converted to the centrosomal orientation of these microtubules, and these changes in the organization of the microtubule cytoskeleton coincides temporally with many adhesive and migratory traits acquired by transitioning carcinoma cells [7]. However, changes in microtubule organization/orientation also occur during neuronal polarity and embryonic development [8][9]. Intriguingly, microtubule polymerization and growth inversely regulate focal adhesion assembly [10]; however, how this coordinated interplay between microtubule growth and focal adhesion assembly accomplishes productive cell adhesion and migration is poorly understood. Moreover, there is an emerging link between microtubules, cell polarity and phosphoinositide signaling. Despite microtubules arising as a target for the therapeutic treatment of cancer soon after the start of modern chemotherapy [11], the functional relationship between the therapeutic targeting of microtubules and focal adhesion assembly/focal adhesion signaling has not been well mechanistically defined.
2. Distinct Orientation and Organization of Microtubules in Epithelial vs. Mesenchymal Cells
Microtubules constitute one of the most prominent cytoskeletal elements in mammalian cells and provide structural support as well as a trafficking platform for the transport of intracellular vesicles and organelles [12]. An individual microtubule is a 25-nm-diameter hollow tube made of 13 tubulin filaments, and each filament is a heterodimer of α- and β-tubulin organized in a head-to-tail fashion [12]. This results in a polarized structure of microtubules, with α-tubulin at one end (minus end) and β-tubulin at the other end (plus end) (Figure 1A) extending up to several millimeters in length. The microtubule minus end exhibits slow dynamics, whereas the plus end grows and shrinks rapidly and interacts with different intracellular molecules and structures [12]. Overall, microtubules are highly dynamic structures that undergo repeated cycles of polymerization (growth) and depolymerization (shrinkage) and are finely tuned to the needs of the cells and tissues.
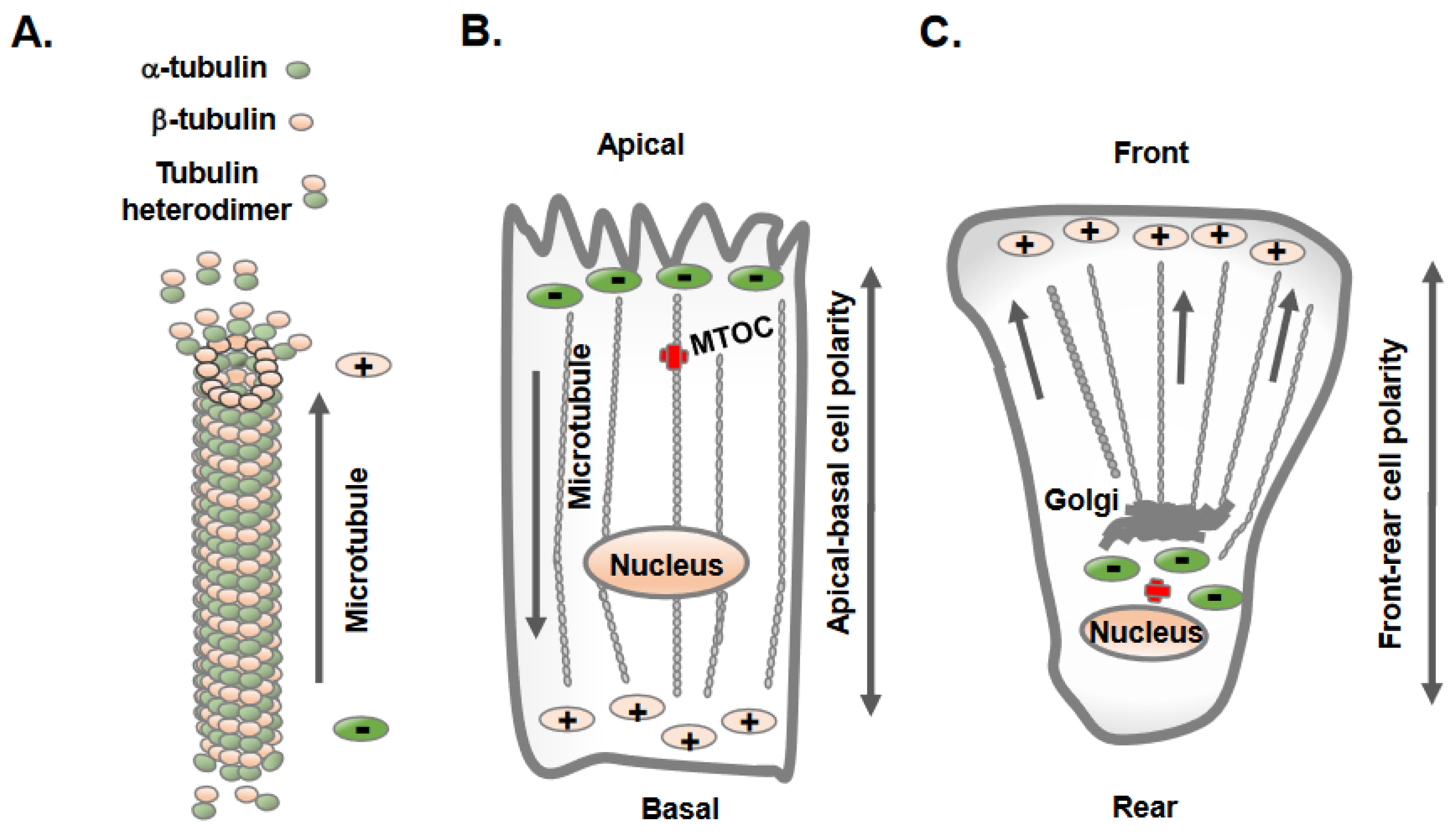
Figure 1. Epithelial and mesenchymal cells display distinct orientation and organization of microtubules. (A) Microtubules are hollow tubes composed of 13-individual polymers of tubulin; each tubulin molecule is heterodimer of α-tubulin and β-tubulin. The polymerization and growth of microtubules often takes place at the plus end with β-tubulin, whereas the minus end contains α-tubulin. (B) Epithelial cells maintain apical-basolateral cell polarity, with the microtubule minus ends located at apical regions and plus ends located at the basolateral regions. (C) Mesenchymal cells acquire front-rear cell polarity and microtubules extend to the cell periphery from the cell center.
The intracellular sites from where microtubule nucleation and growth begin are known as microtubule-organizing centers (MTOCs) [13]. One of the distinctive features observed in epithelial and mesenchymal cells is the position of the MTOC and the overall orientation, growth, and organization of the microtubules [13][14]. Polarized epithelial cells contain non-centrosomal MTOCs at apical membrane regions from where microtubules plus ends orient and extend towards basolateral cell membranes (Figure 1B). In contrast, mesenchymal cells contain dominant centrosomal MTOCs in the vicinity of the nucleus and Golgi apparatus, from which a radial array of microtubule plus ends are oriented towards the cell periphery (Figure 1C) [13]. A unique non-centrosomal MTOC at an apical site result in the alignment of long and numerous microtubules along the apical-to-basal axes of polarized epithelial cells. This unique organization of microtubules in epithelial cells was initially discovered in the late 1980s using hook decoration methods and electron microscopy [15]. This arrangement of microtubules allows the sorting of specific membrane components via directing vesicle trafficking to distinct apical and basolateral regions of the plasma membrane [16].
The mechanisms by which MTOC and centrosomes gain or adopt an off-center position toward apical or intercellular junctions in polarized epithelial cells are not precisely understood [17]. They may arise from the microtubule nucleating activity of the γ-tubulin ring complex (γTuRC) near apical regions in epithelial cells [13]. Similarly, they may originate from microtubule release at the centrosomes; microtubule minus ends is subsequently captured and anchored at apical non-centrosomal sites, generating apico-basal arrays of microtubules [6][13]. The analysis of microtubule regrowth in polarized MDCK cells following nocodazole treatment shows the centrosomal nucleation of microtubules and the initial aster formation, which is subsequently replaced by apico-basal microtubule arrays [13]. Centrosome decentering to apical regions may also result from pulling forces from a defined part of the cell periphery [13]. For example, Par3 is present along epithelial cell junctions and is capable of recruiting the dynein motor protein, which pulls on microtubules, and thereby, can direct the centrosome position to apical regions, as seen during planar cell polarity establishment [18]. Next, major polarity regulators (PAR3, PAR6 and aPKC) and E-cadherin-mediated adherent junctions greatly influence the organization of apical non-centrosomal MTOCs in epithelial cells [13]. Notably, there are a few microtubules minus ends interacting proteins (-Tips), such as γ-tubulin, and calmodulin-regulated spectrin-associated proteins (CAMSAPs) members CAMSAP2, CAMSAP3, spectraplakin and ninein. CAMSAP and ninein represent two central mechanisms by which microtubule minus ends attach to cortical regions of the epithelial tissues in mammalian cells [19]. The re-localization of ninein to apical non-centrosomal MTOC sites helps to anchor microtubule minus ends at the apical membrane of polarizing epithelial cells [19].
Unlike epithelial cells, mesenchymal cells contain radial arrays of microtubules originating from centrosomal MTOCs in proximity to the nucleus and Golgi, which is the principal site of microtubule nucleation and growth (Figure 1C). Mesenchymal cells contain shorter and less numerous microtubules, with their centrosome localized at the cell center [20]. A typical mammalian cell centrosome contains a centrally localized centriole pair in perpendicular orientation with each other and is surrounded by a centrosomal matrix called pericentriolar material (PCM). The mass spectrometric analysis of purified centrosomes shows a large number of proteins that include, α-tubulin, β-tubulin, γ-tubulin, γ-tubulin complex components 1-6, centrin 2 and 3, AKAP450, pericentrin/kendrin, ninein, pericentriolar material 1 (PCM1), centriolin, CLIP-associating proteins CLASP1 and CLASP2, EB1, centractin, myomegalin, Cdk1, dynein intermediate chain, dynein light chain, p150Glued, dynactin and Hsp73 [21].
3. Reversion of MTOC from Non-Centrosomal to Centrosomal Positioning in Epithelial Cells Transitioning to Mesenchymal Cells
The dissolution of the apical-basolateral polarity axes of epithelial cells and establishment of a front-rear polarity axis are accomplished via the EMT process [22]. Along with the dissolution of cell–cell adherent junctions and cell polarity, the change from non-centrosomal to centrosomal MTOCs (centrosome repositioning) and the development of radially oriented arrays of microtubules from the centrosome at the cell center are prominent features observed in epithelial cells converting to mesenchymal cells [7]. Overall, this process is known as a polarity reversal and is an early and a key feature of the EMT [7]. The decades-old literature shows synchrony between centrosomal MTOC repositioning and epithelial cell migration, supporting the notion that internal polarity reversal is instrumental for cell migration [23]. The process of centrosomal MTOC repositioning concomitant with the changes in protein expression and induction of EMT is elegantly demonstrated in MCF10A and MDCK epithelial cells following TGF-β treatment in vitro (Figure 2) [7].
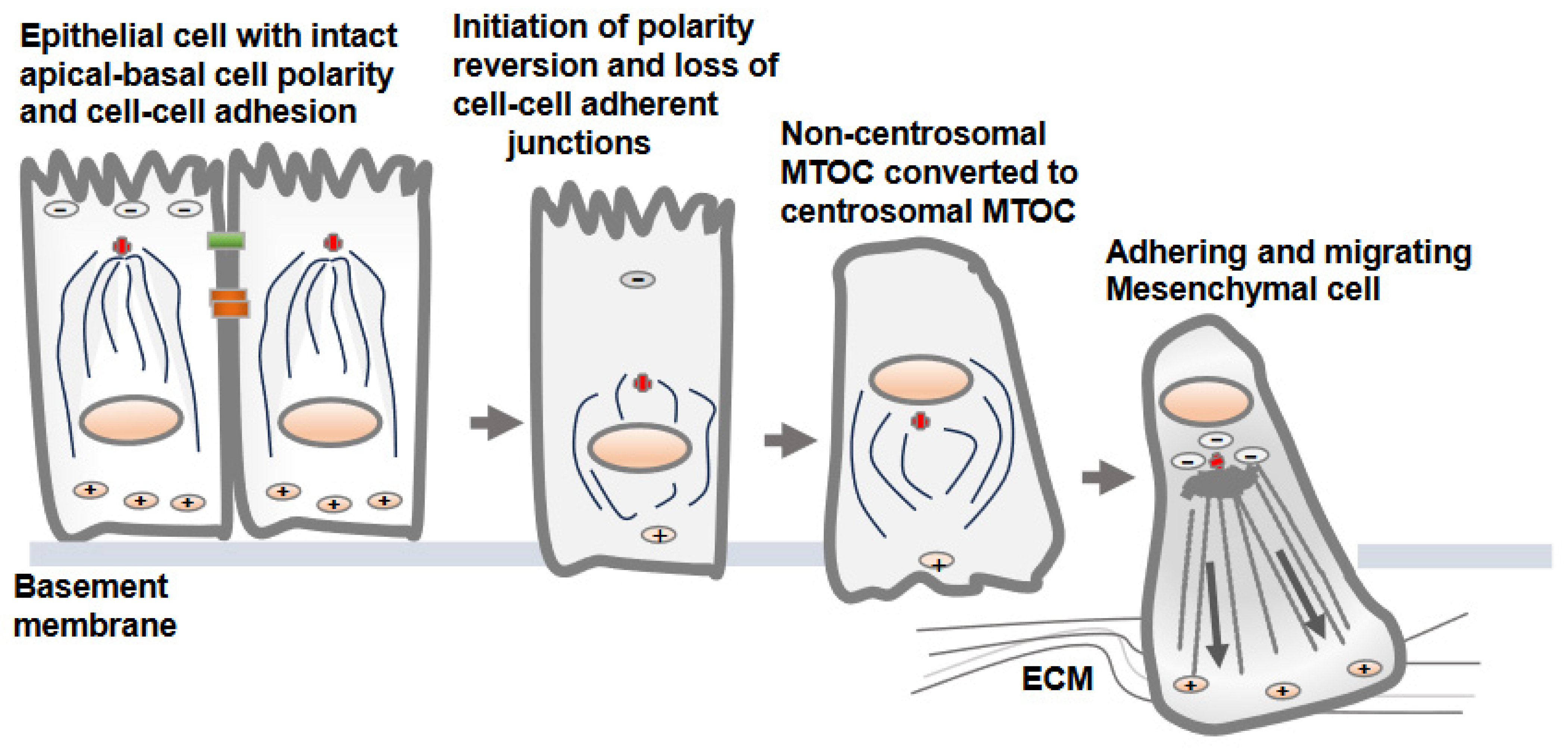
Figure 2. Non-centrosomal MTOC in epithelial cells adopts a centrosomal MTOC position as epithelial cells transition to mesenchymal cells. There is no well-defined mechanism by which non-centrosomal MTOC is reverted to centrosomal MTOC as epithelial cells convert into mesenchymal cells. The development of centrosomal MTOC from non-centrosomal MTOC as epithelial cell convert into mesenchymal cells is referred as cell polarity reversion. Upon loss of the cell–cell adherent junctions and apical-basolateral cell polarity, the MTOC is gradually developed at the cell center, from which polarized microtubules radiate towards the cell periphery. This is accompanied by gain of adhesive and migratory traits via transitioning cells.
There is no precise mechanism explaining how the non-centrosomal MTOC in apical regions is translocated to a centrosomal localization as epithelial cells transition to mesenchymal cells [13][16]. Decreases in microtubule nucleation, polymerization and stabilization along adherent junctions and apical regions are possible mechanisms that may lead to the displacement of non-centrosomal MTOC to the centrosome adjacent to the nucleus and Golgi structures [6]. One of the critical factors is a decrease in the localization of cortical polarity components Par3 and podocalyxin in intercellular junctions and the reversion of the internal polarity axis [4]. Par3 is present along the epithelial cell junction and is capable of recruiting dynein, which pulls on microtubules, and thus, can determine the centrosome position during planar cell polarity establishment. The amount of polymerized tubulin also contributes to the transition from an off-centered microtubule network in epithelial cells to a centered conformation in mesenchymal cells [16].
4. Microtubule Assembly and Its Inverse Relation with Focal Adhesion Assembly: Coordinated Interplay in Cell Adhesion and Cell Migration
Once apical-basolateral polarity is lost, transitioning cells establish front-rear cell polarity along with prominent centrosomal MTOC organization and radially orientated microtubules towards the cell periphery [24][25]. This conversion prepares the cells for adhesion and migration along the extracellular matrix proteins of interstitial tissues [26]. Intriguingly, the dynamics of microtubule polymerization and growth are inversely correlated with the assembly of the focal adhesions [10][27] (Figure 3). This necessitates the coordinated interplay between microtubule polymerization/depolymerization with focal adhesion assembly/disassembly to accomplish successful cell migration. The dynamic assembly and disassembly of focal adhesion at leading and trailing edges of migrating cells is one of the most extensively studied topic in cell adhesion and cell migration [28][29]. The assembly of focal adhesions at the leading edges is accompanied by the disassembly of the focal adhesions at the trailing edges, and these processes continue as the cells migrate [29].
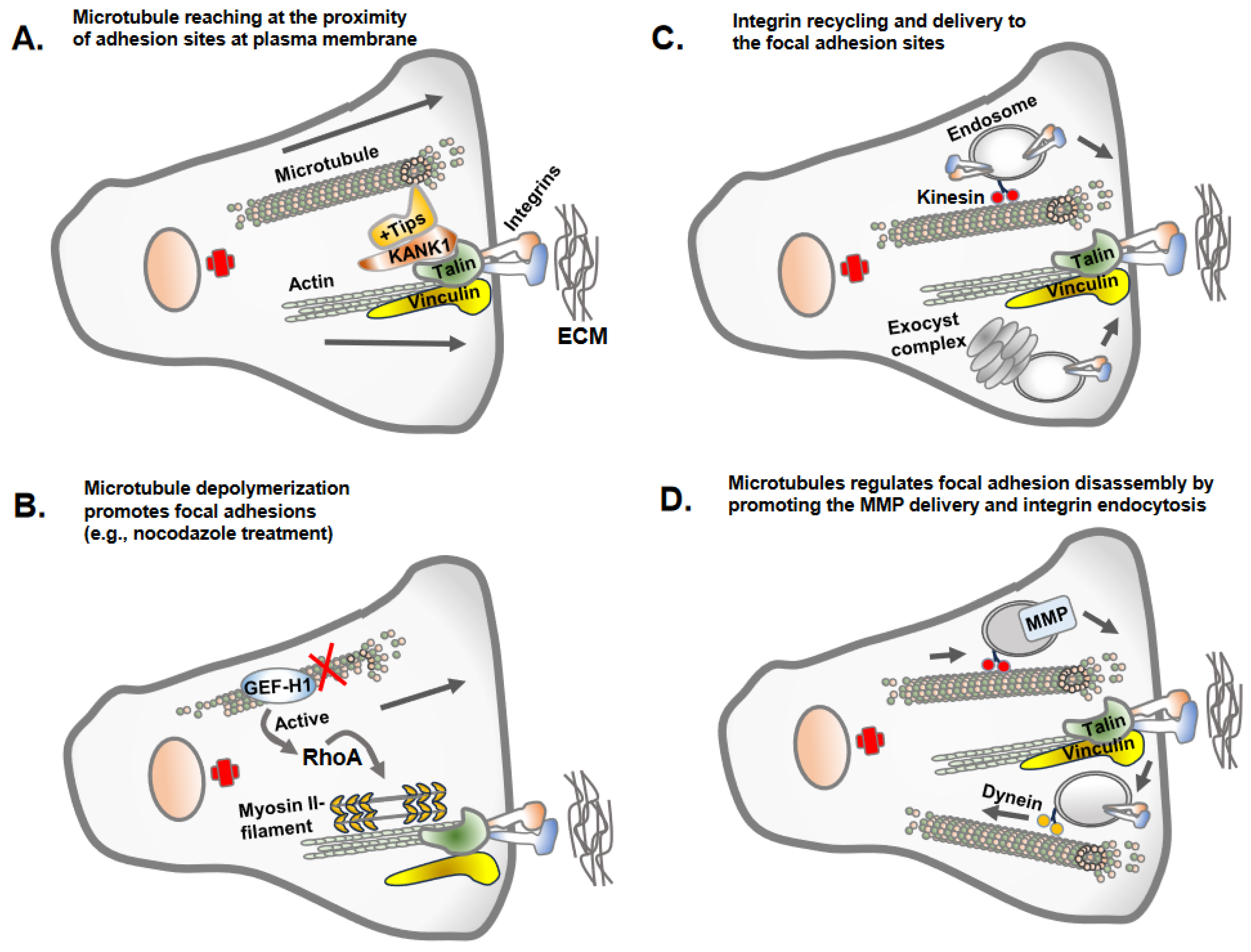
Figure 3. Microtubule regulation of focal adhesions. There are different mechanisms by which the microtubule regulates focal adhesions. (A) Microtubules often do not reach the site of focal adhesions due to the dense actin cytoskeleton but make indirect contact via KANK1 or +Tips proteins, stabilizing the plus ends of the microtubules. (B) The key mechanism by which microtubule polymerization or growth inversely regulate focal adhesions is due to the activity of GEF-H1, the guanine exchange factor H1 for RhoA GTPases. GEF-H1 bound to microtubules is in an inactivated state. GEF-H1 is activated once released from depolymerized microtubules (e.g., nocodazole treatment of cells) and activates RhoA GTPase, which promotes actin polymerization and contractility and increased focal adhesion assembly. (C) Microtubules as a track for endosomal trafficking promote the delivery of the newly synthesized or recycling integrins to newly forming focal adhesion sites. An evolutionary conserved vesicle trafficking complex, exocyst, in association with microtubules may also deliver integrin molecules to focal adhesion sites. (D) Microtubules serve as a track for endosomal trafficking of matrix metalloprotease (e.g., MT1-MMP) at the site of focal adhesion, leading to the disassembly of the focal adhesions. The integrins once internalized from adhesion sites undergo endosomal trafficking along microtubules tracks.
Microtubule dynamics and orientation guide overall cell polarity and leading-edge protrusions [29]. Similarly, the adhesion complexes establish the leading edges of migrating cell fronts interacting with extracellular matrix proteins [30]. Thus, the establishment of front-rear cell polarity and cell migration is an integrated function of microtubule and cell adhesion complexes. Importantly, the dynamics of microtubule polymerization and depolymerization differ at the leading edge and cell rear and are carefully coordinated via adhesion assembly and disassembly [31]. The growing microtubule likely gains access to focal adhesion sites at the leading and trailing edges using actin stress fibers as tracks, with the aid of crosslink proteins MACF1/ACF7, KANK1 and Talin [32][33]. However, the direct interrogation of microtubule plus ends at focal adhesion sites remains unclear due to the presence of denser arrays of the actin cytoskeleton. Microtubules growth/retraction may require multiple repetitions at focal adhesion sites to accomplish focal adhesion turnover [29][31]. The process of microtubule disassembly or catastrophes at focal adhesions is triggered by a biochemical mechanism involving stathmin and catastrophe-inducing molecules, such as kinesin-13 family members MCAK [34]. Furthermore, microtubule growth and capture nearby focal adhesions, leading to the disassembly of focal adhesions via different mechanisms, including clathrin-mediated endocytosis, NBR1-mediated autophagy and the delivery of exocytic vesicles carrying matrix metalloproteases that sever integrin–ECM connection sites [35].
References
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374.
- Martin-Belmonte, F.; Perez-Moreno, M. Epithelial cell polarity, stem cells and cancer. Nat. Rev. Cancer 2011, 12, 23–38.
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548.
- Jung, H.Y.; Fattet, L.; Tsai, J.H.; Kajimoto, T.; Chang, Q.; Newton, A.C.; Yang, J. Apical-basal polarity inhibits epithelial-mesenchymal transition and tumour metastasis by PAR-complex-mediated SNAI1 degradation. Nat. Cell Biol. 2019, 21, 359–371.
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell adhesion in cancer: Beyond the migration of single cells. J. Biol. Chem. 2020, 295, 2495–2505.
- Akhmanova, A.; Kapitein, L.C. Mechanisms of microtubule organization in differentiated animal cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 541–558.
- Burute, M.; Prioux, M.; Blin, G.; Truchet, S.; Letort, G.; Tseng, Q.; Bessy, T.; Lowell, S.; Young, J.; Filhol, O.; et al. Polarity Reversal by Centrosome Repositioning Primes Cell Scattering during Epithelial-to-Mesenchymal Transition. Dev. Cell 2017, 40, 168–184.
- Thyagarajan, P.; Feng, C.; Lee, D.; Shorey, M.; Rolls, M.M. Microtubule polarity is instructive for many aspects of neuronal polarity. Dev. Biol. 2022, 486, 56–70.
- Moorhouse, K.S.; Gudejko, H.F.; McDougall, A.; Burgess, D.R. Influence of cell polarity on early development of the sea urchin embryo. Dev. Dyn. 2015, 244, 1469–1484.
- Ezratty, E.J.; Partridge, M.A.; Gundersen, G.G. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat. Cell Biol. 2005, 7, 581–590.
- Wordeman, L.; Vicente, J.J. Microtubule Targeting Agents in Disease: Classic Drugs, Novel Roles. Cancers 2021, 13, 5650.
- Brouhard, G.J.; Rice, L.M. Microtubule dynamics: An interplay of biochemistry and mechanics. Nat. Rev. Mol. Cell Biol. 2018, 19, 451–463.
- Bellett, G.; Carter, J.M.; Keynton, J.; Goldspink, D.; James, C.; Moss, D.K.; Mogensen, M.M. Microtubule plus-end and minus-end capture at adherens junctions is involved in the assembly of apico-basal arrays in polarised epithelial cells. Cell Motil. Cytoskelet. 2009, 66, 893–908.
- Sanchez, A.D.; Feldman, J.L. Microtubule-organizing centers: From the centrosome to non-centrosomal sites. Curr. Opin. Cell Biol. 2017, 44, 93–101.
- Russell, D.G.; Burns, R.G. The polar ring of coccidian sporozoites: A unique microtubule-organizing centre. J. Cell Sci. 1984, 65, 193–207.
- Zhou, P.; Yang, G.; Xie, W. Organization of cortical microtubules in differentiated cells. J. Cell Physiol. 2023, 238, 1141–1147.
- Schatten, H. Transitions from Centrosomal to Non-centrosomal Microtubule Organization During Cellular Polarization. Adv. Anat. Embryol. Cell Biol. 2022, 235, 75–79.
- Schmoranzer, J.; Fawcett, J.P.; Segura, M.; Tan, S.; Vallee, R.B.; Pawson, T.; Gundersen, G.G. Par3 and dynein associate to regulate local microtubule dynamics and centrosome orientation during migration. Curr. Biol. 2009, 19, 1065–1074.
- Zheng, Y.; Buchwalter, R.A.; Zheng, C.; Wight, E.M.; Chen, J.V.; Megraw, T.L. A perinuclear microtubule-organizing centre controls nuclear positioning and basement membrane secretion. Nat. Cell Biol. 2020, 22, 297–309.
- Burakov, A.V.; Nadezhdina, E.S. Centering and Shifting of Centrosomes in Cells. Cells 2020, 9, 1351.
- Xie, B.; Pu, Y.; Yang, F.; Chen, W.; Yue, W.; Ma, J.; Zhang, N.; Jiang, Y.; Wu, J.; Lin, Y.; et al. Proteomic Mapping and Targeting of Mitotic Pericentriolar Material in Tumors Bearing Centrosome Amplification. Cancer Res. 2022, 82, 2576–2592.
- Banerjee, P.; Xiao, G.Y.; Tan, X.; Zheng, V.J.; Shi, L.; Rabassedas, M.N.B.; Guo, H.F.; Liu, X.; Yu, J.; Diao, L.; et al. The EMT activator ZEB1 accelerates endosomal trafficking to establish a polarity axis in lung adenocarcinoma cells. Nat. Commun. 2021, 12, 6354.
- Carney, P.R.; Couve, E. Cell polarity changes and migration during early development of the avian peripheral auditory system. Anat. Rec. 1989, 225, 156–164.
- Nelson, W.J. Remodeling epithelial cell organization: Transitions between front-rear and apical-basal polarity. Cold Spring Harb. Perspect. Biol. 2009, 1, a000513.
- Godde, N.J.; Galea, R.C.; Elsum, I.A.; Humbert, P.O. Cell polarity in motion: Redefining mammary tissue organization through EMT and cell polarity transitions. J. Mammary Gland. Biol. Neoplasia 2010, 15, 149–168.
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776.
- Seetharaman, S.; Etienne-Manneville, S. Microtubules at focal adhesions—A double-edged sword. J. Cell Sci. 2019, 132, jcs232843.
- Zhao, Y.; Wang, Y.; Sarkar, A.; Wang, X. Keratocytes Generate High Integrin Tension at the Trailing Edge to Mediate Rear De-adhesion during Rapid Cell Migration. iScience 2018, 9, 502–512.
- Mavrakis, M.; Juanes, M.A. The compass to follow: Focal adhesion turnover. Curr. Opin. Cell Biol. 2023, 80, 102152.
- Seetharaman, S.; Etienne-Manneville, S. Cytoskeletal Crosstalk in Cell Migration. Trends Cell Biol. 2020, 30, 720–735.
- Broussard, J.A.; Webb, D.J.; Kaverina, I. Asymmetric focal adhesion disassembly in motile cells. Curr. Opin. Cell Biol. 2008, 20, 85–90.
- Zhao, A.J.; Montes-Laing, J.; Perry, W.M.; Shiratori, M.; Merfeld, E.; Rogers, S.L.; Applewhite, D.A. The Drosophila spectraplakin Short stop regulates focal adhesion dynamics by cross-linking microtubules and actin. Mol. Biol. Cell 2022, 33, ar19.
- Li, X.; Goult, B.T.; Ballestrem, C.; Zacharchenko, T. The structural basis of the talin-KANK1 interaction that coordinates the actin and microtubule cytoskeletons at focal adhesions. Open Biol. 2023, 13, 230058.
- Moon, H.H.; Kreis, N.N.; Friemel, A.; Roth, S.; Schulte, D.; Solbach, C.; Louwen, F.; Yuan, J.; Ritter, A. Mitotic Centromere-Associated Kinesin (MCAK/KIF2C) Regulates Cell Migration and Invasion by Modulating Microtubule Dynamics and Focal Adhesion Turnover. Cancers 2021, 13, 5673.
- Kenific, C.M.; Wittmann, T.; Debnath, J. Autophagy in adhesion and migration. J. Cell Sci. 2016, 129, 3685–3693.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
745
Revisions:
2 times
(View History)
Update Date:
09 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





