Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sahr Lamin Sumana | -- | 3418 | 2023-10-07 05:02:42 | | | |
| 2 | Rita Xu | Meta information modification | 3418 | 2023-10-07 05:10:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sumana, S.L.; Chen, H.; Shui, Y.; Zhang, C.; Yu, F.; Zhu, J.; Su, S. Dietary Selenium. Encyclopedia. Available online: https://encyclopedia.pub/entry/49894 (accessed on 16 January 2026).
Sumana SL, Chen H, Shui Y, Zhang C, Yu F, Zhu J, et al. Dietary Selenium. Encyclopedia. Available at: https://encyclopedia.pub/entry/49894. Accessed January 16, 2026.
Sumana, Sahr Lamin, Huangen Chen, Yan Shui, Chengfeng Zhang, Fan Yu, Jian Zhu, Shengyan Su. "Dietary Selenium" Encyclopedia, https://encyclopedia.pub/entry/49894 (accessed January 16, 2026).
Sumana, S.L., Chen, H., Shui, Y., Zhang, C., Yu, F., Zhu, J., & Su, S. (2023, October 07). Dietary Selenium. In Encyclopedia. https://encyclopedia.pub/entry/49894
Sumana, Sahr Lamin, et al. "Dietary Selenium." Encyclopedia. Web. 07 October, 2023.
Copy Citation
Dietary selenium (Se) is an essential component that supports fish growth and the immune system. It emphasizes that optimal dietary Se levels are necessary for healthy biological processes in fish, such as growth, reproduction, and immunity. Since organic Se appears to be the most ideal for fish due to its low toxicity, environmental safety, and efficient fish culture, it explores the potential sources and forms of Se.
dietary Se effects
growth performance
immune functions
1. Introduction
Dietary Se is vital for the growth and immune system of fish. Dietary Se is a micronutrient that functions as a component of important selenoproteins for both humans and animals [1][2]. These selenoproteins are involved in various biological processes, including antioxidant defense, thyroid hormone metabolism, and immune function [3]. Se as a nutritional element is obtained in the aquatic environment from two main sources: feed and fertilizer. In areas where waste and other activities from agriculture, industries, mining, and other natural events are carried out, Se concentrations might seem higher because of closer point sources. However, human activities can transfer Se into aquatic food webs through pathway concentrations in seleniferous environments [4][5][6]. Increases in dietary Se can, however, upset the aquatic food chain, and overexposure may cause disruption in fish tissues, which is harmful to human consumption both directly and indirectly.
Aquatic animals may be subjected to biotic and abiotic stresses during the farming season [7]. The most important factor that helps to counteract these stressors and promote high productivity and well-being is a nutritionally balanced aquatic diet [8]. Another important measure to ensure the nutritional value of aquafeeds is the addition of microelements such as Se [9]. Se aids many biological functions in the bodies of animals, including those of an antioxidant, a metabolic stimulator, and an immunostimulant [8]. In fact, Se serves as a precursor for a number of metabolites involved in biological processes in the body [10]. The immune and antioxidant systems benefit from the role of Se in the synthesis of selenoproteins [11]. Moreover, it helps regulate liver and kidney functions as crucial organs for the release of body toxins and nitrogen residues [12]. According to Swain et al. [13], Se is crucial for the biological functions of the fish’s body. Nutrition is one of several elements that influence the growth and development of fish in aquatic environments. Selenium is a crucial dietary component that has a tremendous impact on fish growth. Understanding the effects of dietary selenium on fish growth is critical for sustainable aquaculture practices and maintaining ecological balance in aquatic ecosystems. Additionally, several fish species have been found to have better overall growth when their diets contain adequate levels of selenium. According to Wang et al. [14], adequate selenium supplementation in feed has been associated with improved feed efficiency, weight gain, and growth rates.
Additionally, dietary Se enhances immune responses and increases the production of immune-related molecules, such as interleukin-10 (IL-10) and immunoglobulin A (IgA), which are critical for immune regulation and defense against infection. Zhai et al. [15] found that Se supplementation has a positive effect on the immune system and intestinal barrier, reducing permeability and pathogen entry. Se also affects the function of various immune cells in fish, such as macrophages and lymphocytes, as it enhances the phagocytic activity of macrophages so that they can effectively engulf and eliminate pathogens [16]. It also modulates the production of pro-inflammatory cytokines, such as interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α), which are involved in the immune response against pathogens [17]. In addition, Se enhances lymphocyte proliferation and antibody production, promoting a robust immune response in fish. Se has been found to regulate the expression of immune-related genes in fish. For example, studies have shown that dietary Se supplementation promotes the expression of genes involved in the immune response, such as Toll-like receptors (TLRs) and cytokines [14]. These immune genes are needed for both the identification of pathogens and the start of immune reactions. Rainbow trout fed abundant organic Se (Se-Plex®) strengthened their immunity with 4 mg/kg of dietary Se which triggered a crucial response in the head kidney (HK) of rainbow trout and upregulated genes [18]. Rathore et al. [19] conducted a study on nano-Se supplementation in the diet of Nile tilapia, focusing on Se assimilation and the expression of immunoregulated selenoproteins, which showed enhanced Se absorption and utilization, while altered expression levels of immunoregulated selenoproteins were observed, suggesting possible effects on the immune system. In another study by Fontagné-Dicharry et al. [20], dietary Se was found to significantly affect the antioxidant status of rainbow trout fry and parameters related to oxidative stress. Generally, fish feed meal is a vital source of dietary Se in the aquatic environment that helps strengthen stress, inflammation, and infection response [21].
Se in fish feed should be used in the correct dosage, which should not exceed the levels recommended by the European Union. According to previous studies, the recommended Se dose in fish feed falls between 0.1 and 2.00 mg/kg; anything above this can have negative consequences [22]. Also, the European Union has enacted legislation stating that Se supplementation with organic Se should not exceed 0.5 mg/kg in feed [23][24][25].
Furthermore, the insight gained from knowledge about Se contents in fish feeds may ultimately lead to maximizing profitability for the feed industry by increasing fish farmers’ confidence and the demand for fish feed meal [26]. Adequate Se-rich feed from the feed industry supports the fish immune system, prevents disease, promotes fast growth, and ensures high meat quality [27][28]. By providing Se-enriched feeds, the feed industry can help prevent disease outbreaks and promote fish welfare. The feed industry can contribute to Se biofortification, i.e., increasing the Se concentrations in fish products for human consumption. Se-enriched feeds can help address Se deficiencies in regions where soils are naturally low in Se [29]. This ensures that fish farmers have access to Se-rich fish feed, which benefits their health and well-being. According to Wang et al. [26], Se can guarantee better fish health and secure food production, even though several countries have implemented limitations on the Se concentration of fish feed. The feed industry must adhere to Se contents based on fish species acceptability and standards to improve reputation, trust, and profitability. Se is essential for fish nutrition, health, disease prevention, biofortification, and regulatory compliance, enhancing the industry’s overall performance.
2. Sources of Se and Their Effects on the Aquatic Environment
Se is a naturally occurring element that interacts with fish’s environment through anthropogenic and natural means (see Figure 1). Thus, Se concentration in soil and aquatic environments depends on anthropogenic activities (industrial, mining, agriculture inputs, sewage sludge, oils, power plants, and mines) and natural occurrences (weathering rocks, volcanic eruptions, and atmospheric processes). There are ways in which Se can directly and indirectly get into the soil and aquatic environment, including erosion, runoff, and leaching that release coal ash into water bodies, particularly in Se-rich regions [4][30]. However, it also gets into the aquatic environment directly from mine discharges, agricultural fields, waste water, sewage disposals, and industrial discharges [6]. Khoshnood [31] highlighted the most frequent contaminants, such as heavy metals and pesticides, causing harm to fish in aquatic environments. According to Tan et al. [32], 40% of Se in the atmosphere and aquatic environment comes from industrial and mining activities. This percentage of Se was primarily influenced by human activities like burning coal and crude oil, which serves as an influencing factor [32]. In addition, these activities by humans have introduced high Se levels as micronutrient elements to the environment, causing imbalances in the food chain. The level of Se concentration in the aquatic environment in a particular area depends on a number of factors, such as the increase in demand for agriculture, industrial processes, mining power plants, chemical wastes deposited in the environment, from which Se comes from different sources, and the release of pollutants into the aquatic system [33][34]. Fish in the aquatic environment perform all metabolism processes, including Se intake, which may be carried out orally or assimilated into muscle and liver tissues.
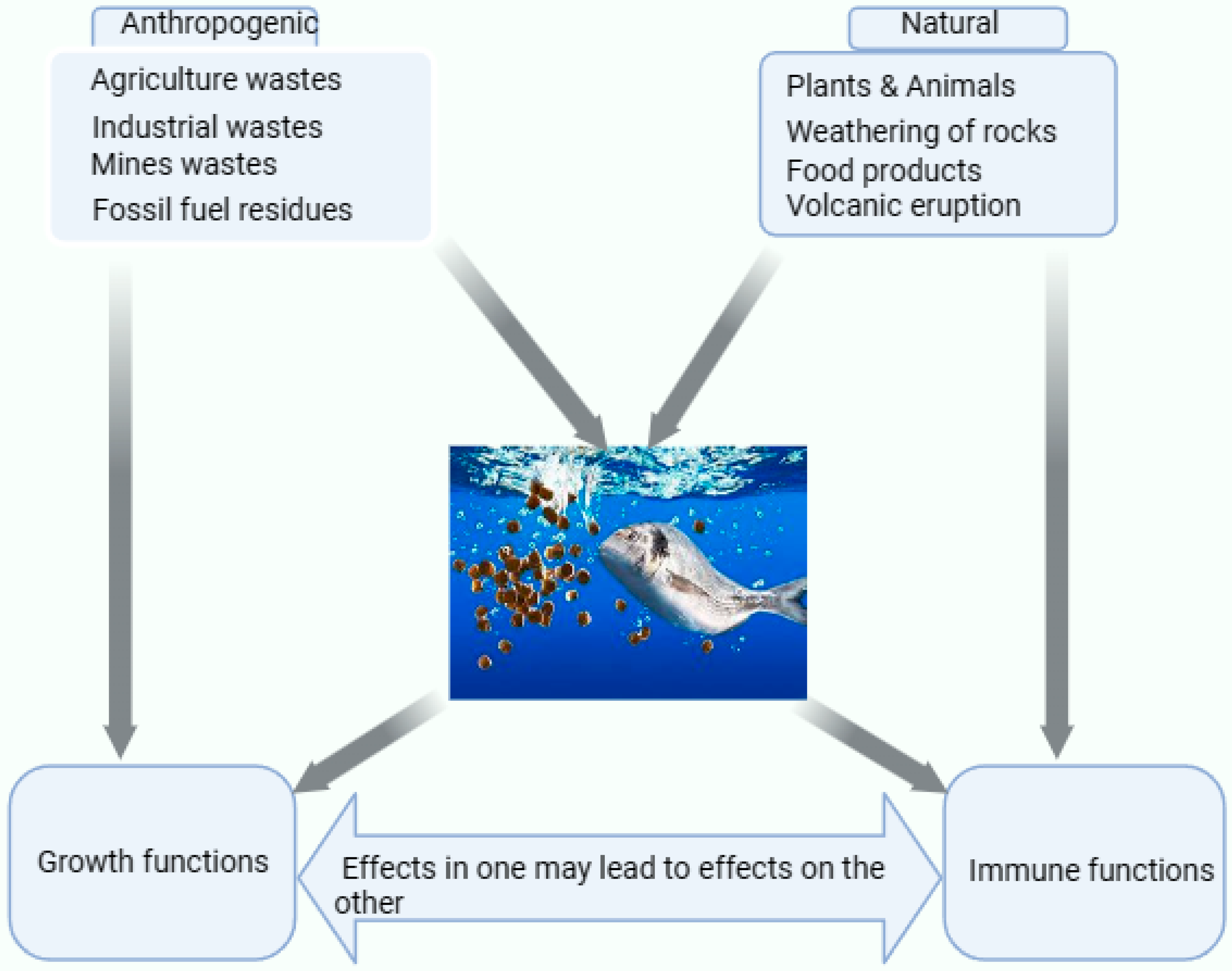
Figure 1. The sources of Se and their influences in the aquatic environment with the fish. Se in the aquatic environment may be derived from artificial (industrial wastes, fossil fuel residues, and mines) and natural sources (plant and animal products, weathering of rocks, volcanic eruptions, and food products). The tissues responsible for Se intake include the gills, muscles, liver, and kidney. The Se obtained in the aquatic environment could either influence their biological functions, such as growth and immunity, which have an inverse relationship wherein the impact on growth will influence immunity, or vice versa.
Fish species make excellent models for detecting genotoxic chemicals in aquatic ecosystems [35][36] due to their abundance and specific habitats [37]. Most toxicity levels are based on uptake in the aquatic environment, which is substantially lower in natural populations than uptake through the food chain. Se concentrations in soils vary depending on the location, activities, type, and nature of the soils. Tan et al. [38] reported that Se content in various soil types varies from 1.07 to 6.69%. Thus, natural processes (physical and mechanical) occurring in the soil could lead to an uneven distribution of Se in the environment. In addition, the Se content in the soils of many countries varies. Studies have found Se levels of 0.4 mg/kg in most parts of the world’s soil, including North America, Ireland, Australia, and Israel, as well as in some Chinese provinces (72% of the area of China), New Zealand, and a considerable part of Europe [39][40] (Table 1).
Table 1. Summarizing the sources and effects of the dispensation of Se in the aquatic environment.
| Sources of Se | Dispensation of Se in the Aquatic Environment | Effects of Action | Refs. |
|---|---|---|---|
| Agriculture | Derived from mining sites, fertilizer application, and sewage sludge (municipal). | It enhances Se in the aquatic environment through surface runoff, infiltration, leaching, and percolation. | [41][42] |
| Natural activities of parent rocks | Parents’ rocks weathering contributes to Se presence in environment. | Rocks contain various metals, and Se is obtained from their weathering and gets into the surface soils and groundwater of the aquatic environment | [41][42] |
| Industrial activities | Crude oil, coal, fossil fuels, nuclear fuel, mining, and smelting processes elevate Se levels in the soil and aquatic environment. | Several industries are located along aquatic environments for water and in most cases, dumping can easily occur. | [40][43] |
3. Forms of Dietary Se Intake and Their Effects on Fish Species
Fish species absorb dietary Se in various forms, such as organic and inorganic [44][45][46]. These forms of dietary Se impact fish’s biological functions. The forms of dietary Se aid fish species in several ways depending on their health, size, species, feed composition, and experiment conditions [47]. The biological functions of fish in their aquatic environment may be supported by dietary Se use. However, the majority of dietary Se absorption by fish species is efficient due to their bulkiness or minute size [48].
As for Se in the diet of fish, there are a few different forms commonly used as inorganic, such as Na2SeO3 or sodium selenate (Na2SeO4) (Table 2). They differ in toxicity, with Na2SeO3 being slightly less toxic than Na2SeO4 [49]. Na2SeO3 is commonly used in fish feed and is also found in plant foods, meats, seafood, and dietary supplements that provide numerous health benefits, while Na2SeO4 is an insecticide used in agriculture to control insects and can be used as a fungicide [50]. Both forms are readily absorbed by fish and can meet their Se requirements. Na2SeO3 plays an important role in fish nutrition and tissues, as a study by Rathore et al. [19] showed that supplementing fish feed with inorganic Se positively affects Se content in Nile tilapia tissues.
Organic forms of dietary Se also include selenomethionine (SeMet) and selenocysteine (SeCyst). The two forms often come from natural sources such as yeast or plants. According to a study by Penglase et al. [51], fish feed enriched with Se provides fish with a higher proportion of essential nutrients and thus promotes their accumulation in their tissues. Additionally, dietary organic Se in fish feed has resulted in strengthened retention and increased Se levels in the fish. Since fish tissues accumulate both essential and non-essential trace elements [52], it is important to have an understanding of the various Se forms and their effects on fish.
SeMet is one of the essential amino acids. Fish can obtain SeMet from their diet, and it is known to play a crucial role in their overall health and biological functions such as growth and the immune system. A study by Liu et al. [53] demonstrated that SeMet supplementation in grass carp diets contributed to fostering growth performance, immune response, and antioxidant capacity.
SeCyst is an amino acid that incorporates Se into selenoproteins, thereby affecting reproduction, egg quality, and spawning performance in fish [26]. It is important for protecting fish from oxidative stress and maintaining cellular health, as shown by Kumar et al. [54]. In addition, SeCyst is an important component of antioxidant enzymes such as GPxs.
Table 2. Forms of dietary Se and their effects on some fish species.
| Forms of Dietary Se Intake | Effect | Refs. |
|---|---|---|
| Na2SeO3 | Its supplementation in Mahseer fish shows better growth and biochemical health. | [55] |
| It enhances African catfish growth. | [56][57] | |
| Na2SeO3, a Se dietary supplement, improves fish growth, antioxidant capacity, and immune response in fish, including Nile tilapia. | [58] | |
| It supports the growth and survival rates of rainbow trout. | [59] | |
| SeMet | It supports better absorption and more bioavailability in species such as rainbow trout, fathead minnows, channel catfish, and Atlantic salmon. | [60] |
| It positively enhances innate immunity by improving serum lysozyme activity and GPxs activities in the liver and muscle tissues of Mahseer fish. | ||
| It causes better protective influences during stressful conditions with Medaka. | [55] | |
| Se content elevates the healthy growth of common carp. | [26] | |
| At 0.6 mg/kg, it supports the growth and immunity of goldfish. | [56] | |
| It stimulates the larval growth of rainbow trout fish. | [61] | |
| It promotes the growth potential of African catfish. | [57] | |
| Se Nanoparticles (nSe) | It enhances growth and improves antioxidant enzyme activities in juvenile black sea bream. | [14] |
| It increases the growth and immunity of goldfish at 0.6 mg/kg. | [56] | |
| It can alleviate hepatopancreas injury in high-fat diets and enhance the survival of grass carp. | [62] | |
| Na2SeO4 | It enhances the growth performance of African catfish. | [57] |
4. Se Characteristics in the Soil and Their Influences on the Aquatic Environment
Se is a chemical element that can naturally occur in soil. Soil serves as the primary source of Se, influencing its content in food, vegetables, and drinking water [38]. Hence, the amount of Se in the soil directly influences the amount of Se in fish. Soil parent material, physical and chemical properties, and other factors like soil type and land use could influence Se distribution in soil [63]. Se distribution in soil varies geographically and depends on factors such as parent material, climate, and land use practices. It can be found in varying concentrations worldwide (see Table 3), with higher levels typically found in regions with seleniferous deposits [63]. The bioavailability of Se in soil depends on its chemical form, as it can exist in different oxidation states (−2 to +6), with selenate (SeO4−) and selenite (SeO3−) being the most common forms. Na2SeO3 is more mobile and bioavailable, while SeO4− is prone to adsorption and immobilization in soil [64]. Soil contains various sources and forms of Se, including Se sulfides and Se ions. It is acidic, oily, and rich in organic matter. Se exists in various organic and inorganic forms, with selenopyrite or selenopyrite fixed to organic matter. According to Saha et al. [43], dietary Se exists in selenide (Se2−), SeO32−, and SeO42− forms, found in acidic, low redox soils, predominant in acidic, medium redox soils (0 to 200 mV), and dominant in alkaline, high redox soils (500 mV). Moreover, soil pH, organic matter content, and redox conditions significantly influence Se concentrations in soil. Acidic soils with low organic matter content can increase Se availability, while alkaline or high organic soils may reduce its mobility [65]. The interaction of Se in soil can have impacts on the aquatic environment, including fish populations. When Se-rich soils are eroded or irrigation water containing Se is used, Se can enter nearby water bodies through runoff or leaching. This can result in elevated Se concentrations in aquatic environments [66].
In the soil environment, the ideal Se levels, which support a variety of living organisms, including some fish species, particularly the African catfish in terms of extreme conditions, some crustaceans like crab, lobster, and prawns, as well as mollusks like snails and slugs, should exist. The soil environment serves in the capacity of producing food, transferring energy within food webs, releasing water, and regulating carbon [26][67]. Living organisms, including fish in the soil and aquatic environment, require adequate Se based on their acceptability for their survival and welfare. However, high Se concentration depends on soil types and characteristics such as sedimentary and organic matter, whereas magmatic rocks are poor in Se concentration and have a low Se content [68]. In areas where aquaculture structures are unmodernized, they allow external sources of Se to enter aquatic environments through runoff.
Moreover, authors have reported the global average Se concentration in soils at 0.1 and 2 mg/kg [69], 0.01–0.4 mg/kg [70], and 0.03–2.0 mg kg−1, with an average of 0.40 mg/kg [70][71]. The soil has multiple Se levels, and its characteristics are high, toxic, deficient, marginal, and moderate in levels of 0.40–1.00 mg/kg, 1.00–3.00 mg/kg, >3.00 mg/kg, <0.125 mg/kg, and 0.125–0.175 mg/kg [72], respectively, depending on type and characteristics. However, the average Se concentration level in soil globally falls within the range of 0.01–0.4 mg/kg [73][74]. The levels of Se in the soil vary based on the region or countries, as indicated in Table 3.
Table 3. Se concentration in soil environment and its characteristics.
| Countries | Se Concentrations in Soils (mg/kg) | Refs. |
|---|---|---|
| Belgium | It has low Se content in agricultural soils, with an average of 0.30. | [71][75] |
| China | Its average Se range falls within 0.01–2-Se and most top soils contain 0.06–9.13 | [64][76][77] |
| Denmark | A deficient soil Se level was found to be between 0.1 and 0.6. | [66] |
| England | <0.01–16 | [66] |
| Global Se levels | The average Se range in the world falls between 0.01 and 2.00. | [4][66] |
| Iran | 0.04–0.45 | [78] |
| Japan | It has an average Se range in the soil between 0.05 and 2.80. | [70][71] |
| Netherlands | Aqua regia has average Se contents of 0.12–1.9 for grassland soils and 0.20–1.20 for arable land soils. | [71] |
| Qatar | 0.12–0.77 | [79] |
| Scotland | It has a general average Se level in most soils between 0.12 and 0.88, and sandy soil contains 0.43. | [66][80] |
| Sweden | It has a low average Se content in agricultural soils of 0.30, the same as Belgium. | [71][81] |
5. Se Influences the Biological Functions of Various Diets of Fish Species
Many fish species’ biological processes are positively impacted by dietary Se, including thyroid regulation, growth promotion, improvement of fertility, immune system enhancement, and delay of aging [82][83][84], as well as antioxidant activity [85][86]. In contrast, Mechlaoui et al. [87] discovered that gilthead sea bream fed SeMet had better growth performance and higher antioxidant content than those fed sodium selenite (Na2SeO3). Ma et al. [88] demonstrated that dietary Se supplementation resulted in improved growth of grass carp. Abdel-Tawwab et al. [57] studied African catfish fed diets containing 0.2, 0.4, or 0.6 mg/kg Se and were also exposed to copper toxicity. They found that organic Se improved growth and reduced the negative effects of copper toxicity on physiology. Elia et al. [89] confirmed that fish fed Se-supplemented diets had higher growth rates and Se accumulation than the control group. Zhu et al. [90] investigated the effect of dietary Se on growth, body composition, and hepatic GPxs activity of largemouth bass; the researchers discovered that dietary Se supplementation improved growth and body composition and increased hepatic GPxs activity in largemouth bass, which may have a positive effect on their health. Han et al. [91] found that higher dietary Se content positively affected the overall growth performance of gibel carp compared to diets with lower Se content. Saffari et al. [92] confirmed that dietary Se is important to improve the growth and productivity of common carp and Ashouri et al. [93] reported that dietary Se concentrations of 0.1, 0.2, and 0.4 mg/kg improved the growth of common carp, muscle composition, and antioxidant status. Ibrahim et al. [8] confirmed that Nile tilapia fed nanoselenium (nanoSe) supplements had better growth rates and feed efficiency than those fed Se bulk supplements. Se is also important for biological functions like promoting fish growth, immune function, and health. It also boosts fish immunity, antioxidation, the stabilization of cell membranes [94][95], and the maintenance of normal metabolism in the body [30]. Fish feeding on regulated Se may support fish species performance by contributing to weight gain and specific growth rates in aquatic animals [63][92][93]. Studies on Wuchang bream have shown that Se increases growth hormone (GH) and insulin-like growth factor (IGF)-I levels in serum and messenger ribonucleic acid (mRNA) transcription levels of growth hormone receptor 2 (ghr2) and insulin-like growth factor 1 (igf1) in the liver [96]. It effectively promotes growth in various fish species, including grass carp, gibel carp, and rainbow trout [91][97].
References
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233.
- Hamilton, S.J. Review of selenium toxicity in the aquatic food chain. Sci. Total. Environ. 2004, 326, 1–31.
- Lu, J.; Holmgren, A. Selenoproteins. J. Biol. Chem. 2009, 284, 723–727.
- Ullah, H.; Liu, G.; Yousaf, B.; Ali, M.U.; Irshad, S.; Abbas, Q.; Ahmad, R. A comprehensive review on environmental transformation of selenium: Recent advances and research perspectives. J. Environ. Geochem. Health 2019, 41, 1003–1035.
- USEPA-United States Environmental Protection Agency. Draft Aquatic Life and Aquatic-Dependent Wildlife Se Water Quality Criterion for Freshwaters of California; U.S. EPA, Office of Water, Office of Science and Technology: Washington, DC, USA, 2018.
- Maier, K.J.; Knight, A.W. Ecotoxicology of Se in freshwater systems. Rev. Environ. Contam. Toxicol. 1994, 134, 31–48.
- Dawood, M.A.O.; El Basuini, M.F.; Zaineldin, A.I.; Yilmaz, S.; Hasan, M.T.; Ahmadifar, E.; El Asely, A.M.; Abdel-Latif, H.M.R.; Alagawany, M.; Abu-Elala, N.M.; et al. Antiparasitic and antibacterial functionality of essential oils: An alternative approach for sustainable aquaculture. Pathogens 2021, 10, 185.
- Ibrahim, M.S.; El-gendy, G.M.; Ahmed, A.I.; Elharoun, E.R.; Hassaan, M.S. NanoSe versus bulk Se as a dietary supplement: Effects on growth, feed efficiency, intestinal histology, haemato-biochemical and oxidative stress biomarkers in Nile tilapia (Oreochromis niloticus Linnaeus, 1758) fingerlings. Aquac. Res. 2021, 52, 5642–5655.
- Sarkar, B.; Bhattacharjee, S.; Daware, A.; Tribedi, P.; Krishnani, K.K.; Minhas, P.S. Se nanoparticles for stress-resilient fish and livestock. Nanoscale Res. Lett. 2015, 10, 371.
- Mehdi, Y.; Hornick, J.L.; Istasse, L.; Dufrasne, I. Se in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311.
- Holben, D.H.; Smith, A.M. The diverse role of Se within selenoproteins: A review. J. Am. Diet. Assoc. 1999, 99, 836–843.
- Wang, N.; Tan, H.-Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of micronutrient Se in metabolic diseases: Its role as an antioxidant. Oxid. Med. Cell. Longev. 2017, 2017, 7478523.
- Swain, P.; Das, R.; Das, A.; Padhi, S.K.; Das, K.C.; Mishra, S.S. Effects of dietary zinc oxide and Se nanoparticles on growth performance, immune responses and enzyme activity in rohu, Labeo rohita (Hamilton). Aquac. Nutr. 2019, 25, 486–494.
- Carlson, B.A.; Yoo, M.H.; Shrimali, R.K.; Irons, R.; Gladyshev, V.N.; Hatfield, D.L.; Park, J.M. Role of Se-containing proteins in T-cell and macrophage function. Proc. Nutr. Soc. 2010, 69, 300–310.
- Zhai, Q.; Cen, S.; Li, P.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Effects of Dietary Selenium Supplementation on Intestinal Barrier and Immune Responses Associated with Its Modulation of Gut Microbiota. J. Environ. Sci. Technol. Lett. 2018, 5, 724–730.
- Avery, J.; Hoffmann, P. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203.
- Niu, R.; Yang, Q.; Dong, Y.; Hou, Y.; Liu, G. Selenium metabolism and regulation of immune cells in immune-associated diseases. J. Cell. Physiol. 2022, 237, 3449–3464.
- Pacitti, D.; Lawan, M.M.; Feldmann, J.; Sweetman, J.; Wang, T.; Martin, S.A.M.; Secombes, C.J. Impact of selenium supplementation on fish antiviral responses: A whole transcriptomic analysis in rainbow trout (Oncorhynchus mykiss) fed supranutritional levels of Sel-Plex®. BMC Genom. 2016, 17, 116.
- Rathore, S.S.; Murthy, H.S.; Mamun, M.A.-A.; Nasren, S.; Rakesh, K.; Kumar, B.T.N.; Abhiman, P.B.; Khandagale, A.S. Nano-Se supplementation to ameliorate nutrition physiology, immune response, antioxidant system and disease resistance against Aeromonas hydrophila in monosex Nile tilapia (Oreochromis niloticus). Biol. Trace Elem. Res. 2021, 199, 3073–3088.
- Fontagné-Dicharry, S.; Godin, S.; Liu, H.; Prabhu, P.A.J.; Bouyssière, B.; Bueno, M.; Tacon, P.; Médale, F.; Kaushik, S.J. Influence of the forms and levels of dietary selenium on antioxidant status and oxidative stress-related parameters in rainbow trout (Oncorhynchus mykiss) fry. Br. J. Nutr. 2015, 113, 1876–1887.
- Hoffmann, P.R. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273.
- Gatlin, D.M.; Wilson, R.P. Dietary Se requirement of fingerling channel catfish. J. Nutr. 1984, 114, 627–644.
- European Commission. Commission Implementing Regulation (EU) No 2015/489 of 23 March 2015 concerning the authorisation of selenomethionine produced by Saccharomyces cerevisiae NCYC R645 as a feed additive for all animal species (Text with EEA relevance). Off. J. Eur. Union 2015, 78, 5–8.
- European Commission. Commission Implementing Regulation (EU) No 121/2014 of 7 February 2014 concerning the authorisation of L-selenomethionine as a feed additive for all animal species (Text with EEA relevance). Off. J. Eur. Union 2017, 39, 53–55.
- European Commission. Commission Implementing Regulation (EU) No 847/2014 of 4 August 2014 concerning the authorisation of DL-selenomethionine as a feed additive for all animal species (Text with EEA relevance). Off. J. Eur. Union 2017, 232, 10–12.
- Wang, Y.F.; Chen, P.; Wang, F.H.; Han, W.X.; Qiao, M.; Dong, W.X.; Hu, C.S.; Zhu, D.; Chu, H.Y.; Zhu, Y.G. The ecological clusters of soil organisms drive the ecosystem multifunctionality under long-term fertilization. Environ. Int. 2022, 161, 107133.
- Nip, T.H.M.; Ho, W.Y.; Wong, C.K. Feeding ecology of larval and juvenile black seabream (Acanthopagrus schlegeli) and Japanese seaperch (Lateolabrax japonicus) in Tolo Harbour, Hong Kong. Environ. Biol. Fishes 2003, 66, 197–209.
- Barchielli, G.; Capperucci, A.; Tanini, D. The Role of Selenium in Pathologies: An Updated Review. Antioxidants 2022, 11, 251.
- Rayman, M.P.; Winther, K.H.; Pastor-Barriuso, R.; Cold, F.; Thvilum, M.; Stranges, S.; Guallar, E.; Cold, S. Effect of long-term selenium supplementation on mortality: Results from a multiple-dose, randomised controlled trial. Free Radic. Biol. Med. 2018, 127, 46–54.
- Otter, R.R.; Bailey, F.C.; Fortner, A.M.; Adams, S.M. Trophic status and metal bioaccumulation differences in multiple fish species exposed to coal ash-associated metals. Ecotoxicol. Environ. Saf. 2012, 85, 30–36.
- Khoshnood, Z. Using Biomarkers in Ecotoxicology: What and Why? Focus Sci. 2016, 2, 1–2.
- Tan, L.C.; Nancharaiah, Y.V.; van Hullebusch, E.D.; Lens, P.N.L. Selenium: Environmental significance, pollution, and biological treatment technologies. Biotechnol. Adv. 2016, 34, 886–907.
- Kaur, R.; Dua, A. Scales of freshwater fish Labeo rohita as bioindicators of water pollution in Tung Dhab Drain, Amritsar, Punjab, India. J. Toxicol. Environ. Health Part A 2015, 78, 388–396.
- Byrne, S.; Miller, P.; Waghiyi, V.; Buck, C.L.; von Hippel, F.A.; Carpenter, D.O. Persistent organochlorine pesticide exposure related to a formerly used defence site on St. Lawrence Island, Alaska: Data from sentinel fish and human sera. J. Toxicol. Environ. Health Part A 2015, 78, 976–992.
- Aich, A.; Goswami, A.R.; Roy, U.S.; Mukhopadhyay, S.K. Ecotoxicological assessment of tannery effluent using guppy fish (Poecilia reticulata) as an experimental model: A biomarker study. J. Toxicol. Environ. Health Part A 2015, 78, 278–286.
- Walia, G.K.; Handa, D.; Kaur, H.; Kalotra, R. Ecotoxicological studies on fish, Labeo rohita exposed to tannery industry effluent by using micronucleus test. Nucleus 2015, 58, 111–116.
- Ali, F.K.; El-Shehawi, A.M.; Seehy, M.A. Micronucleus test in fish genome: A sensitive monitor for aquatic pollution. Afr. J. Biotechnol. 2008, 7, 606–612.
- Tan, J.; Zhu, W.; Wang, W.; Li, R.; Hou, S.; Wang, D.; Yang, L. Se in soil and endemic diseases in China. Sci. Total Environ. 2002, 284, 227–235.
- Zhou, X.; Li, Y.; Lai, F. Effects of different water management on absorption and accumulation of Se in rice. Saudi. J. Biol. Sci. 2018, 25, 1178–1182.
- Mayland, H.F.; James, L.F.; Panter, K.E.; Sonderegger, J.L. Se in seleniferous environments. Soil Sci. Soc. Am. J. 1989, 23, 15–50.
- Dixit, R.; Wasiullah; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; et al. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability 2015, 7, 2189–2212.
- Li, H.F.; McGrath, S.P.; Zhao, F.J. Se uptake, translocation and speciation in wheat supplied with selenate or selenite. N. Phytol 2008, 178, 92–102.
- Saha, U.; Fayiga, A.; Sonon, L. Se in the Soil-Plant Environment: A Review. Int. J. Appl. Agric. Sci. 2017, 3, 1–18.
- Janz, D.M.; DeForest, D.K.; Brooks, M.L.; Chapman, P.M.; Gilron, G.; Hoff, D.; Hopkins, W.A.; McIntyre, D.O.; Mebane, C.A.; Palace, V.P.; et al. Se toxicity to aquatic organisms. In Ecological Assessment of Se in the Aquatic Environment; Society of Environmental Toxicology and Chemistry (SETAC): Pensacola, FL, USA, 2010; pp. 139–230.
- Thomas, J.K.; Wiseman, S.; Giesy, J.P.; Janz, D.M. Effects of chronic dietary selenomethionine exposure on repeat swimming performance, aerobic metabolism and methionine catabolism in adult zebrafish (Danio rerio). Aquat. Toxicol. 2013, 130–131, 112–122.
- Albrecht, M.A.; Evans, C.W.; Raston, C.L. Green chemistry and the health implications of nanoparticles. Green Chem. 2006, 8, 417–432.
- Yu, Q.; Xia, C.; Han, F.; Xu, C.; Rombenso, A.; Qin, J.G.; Chen, L. Effect of Different Dietary Selenium Sources on Growth Performance, Antioxidant Capacity, Gut Microbiota, and Molecular Responses in Pacific White Shrimp Litopenaeus vannamei. Aquac. Nutr. 2022, 2022, 5738008.
- Zhang, J.S.; Wang, X.F.; Xu, T.W. Elemental Se at nano size (Nano-Se) as a potential chemo preventive agent with reduced risk of Se toxicity: Comparison with Se-methyl selenocysteine in mice. Toxicol. Sci. 2008, 101, 22–31.
- Franke, K.W.; Moxon, A.L. A comparison of the minimum fatal doses of selenium, tellurium, arsenic, and vanadium. J. Pharmacol. Exp. Ther. 1936, 58, 454–459.
- Lemly Agency for Toxic Substances and Disease Registry (ATSDR, 1996) Toxicological Profile for Selenium (Update); ATSDR: Atlanta, GA, USA, 1996.
- Penglase, S.; Hamre, K.; Ellinngsen, S. Selenium and mercury have a synergistic negative effect on fish reproduction. Aquat. Toxicol. 2014, 149, 16–24.
- Hussein, A.; Khaled, A. Determination of metals in tuna species and bivalves from Alexandria, Egypt. Egypt. J. Aquat. Res. 2014, 40, 9–17.
- Liu, L.; Liang, X.; Li, J.; Fang, J.; Alam, M. Effects of dietary selenium on growth performance and oxidative stress in juvenile grass carp (Ctenopharyngodon idellus). Aquac. Nutr. 2018, 24, 1296–1303.
- Kumar, N.; Krishnani, K.K.; Gupta, S.K.; Singh, N.P. Se nanoparticles enhanced thermal tolerance and maintain cellular stress protection of Pangasius hypophthalmus reared under lead and high temperature. Respir. Physiol. Neurobiol. 2017, 246, 107–116.
- Raza, A. Effects of Graded Levels of Dietary Se Supplementation on the Growth of Juvenile Mahseer (Tor putitora). Master’s Thesis, Quaid-i-Azam University, Islamabad, Pakistan, 2012.
- Jahanbakhshi, A.; Pourmozaffar, S.; Adeshina, I.; Mahmoudi, R.; Erfanifar, E.; Ajdari, A. Se nanoparticle and selenomethionine as feed additives: Effects on growth performance, hepatic enzymes’ activity, mucosal immune parameters, liver histology, and appetite-related gene transcript in goldfish (Carassius auratus). Fish Physiol. Biochem. 2021, 47, 639–652.
- Abdel-Tawwab, M.; Mousa, M.A.A.; Abbass, F.E. Growth performance and physiological response of African catfish, Clarias gariepinus (B.) fed organic selenium prior to the exposure to environmental copper toxicity. Aquaculture 2007, 272, 335–345.
- Chen, H.; Li, J.; Yan, L.; Cao, J.; Li, D.; Huang, G.-Y.; Shi, W.-J.; Dong, W.; Zha, J.; Ying, G.-G.; et al. Subchronic effects of dietary selenium yeast and selenite on growth performance and the immune and antioxidant systems in Nile tilapia Oreochromis niloticus. Fish Shellfish. Immunol. 2020, 97, 283–293.
- Kousha, M.; Yeganeh, S.; Amirkolaie, A.K. Synergistic effect of sodium selenite and Pediococcus acidilactici on growth, intestinal bacterial counts, selenium bioavailability, hepatic enzymes and non-specific immune response in rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2019, 26, 74–87.
- Khan, K.U.; Zuberi, A.; Fernandes, J.B.K.; Ullah, I.; Sarwar, H. An overview of the ongoing insights in Se research and its role in fish nutrition and fish health. Physiol. Biochem. Fish 2017, 43, 1689–1705.
- Vidal, D.; Bay, S.M.; Schlenk, D. Effects of dietary selenomethionine on larval rainbow trout (Oncorhynchus mykiss). Arch Env. Contam Toxicol. 2005, 49, 71–75.
- Liu, G.; Yu, H.; Wang, C.; Li, P.; Liu, S.; Zhang, X.; Zhang, C.; Qi, M.; Ji, H. Nano-Se supplements in high-fat diets relieve hepatopancreas injury and improve survival of grass carp Ctenopharyngodon idella by reducing lipid deposition. Aquaculture 2021, 538, 736580.
- Pan, Z.; Feng, Y.; Wang, M.; Meng, W.; Chen, J. Geochemical characteristics of soil selenium and evaluation of selenium-rich land resources in Guiyang area. Front. Geochem. 2023, 1, 1094023.
- Arocha, M.A.; McCoy, B.J.; Jackman, A.P. VOC immobilization in soil by adsorption, absorption and encapsulation. J. Hazard. Mater. 1996, 51, 131–149.
- Zayed, A.M.; Terry, N. Chromium in the Environment: Factors Affecting Biological Remediation. Plant Soil 2003, 249, 139–156.
- Fordyce, F.M. Selenium Deficiency and Toxicity in the Environment; Elseweir: London, UK, 2012.
- Tolu, J.; Bouchet, S.; Helfenstein, J.; Hausheer, O.; Chékifi, S.; Frossard, E.; Tamburini, F.; Chadwick, O.A.; Winkel, L.H.E. Understanding soil Se accumulation and bioavailability through size resolved and elemental characterization of soil extracts. Nat. Commun. 2022, 13, 6974.
- McNeal, J.M.; Balistrieri, L.S. Geochemistry and occurrence of Se: An overview. In Se in Agriculture and the Environment; Jacobs, L.W., Ed.; SSSA Special Publication: Canberra, Australia, 1989.
- Pezzarossa, B.; Petruzzeli, G. Selenium contamination in soil: Sorption and desorption processes. In Heavy Metals Release in Soils; Selim, H.M., Spark, D.L., Eds.; Lewis Publishers: Boca Raton, FL, USA, 2001; pp. 191–206.
- Yamada, H.; Kamada, A.; Usuki, M.; Yanai, J. Total selenium content of agricultural soils in Japan. Soil Sci. Plant Nutr. 2009, 55, 616–622.
- Supriatin, S.; Weng, L.; Comans, R.N.J. Selenium speciation and extractability in Dutch agricultural soils. Sci. Total Environ. 2015, 532, 368–382.
- Kumar, N.; Krishnani, K.K.; Gupta, S.K.; Sharma, R.; Baitha, R.; Singh, D.K.; Singh, N.P. Immuno-protective role of biologically synthesized dietary Se nanoparticles against multiple stressors in Pangasinodon hypophthalmus. Fish Shellfish Immunol. 2018, 78, 289–298.
- Feinberg, A.; Stenke, A.; Peter, T.; Hinckley, E.-L.S.; Driscoll, C.T.; Winkel, L.H.E. Reductions in the deposition of sulfur and Se to agricultural soils pose risk of future nutrient deficiencies. Commun. Earth Environ. 2021, 2, 101.
- Porcal, P.; Koprivnjak, J.-F.; Molot, L.A.; Dillon, P.J. Humic substances—Part 7: The biogeochemistry of dissolved organic carbon and its interactions with climate change. Environ. Sci. Pollut. Res. 2009, 16, 714–726.
- De Temmerman, L.; Waegeneers, N.; Thiry, C.; Laing, G.D.; Tack, F.; Ruttens, A. Selenium content of Belgian cultivated soils and its uptake by field crops and vegetables. Sci. Total Environ. 2014, 468–469, 77–82.
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Se distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309.
- Dungan, R.S.; Frankenberger, W.T. Microbial transformations of Se and the bioremediation of seleniferous environments. Biorem. J. 1999, 3, 171–188.
- Kieliszek, M.; Bano, I.; Zare, H. A Comprehensive Review on Se and Its Effects on Human Health and Distribution in Middle Eastern Countries. Biol. Trace Elem. Res. 2022, 200, 971–987.
- Shomar, B. Geochemistry of soil and groundwater in arid regions: Qatar as a case study. Groundw. Sustain. Dev. 2015, 1, 33–40.
- Shand, C.A.; Eriksson, J.; Dahlin, A.S.; Lumsdon, D.G. Selenium concentrations in national inventory soils from Scotland and Sweden and their relationship with geochemical factors. J. Geochem. Explor. 2012, 121, 4–14.
- Johnsson, L. Selenium in Swedish Soils. Factors Influencing Soil Content and Plant Uptake; Department of Soil Sciences, Swedish University of Agricultural Sciences: Uppsala, Sweden, 1992.
- Cai, Z.; Zhang, J.; Li, H. Selenium, aging and aging-related diseases. Aging Clin. Exp. Res. 2019, 31, 1035–1047.
- Liu, H.M.; Li, X.M.; Qin, F.; Huang, K. Se suppresses oxidative-stress-enhanced vascular smooth muscle cell calcification by inhibiting the activation of the PI3K/AKT and ERK signaling pathways and endoplasmic reticulum stress. J. Biol. Inorg. Chem. 2014, 19, 375–388.
- Ahsan, U.; Kamran, Z.; Raza, I.; Ahmad, S.; Babar, W.; Riaz, M.; Iqbal, Z. Role of Se in male reproduction—A review. Anim. Reprod. Sci. 2014, 146, 55–62.
- Wolonciej, M.; Milewska, E.; Roszkowska-Jakimiec, W. Trace elements as an activator of antioxidant enzymes. Postep. Hig. Med. 2016, 70, 1483–1498.
- Zhang, X.; Liu, C.; Guo, J.; Song, Y. Se status and cardiovascular diseases: Meta-analysis of prospective observational studies and randomized controlled trials. Eur. J. Clin. Nutr. 2016, 70, 162–169.
- Mechlaoui, M.; Dominguez, D.; Robaina, L.; Geraert, P.-A.; Kaushik, S.; Saleh, R.; Briens, M.; Montero, D.; Izquierdo, M. Effects of different dietary selenium sources on growth performance, liver and muscle composition, antioxidant status, stress response and expression of related genes in gilthead seabream (Sparus aurata). Aquaculture 2019, 507, 251–259.
- Ma, P.; Hu, Z.; Li, L.; Li, D.; Tang, R. Dietary selenium promotes the growth performance through growth hormone-insulin-like growth factor and hypothalamic-pituitary-thyroid axes in grass carp (Ctenopharyngodon idella). Fish Physiol. Biochem. 2021, 47, 1313–1327.
- Elia, A.C.; Prearo, M.; Pacini, N.; Dorrr, A.J.M.; Abete, M.C. Effects of selenium diets on growth, accumulation and antioxidant response in juvenile carp. Ecotoxicol. Env. Saf. 2011, 74, 166–173.
- Zhu, Y.; Chen, Y.; Liu, Y.; Yang, H.; Liang, G.; Tian, L. Effect of dietary selenium level on growth performance, body composition and hepatic glutathione peroxidase activities of largemouth bass Micropterus salmoide. Aquac. Res. 2011, 43, 1660–1668.
- Han, D.; Xie, S.; Liu, M.; Xiao, X.; Liu, H.; Zhu, X.; Yang, Y. The effects of dietary selenium on growth performances, oxidative stress and tissue selenium concentration of gibel carp (Carassius auratus gibelio). Aquac. Nutr. 2011, 17, e741–e749.
- Saffari, S.; Keyvanshokooh, S.; Zakeri, M.; Johari, S.A.; Pasha-Zanoosi, H. Effects of different dietary Se sources (sodium selenite, selenomethionine and nanoSe) on growth performance, muscle composition, blood enzymes and antioxidant status of common carp (Cyprinus carpio). Aquac. Nutr. 2016, 23, 611–617.
- Ashouri, S.; Keyvanshokooh, S.; Salati, A.P.; Johari, S.A.; Pasha-Zanoosi, H. Effects of different levels of dietary Se nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquaculture 2015, 446, 25–29.
- DeForest, D.K.; Brix, K.V.; Elphick, J.R.; Rickwood, C.J.; deBruyn, A.M.H.; Tear, L.M.; Gilron, G.; Hughes, S.A.; Adams, W.J. Lentic, lotic, and sulfate-dependent waterborne Se screening guidelines for freshwater systems. Environ. Toxicol. Chem. 2017, 36, 2503–2513.
- Fernandes, I.O.; Oliveira, M.L.d.; Delabie, J.H.C. Description of two new species in the Neotropical Pachycondyla foetida complex (Hymenoptera: Formicidae: Ponerinae) and taxonomic notes on the genus. Myrmecol. News 2014, 19, 133–163.
- Guo, H.; Lin, W.; Hou, J.; Wang, L.; Zhang, D.; Wu, X.; Li, L.; Li, D. The Protective Roles of Dietary Selenium Yeast and Tea Polyphenols on Growth Performance and Ammonia Tolerance of Juvenile Wuchang Bream (Megalobrama amblycephala). Front. Physiol. 2018, 9, 1371.
- Zheng, L.; Feng, L.; Jiang, W.D.; Wu, P.; Tang, L.; Kuang, S.Y.; Zeng, Y.Y.; Zhou, X.Q.; Liu, Y. Selenium deficiency impaired immune function of the immune organs in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2018, 77, 53–70.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
765
Revisions:
2 times
(View History)
Update Date:
07 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




