Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Astrid Gerlinde Petzoldt | -- | 3097 | 2023-09-28 16:26:27 | | | |
| 2 | Fanny Huang | Meta information modification | 3097 | 2023-10-09 09:00:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Petzoldt, A.G. Presynaptic Precursor Vesicle Biogenesis. Encyclopedia. Available online: https://encyclopedia.pub/entry/49778 (accessed on 07 February 2026).
Petzoldt AG. Presynaptic Precursor Vesicle Biogenesis. Encyclopedia. Available at: https://encyclopedia.pub/entry/49778. Accessed February 07, 2026.
Petzoldt, Astrid G.. "Presynaptic Precursor Vesicle Biogenesis" Encyclopedia, https://encyclopedia.pub/entry/49778 (accessed February 07, 2026).
Petzoldt, A.G. (2023, September 28). Presynaptic Precursor Vesicle Biogenesis. In Encyclopedia. https://encyclopedia.pub/entry/49778
Petzoldt, Astrid G.. "Presynaptic Precursor Vesicle Biogenesis." Encyclopedia. Web. 28 September, 2023.
Copy Citation
The faithful formation and, consequently, function of a synapse requires continuous and tightly controlled delivery of synaptic material. At the presynapse, a variety of proteins with unequal molecular properties are indispensable to compose and control the molecular machinery concerting neurotransmitter release through synaptic vesicle fusion with the presynaptic membrane. As presynaptic proteins are produced mainly in the neuronal soma, they are obliged to traffic along microtubules through the axon to reach the consuming presynapse.
presynaptic precursor vesicles
Rab2
1. Introduction
A neuron is a highly polarised cell with the cell body often distant from its synaptic terminals, where synapses are formed and maintained. Robust presynapse formation, maturation, and maintenance depend on the continuous delivery of presynaptic proteins from the neuronal soma, the predominant site of protein production. In the neuronal soma, newly synthetised presynaptic proteins are packaged into presynaptic transport vesicles or presynaptic precursor vesicles (PVs, this general term is used to unitingly denominate all forms of presynaptic protein-transporting vesicles or organelles), exported from the soma to traffic anterogradely on polarised microtubules (MTs) along the axon to the consuming presynapse (Figure 1) [1][2][3][4][5][6][7][8][9].
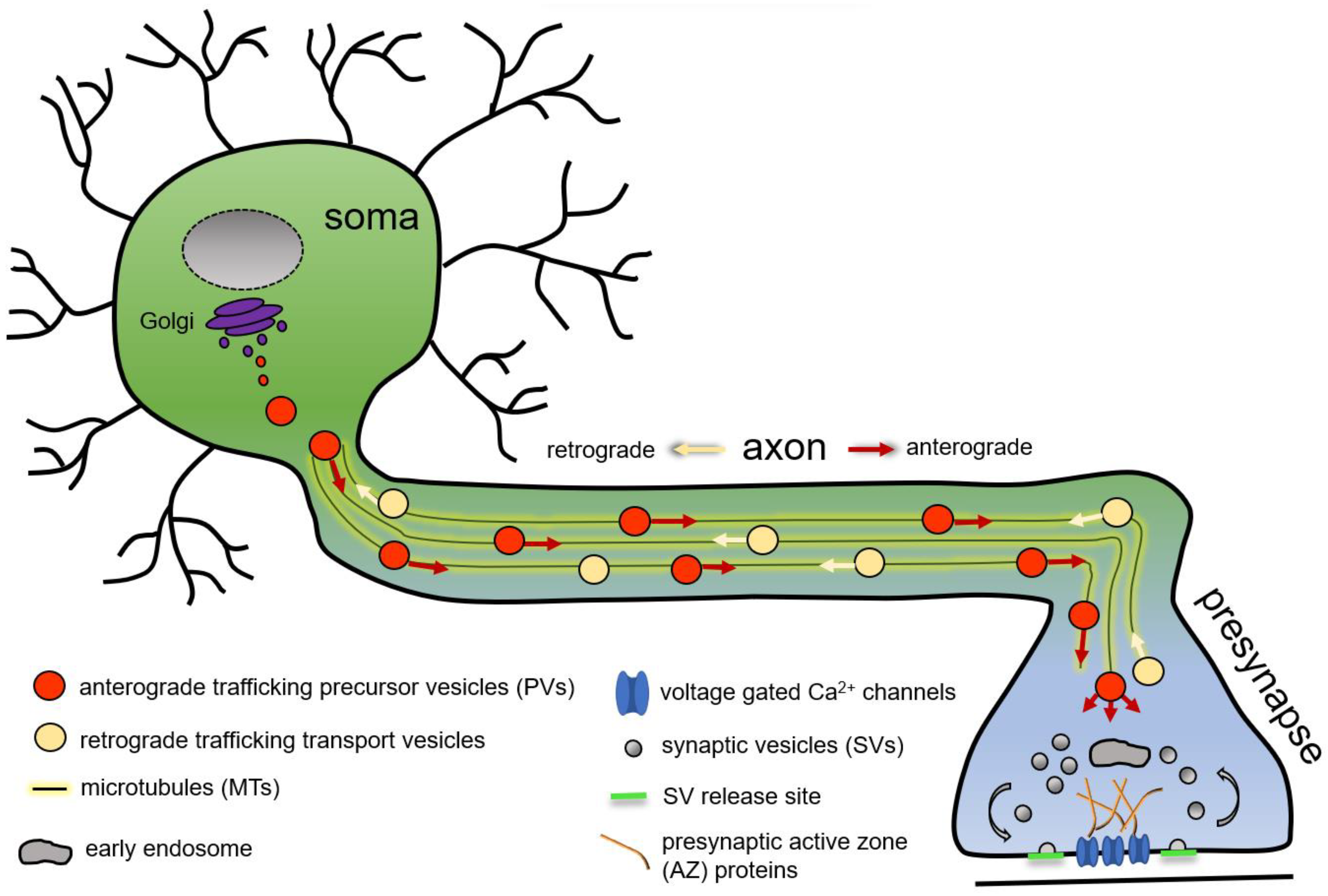
Figure 1. Schematic representation of microtubule-based axonal presynaptic precursor vesicle transport of presynaptic proteins from the neuronal soma to the consuming presynapse.
Of note, although protein biosynthesis predominantly occurs in the neuronal soma, growing evidence is raised that pre- and postsynaptic local mRNA translation and protein synthesis significantly contribute to activity-dependent synapse plasticity mechanisms [10][11][12][13]. Nonetheless, despite the fact that mRNA transcripts are detected in axons and growth cones [14], presynaptic protein transcripts are not sufficiently enriched to solely account for the extensive protein demand of synaptic terminals [7][15].
2. Presynaptic Precursor Vesicle Cargo, Morphology, and Transport Arrangements
2.1. Precursor Vesicle Cargo: Presynaptic Proteins
The presynapse is composed of a variety of structurally and functionally highly diverse proteins, including synaptic vesicle (SV) proteins with integral membrane and membrane-associated proteins, cytosolic active zone (AZ) proteins and release factors, ion-channels, adhesion molecules, and presynaptic membrane proteins coordinating vesicle exo- and endocytosis [2][3][8][16]. In numbers, it was estimated that an average presynapse in the vertebrate brain contains as many as approximately 300,000 molecules [17].
Scaffold proteins structure the presynaptic AZ, the site of SV fusion and neurotransmitter release, by defining SV release sites, clustering voltage-gated calcium channels (VGCCs or Cavs) in the centre of the AZ, and tethering SVs [16][18][19]. They comprise RIM-BP (Rab3-interacting molecule binding protein), the ELKS/CAST protein family (glutamic acid (E), lysine (K), leucine (L), and serine (S)-rich protein = ELKS; cytomatrix at the active zone (CAZ)-associated structural protein = CAST), including the ELKS/CAST homologue Bruchpilot (BRP) in Drosophila, the vertebrate specific proteins Piccolo and Bassoon, as well as Liprin-α/Syd-2 (LAR-interacting protein = Liprin; synapse defective 2 = Syd-2), Syd-1, and Spinophilin/Neurabin [16][18][19][20]. Release factors such as RIM (Rab3-interacting molecule) and (M)Unc13 (uncoordinated movement 13) concert the SV release, and (M)Unc18 (uncoordinated movement 18) and complexin regulate SNARE (soluble N-ethylmaleimide-sensitive-factor attachment receptor) complex activity [16][21][22][23][24]. Apart from these cytosolic proteins, several integral membrane proteins localise to different membranes and organelles at the presynapse. Fusion of SVs is triggered by local Ca2+ influx through voltage-gated Ca2+ channels upon arrival of an action potential and is executed by the SNARE-complex with its proteins residing in the SV (Synaptobrevin/Vamp) and presynaptic membrane (SNAP-25 = synaptosomal-associated protein, 25 kDa and Syntaxin) [23][24]. The SV membrane is crowded with integral membrane proteins [25] including Synaptotagmins as Ca2+ sensors, VGlut (vesicular glutamate transport protein), Synaptophysin, Synaptogyrin, SV2 (synaptic vesicle protein 2), and associated or peripheral membrane proteins, e.g., Rab3 (Ras-associated binding), CSP (cysteine string protein), and Synapsin [2][8][23][24]. Presynaptic transmembrane proteins further include synaptic adhesion molecules, e.g., N-cadherin and Neurexin, forming trans-synaptic connections to the postsynapse [2][3][16][23].
This plurality of presynaptic proteins, including integral or peripheral membrane and cytoplasmic proteins, is transported speedily by PVs to the synapse, as synapse formation itself is a rapid process and occurs within 30–60 min in vertebrate neurons after initial axodendritic contact formation [4][26], and in C. elegans, accumulation of presynaptic proteins in nascent synaptic specialisations occurs even within 5 min [27]. The fast delivery and synapse formation kinetic raise several conceptual questions: Are presynaptic proteins transported individually or in stoichiometric preassembled units? What is the cargo of a single PV? Are different PV types transporting different protein assortments, e.g., SV versus AZ proteins or membrane proteins versus cytosolic proteins? Would a difference in cargo load require different PV morphologies or are PVs uniform in size and shape? Is each individual PV transported singly by a specific kinesin or do PVs assemble into packets for axonal transport and delivery? None of these questions have been answered unambiguously to date; however, several concepts have been proposed, and a multiplicity of experimental data were collected over the last decades, spanning the different genetic model systems.
2.2. Presynaptic Precursor Vesicles: Ultrastructure and Cargo in Fixed Cells
Early studies in 1989 visualised by electron microscopy (EM) synapses of the mouse CNS (central nervous system) and described “granulated” vesicles of 60–80 nm size and “empty” vesicles with varying size, morphology, and electron density localising preferentially near the presynaptic membranes, suggested to deliver presynaptic material [28]. Of note, the granulated vesicles are thought to be distinct from the neuropeptide containing “classical” dense core vesicles (DCVs) [28][29]. Subsequent EM studies in cultured hippocampal neurons observed 80 nm dense cored vesicles stringed on MTs in the vicinity of nascent synapses [29], and a similar PV scenario is observable at neuromuscular synapses in Drosophila. First PV cargos were identified: the AZ scaffold protein Piccolo was detected by immunogold labelling on the outside of 80 nm granulated (dense cored) vesicles, hence the denomination of the characterised vesicles as Piccolo transport vesicles (PTVs) [29]. This EM study further suggests that the presynaptic cytoplasmic proteins localise to the outside of the precursor vesicle and are not transported in the vesicular lumen. The mechanisms by which the cargo proteins are hooked on the membrane are currently not known. Immunoisolated Piccolo-PVs were biochemically positive for other presynaptic proteins, including the AZ scaffold protein Bassoon, the presynaptic membrane SNARE proteins Syntaxin and SNAP-25, and the cell adhesion molecule N-cadherin, while SV proteins (Synaptobrevin/Vamp, Synaptotagmin) were not detected [29]. Immunoisolated Bassoon-PVs were positive for RIM1 and ELKS/CAST, but not for (M)Unc13-1 or Synaptophysin [30]. Using the same technique, electron micrographs of immunoisolated PVs were additionally positive for the cytoplasmic SV release controlling proteins (M)Unc18, (M)Unc13, and RIM, the peripheral SV membrane protein Rab3, and the α1 and β1 subunits of the N-type Ca2+ channel [31]. These findings were confirmed by confocal immunofluorescence microscopy showing a partial overlap of Piccolo-positive puncta with Rab3, (M)Unc18, and RIM, and immunoisolated Piccolo-PV were positive for Bassoon, RIM, and (M)Unc18, as detected by immuno-EM analysis [31].
Appart from the detection of single PVs and the identification of their cargo, several observations suggest that axonal PV transport occurs in packets or aggregates of differently shaped PVs. In hippocampal neurons, using correlating fluorescent light microscopy and retrospective EM, “transport packets” were observed, composed of several separate membranous organelles including dense cored vesicles (70 ± 10 nm diameter), pleiomorphic clear cored vesicles of 42 ± 15 nm × 22 ± 13 nm size, and tubulovesicular membrane-structures [4]. These packets transported Synaptobrevin/Vamp, voltage-dependent calcium channel subunits, SV proteins (SV2, Synapsin 1), and an endocytosis protein (Amphiphysin 1) [4]. A subsequent study immunolabelling PVs in neuronal cell cultures identified, by EM, aggregates of one to two 70 nm sized dense cored vesicles surrounding five to six clear cored and smaller vesicles, which occure with a 70% frequency at young presynapses within a 200 nm radius of the presynaptic centre [32]. Within the PV aggregates, SV membrane proteins Synaptobrevin/Vamp, SV2, Synaptotagmin/p65, and the SV-associated protein Synapsin 1 localised to the clear cored 45 nm vesicles, but also to the larger dense cored vesicles, while the presynaptic membrane protein SNAP-25 was not detected in the aggregates [32]. Filamentous structures or spikes seemed to connect the single vesicles. Single 70 nm sizeddense cored vesicles positive for Bassoon and Piccolo were observed at a lower frequency of 17%,, with a decreasing frequency from young to old synapses [32]. Interestingly, when quantifying protein labelling on single 70 nm sized dense cored PVs, Bassoon (5%) and Piccolo (17%) were, compared to SV2 (88%) and Synaptotagmin/p65 (56%), less frequently present, indicating that individual PVs are unlikely to account for the majority of presynaptic active zone protein transport [32].
Studies in mouse retinal photoreceptor ribbon synapses using STED (stimulated emission depletion) microscopy and immuno-EM also identified assembled PV transport units with a total diameter of 130 nm composed of multiple vesicles positive for Bassoon, Piccolo, Ribeye, and RIM, but not for (M)Unc13 and ELKS/CAST [33]. It is assumed, that these units represent an early stage in the formation of synaptic ribbons. The diverse and varying observations suggest that PVs can probably traffic as single entities and, possibly more frequently, as macromolecular complexes of preassembled presynaptic proteins in PV packets composed of several different PV types to supply the presynapse with the required components (summarised also in [2][5][8]).
A possibility to visualise PVs in higher numbers compared to occasional axonal transport events arises when the transporting kinesin or kinesin adaptor (for details, see also later paragraphs) are depleted, inducing an accumulation of “transport arrested” PVs in the neuronal soma. In knockout mice for the anterograde kinesin-3 motor KIF1A, small, clear cored vesicles accumulated in clusters in the neuronal soma [34]. Similarly, knockout of the kinesin adaptor Arl8 in Drosophila leads to an accumulation of PV clusters in the neuronal soma [35]. Electron micrographs here reveal uniform-shaped vesicles of ~70 nm diameter with all shades from clear to dense cored vesicles. Immunogold labelling confirmed both AZ proteins (BRP) and SV proteins (Synaptotagmin 1) as PV cargo [35]. Future comparative analysis between species might help to unify the different observations into a comprehensive model.
2.3. Live Imaging of Presynaptic Precursor Vesicle Trafficking
Additionally tothe immobile snapshots of PVs at the presynapse, in the axon, or the soma, live imaging analysis provides valuable insights into the cargo load of PVs, easily identified by co-trafficking analysis of fluorescently tagged proteins. The first live imaging experiments overexpressing GFP-tagged Bassoon already revealed that single PVs carry 50% of Bassoon, Piccolo, and RIM protein incorporated in a mature synapse, suggesting that two to three PVs deliver sufficient protein for a single synapse formation event [31]. However, a high observed variance suggests that AZ assembly is not a classical “quantal” process, albeit based on presynaptic material delivery through integer numbers of PVs [31]. In later studies, co-trafficking of Bassoon and ELKS/CAST, but not (M)Unc13, was observed [36]. Neurexin and Ca2+ channels, as shown by a study in hippocampal neurons, described epitope-tagged Neurexin co-trafficking with RIM1α and N-type Ca2+ channels, but not Bassoon [37]. Additionally, fluorescently tagged Neurexin showed a 60–75% co-labelling with the SV proteins Synaptophysin, Synapsin, and Synaptotagmin [38]. In Drosophila motoneurons, the AZ proteins BRP and RIM-BP are co-transported, as detected by intravital axonal imaging [39], and in C. elegans, in vivo analysis described co-transport of two SV proteins, Rab3 and Synaptogyrin [40]. As a summarising model integrating EM immunolabelling and life imaging data, the existence of two or three PV types with distinct cargos was suggested: (i) large, dense cored, 70–80 nm sized AZ-protein-transporting PVs, denominated PTVs or AZ precursor vesicles, charged with ELKS/CAST, Bassoon, Piccolo; (ii) small, clear cored, 30–40 nm sized SV-protein-transporting PVs, denominated SVP (synaptic vesicle precursors) transporting Synaptobrevin/Vamp, SV2, Synaptotagmin/p65, Synapsin, possibly also voltage-gated Ca2+ channels, RIM, and Neurexin; and (iii) only (M)Unc13 specific PVs [2][8][9][20][41].
Recent studies investigated co-trafficking of both SV and AZ proteins. An in vivo study in both vertebrate and invertebrate systems revealed axonal co-trafficking of presynaptic AZ and SV proteins, with Bassoon and VGlut (vesicular glutamate transport protein) in hippocampal neurons and the ELKS/CAST homologue Bruchpilot and Synaptotagmin 1 in Drosophila motoneurons [35]. Also, in C. elegans, dual-colour live imaging showed co-transport of RIM/Unc10 with Rab3 (95% of all RIM/Unc10-positive PVs) and Synaptogyrin, and Liprin-α/Syd-2 and Rab3 co-trafficking [42]. The observed AZ/SV “co-trafficking” could either result from single PVs carrying both cargos, in agreement with the immuno-EM analysis showing both AZ and SV proteins on a single PV [32] or represent the multivesicular “transport packets” of different PVs with either SV or AZ protein cargo [32][41]. Alternatively, it has been suggested that additional maturation steps during the axonal transport prior to presynaptic protein incorporation possibly constitute SV and AZ containing PVs [2]. However, recent live imaging of AZ (ELKS-1, Liprin-α/Syd-2, RIM/Unc10) and SV proteins (Rab3) in C. elegans revealed different trafficking kinetics for the two protein groups in the area behind a growth cone [27]. AZ proteins showed a slow and infrequent transport of large puncta, compared to SV proteins, and did not feed directly into forming synapses, but into a diffuse pool, suggesting that AZ and SV proteins use distinct PVs with distinct motor proteins during axonal transport.
Finally, it should be noted that next to the delivery of newly synthesised proteins from the soma, local shuffling of presynaptic material occurs, both between individual AZs, and between AZ and extrasynaptic, cytosolic reservoir pools [7][43][44][45][46]. This local rearrangement of presynaptic material does not require an “on locus” delivery of a precise number of PVs to form an individual synapse. Possibly, AZ proteins tend to be transported in quantal large PVs to these local reservoir pools, while SV proteins traffic on smaller precursor vesicles directly to the nascent or growing presynapse [7]. However, presynaptic cargo needs to be delivered to either the local reservoir pool or directly to the consuming presynapse, and distinct, but not exclusive, modes of protein supply might be utilised during synaptogenesis, possibly dependent on the physiological properties of a synapse (tonic versus phasic synapses) and/or the developmental status of the synapse (initial synapse seeding versus nascent synapse growth versus mature synapse maintenance).
3. Presynaptic Precursor Vesicle Biogenesis
Assembly of PVs occurs in the neuronal soma, and although the underlying molecular pathways controlling formation of the membranous transport organelles have been intensely studied, the current biogenesis model is still fragmentary. A set of early studies in cultured mouse neurons provided evidence that PVs originate from the trans-Golgi [31][36][47]. Proteins are processed in the Golgi and subsequently sorted at the trans-Golgi on different carriers or transport vesicles, and finally exported towards their specific destination, e.g., plasma membrane or the endo/lysosomal system [48][49][50]. It has been estimated that in cells of cultured cell lines, roughly 4000 proteins per second are leaving the Golgi [51]. In cultured hippocampal neurons, endogenous Bassoon and Piccolo colocalise with the trans-Golgi marker (TGN38) upon controlled cooling-induced attenuation of Golgi-export, causing an increase of the Golgi-associated Bassoon protein fraction and a decrease of Bassoon level in axons, while drug-induced Golgi disruption dispersed Bassoon in the cytoplasm [47]. Later studies utilising the same experimental paradigm to attenuate Golgi-export showed a diverse Golgi localisation pattern for different presynaptic proteins: the AZ protein ELKS2/CAST localised similar to Piccolo and Bassoon at the trans-Golgi, while (M)Unc13 localised to the cis-Golgi and RIM1α was not detected at the Golgi [36]. Neurexin was shown to be enriched in the endoplasmatic reticulum (ER)/Golgi, and ER/Golgi export depends on the PDZ-binding motif (postsynaptic density protein (PSD95), Drosophila disc large tumour suppressor (Dlg1), and zonula occludens-1 protein (zo-1)) of the Neurexin C-terminus [37]. Furthermore, voltage-gated Ca2+ channels are thought to be packaged on PVs at the trans-Golgi and transported towards the presynapse [52], and for N-type voltage-gated calcium (CaV2.2) channels, trafficking from the trans-Golgi is mediated by the Adaptor protein complex-1 protein (AP-1) [53].
A recent study on Drosophila identified the small, Golgi-related GTPase Rab2 (Unc108 in C. elegans) as a crucial regulator of the early steps of PV biogenesis at the trans-Golgi [54]. Presynaptic precursors accumulate at the trans-Golgi upon Rab2 knockdown and synaptic terminals show reduced presynaptic protein level. Interestingly, ultrastructural EM analysis of these maturation arrested PVs in the soma showed a large subfraction of elongated, sometimes tubular clear cored vesicles of 40–60 nm size and a small population of circular, large, dense cored vesicles of roughly 80 nm size [54]). Immunofluorescent co-labelling revealed an interesting cargo segregation within the ectopic PV clusters: a large PV fraction was co-positive for synaptic vesicle proteins (VGlut, Sytaptotagmin 1), the endocytic machinery (Dap160/intersectin, dynamin-associated protein 160 kDa), and the lysosomal marker Lamp1 opposed to small PV fraction co-positive for the presynaptic AZ proteins BRP, RIM-BP, and (D)Unc13A (Drosophila Unc13), homologue of the vertebrate (M)Unc13. Thus, possibly already in this early step of PV biogenesis during protein export from the trans-Golgi, two types of PV are differentiated: 40–60 nm sized clear cored synaptic vesicle transporting PVs and 80 nm sized dense cored AZ-protein-transporting PVs [20][31][32][41]. However, without ultrastructural labelling analysis, this remains an assumption.
Small GTPases including Rab2 recruit in their activated GTP-bound state effector proteins to dedicated membranes to concert vesicle fusion and/or fission events [55][56], and might hence play a central role in the organisation of vesicle formation and trafficking at the trans-Golgi [57].
Early studies described Rab2 as a Golgi-resident to act in anterograde and retrograde ER-Golgi trafficking [57][58][59]. However, several lines of evidence expand Rab2 function to vesicular biogenesis pathways at the trans-Golgi, including PV biogenesis but also the formation of neuronal DCVs. DCVs have a typical size of roughly 43 nm diameter with a dense core undergoing a complex maturation sequence to form mature neuropeptide transporting organelles [60][61][62]. In the C. elegans system, Rab2/Unc108 is involved in neuronal DCV biogenesis at the trans-Golgi, as DCV morphology (size and shape) and PI(3)P-dependent cargo sorting during DCV maturation is aberrant in rab2−/− mutants [63][64]. Importantly, Rab2 effectors or interactors, such as RIC-19 (resistance to inhibitors of cholinesterase-19), Rund-1 (RUN domain-containing protein 1), CCCP-1/Golgin104 (conserved coiled-coil protein 1), and TBC-8 (Tre2/Bub2/Cdc16 domain containing protein-8), are implied in DCV maturation at the trans-Golgi [63][65][66][67]. Also, axonal transport of DCVs and lysosomes in Drosophila neurons depends on Rab2 [66]. Finally, Rab2 was shown to be required for autophagosome and endosome maturation as well as lysosome function in non-neuronal Drosophila tissue [68], and endo-lysosomal fusion and the delivery of the lysosomal protein Lamp1 to late endosomes [69]. Interestingly, PVs transport was shown to depend on the lysosomal kinesin adaptor Arl8, and PV membranes contain lysosomal membranes proteins such as Lamp1 or Spinster (for details, see last paragraph) [35][70]. As lysosomal proteins, e.g., Lamp1, are exported from the trans-Golgi on lysosomal membrane protein (LMP) carriers, a process mediated by Rab2 [69] and, Rab2 is implied in lysosome formation, while PVs share a lysosome-like membrane identity, e.g., contain Lamp1 [35], Rab2 might at the trans-Golgi organise PV biogenesis using components of the other Golgi export pathways, or, as part of the PV maturation process, mediate fusion events with endo-lysosomal organelles.
In summary, these findings place the small GTPase Rab2 at a potent cross-section of three organelle biogenesis pathways, intersecting PV, DCV, and endo-lysosome biogenesis and trafficking, potentially acting here as a signpost or decision-maker concerting an interplay of trans-Golgi export routes, possibly using components of the hitherto independently described molecular pathways to orchestrate PV biogenesis.
References
- Guedes-Dias, P.; Holzbaur, E.L.F. Axonal transport: Driving synaptic function. Science 2019, 366, eaaw9997.
- Rizalar, F.S.; Roosen, D.A.; Haucke, V. A Presynaptic Perspective on Transport and Assembly Mechanisms for Synapse Formation. Neuron 2021, 109, 27–41.
- Petzoldt, A.G.; Lützkendorf, J.; Sigrist, S.J. Mechanisms controlling assembly and plasticity of presynaptic active zone scaffolds. Curr. Opin. Neurobiol. 2016, 39, 69–76.
- Ahmari, S.E.; Buchanan, J.; Smith, S.J. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat. Neurosci. 2000, 3, 445–451.
- Maeder, C.I.; Shen, K.; Hoogenraad, C.C. Axon and dendritic trafficking. Curr. Opin. Neurobiol. 2014, 27, 165–170.
- Chia, P.H.; Li, P.; Shen, K. Cell biology in neuroscience: Cellular and molecular mechanisms underlying presynapse formation. J. Cell Biol. 2013, 203, 11–22.
- Ziv, N.E. Maintaining the active zone: Demand, supply and disposal of core active zone proteins. Neurosci. Res. 2018, 127, 70–77.
- Ziv, N.E.; Garner, C.C. Cellular and molecular mechanisms of presynaptic assembly. Nat. Rev. Neurosci. 2004, 5, 385–399.
- Bury, L.A.; Sabo, S.L. Building a Terminal: Mechanisms of Presynaptic Development in the CNS. Neuroscientist 2016, 22, 372–391.
- Hafner, A.S.; Donlin-Asp, P.G.; Leitch, B.; Herzog, E.; Schuman, E.M. Local protein synthesis is a ubiquitous feature of neuronal pre- and postsynaptic compartments. Science 2019, 364, eaau3644.
- Donlin-Asp, P.G.; Polisseni, C.; Klimek, R.; Heckel, A.; Schuman, E.M. Differential regulation of local mRNA dynamics and translation following long-term potentiation and depression. Proc. Natl. Acad. Sci. USA 2021, 118, e2017578118.
- Fernandez-Moya, S.M.; Bauer, K.E.; Kiebler, M.A. Meet the players: Local translation at the synapse. Front. Mol. Neurosci. 2014, 7, 84.
- Sigrist, S.J.; Thiel, P.R.; Reiff, D.F.; Lachance, P.E.; Lasko, P.; Schuster, C.M. Postsynaptic translation affects the efficacy and morphology of neuromuscular junctions. Nature 2000, 405, 1062–1065.
- Biever, A.; Donlin-Asp, P.G.; Schuman, E.M. Local translation in neuronal processes. Curr. Opin. Neurobiol. 2019, 57, 141–148.
- Kosik, K.S. Life at Low Copy Number: How Dendrites Manage with So Few mRNAs. Neuron 2016, 92, 1168–1180.
- Sudhof, T.C. The presynaptic active zone. Neuron 2012, 75, 11–25.
- Wilhelm, B.G.; Mandad, S.; Truckenbrodt, S.; Krohnert, K.; Schafer, C.; Rammner, B.; Koo, S.J.; Classen, G.A.; Krauss, M.; Haucke, V.; et al. Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science 2014, 344, 1023–1028.
- Gundelfinger, E.D.; Fejtova, A. Molecular organization and plasticity of the cytomatrix at the active zone. Curr. Opin. Neurobiol. 2012, 22, 423–430.
- Petzoldt, A.G.; Sigrist, S.J. Synaptogenesis. Curr. Biol. 2014, 24, R1076–R1080.
- Gundelfinger, E.D.; Reissner, C.; Garner, C.C. Role of Bassoon and Piccolo in Assembly and Molecular Organization of the Active Zone. Front. Synaptic Neurosci. 2015, 7, 19.
- Walter, A.M.; Böhme, M.A.; Sigrist, S.J. Vesicle release site organization at synaptic active zones. Neurosci. Res. 2018, 127, 3–13.
- Sudhof, T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004, 27, 509–547.
- Rizo, J.; Xu, J. The Synaptic Vesicle Release Machinery. Annu. Rev. Biophys. 2015, 44, 339–367.
- Sudhof, T.C. Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron 2013, 80, 675–690.
- Takamori, S.; Holt, M.; Stenius, K.; Lemke, E.A.; Grønborg, M.; Riedel, D.; Urlaub, H.; Schenck, S.; Brügger, B.; Ringler, P.; et al. Molecular anatomy of a trafficking organelle. Cell 2006, 127, 831–846.
- Friedman, H.V.; Holt, M.; Stenius, K.; Lemke, E.A.; Gronborg, M.; Riedel, D.; Urlaub, H.; Schenck, S.; Brugger, B.; Ringler, P.; et al. Assembly of new individual excitatory synapses: Time course and temporal order of synaptic molecule recruitment. Neuron 2000, 27, 57–69.
- Lipton, D.M.; Maeder, C.I.; Shen, K. Rapid Assembly of Presynaptic Materials behind the Growth Cone in Dopaminergic Neurons Is Mediated by Precise Regulation of Axonal Transport. Cell Rep. 2018, 24, 2709–2722.
- Vaughn, J.E. Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse 1989, 3, 255–285.
- Zhai, R.G.; Vardinon-Friedman, H.; Cases-Langhoff, C.; Becker, B.; Gundelfinger, E.D.; Ziv, N.E.; Garner, C.C. Assembling the presynaptic active zone: A characterization of an active one precursor vesicle. Neuron 2001, 29, 131–143.
- Ohtsuka, T.; Takao-Rikitsu, E.; Inoue, E.; Inoue, M.; Takeuchi, M.; Matsubara, K.; Deguchi-Tawarada, M.; Satoh, K.; Morimoto, K.; Nakanishi, H.; et al. Cast: A novel protein of the cytomatrix at the active zone of synapses that forms a ternary complex with RIM1 and munc13-1. J. Cell Biol. 2002, 158, 577–590.
- Shapira, M.; Zhai, R.G.; Dresbach, T.; Bresler, T.; Torres, V.I.; Gundelfinger, E.D.; Ziv, N.E.; Garner, C.C. Unitary assembly of presynaptic active zones from Piccolo-Bassoon transport vesicles. Neuron 2003, 38, 237–252.
- Tao-Cheng, J.H. Ultrastructural localization of active zone and synaptic vesicle proteins in a preassembled multi-vesicle transport aggregate. Neuroscience 2007, 150, 575–584.
- Regus-Leidig, H.; Tom Dieck, S.; Specht, D.; Meyer, L.; Brandstatter, J.H. Early steps in the assembly of photoreceptor ribbon synapses in the mouse retina: The involvement of precursor spheres. J. Comp. Neurol. 2009, 512, 814–824.
- Yonekawa, Y.; Harada, A.; Okada, Y.; Funakoshi, T.; Kanai, Y.; Takei, Y.; Terada, S.; Noda, T.; Hirokawa, N. Defect in synaptic vesicle precursor transport and neuronal cell death in KIF1A motor protein-deficient mice. J. Cell Biol. 1998, 141, 431–441.
- Vukoja, A.; Rey, U.; Petzoldt, A.G.; Ott, C.; Vollweiter, D.; Quentin, C.; Puchkov, D.; Reynolds, E.; Lehmann, M.; Hohensee, S.; et al. Presynaptic Biogenesis Requires Axonal Transport of Lysosome-Related Vesicles. Neuron 2018, 99, 1216–1232.e7.
- Maas, C.; Torres, V.I.; Altrock, W.D.; Leal-Ortiz, S.; Wagh, D.; Terry-Lorenzo, R.T.; Fejtova, A.; Gundelfinger, E.D.; Ziv, N.E.; Garner, C.C.; et al. Formation of Golgi-derived active zone precursor vesicles. J. Neurosci. 2012, 32, 11095–11108.
- Fairless, R.; Masius, H.; Rohlmann, A.; Heupel, K.; Ahmad, M.; Reissner, C.; Dresbach, T.; Missler, M. Polarized targeting of neurexins to synapses is regulated by their C-terminal sequences. J. Neurosci. 2008, 28, 12969–12981.
- Neupert, C.; Schneider, R.; Klatt, O.; Reissner, C.; Repetto, D.; Biermann, B.; Niesmann, K.; Missler, M.; Heine, M. Regulated Dynamic Trafficking of Neurexins Inside and Outside of Synaptic Terminals. J. Neurosci. 2015, 35, 13629–13647.
- Siebert, M.; Bohme, M.A.; Driller, J.H.; Babikir, H.; Mampell, M.M.; Rey, U.; Ramesh, N.; Matkovic, T.; Holton, N.; Reddy-Alla, S.; et al. A high affinity RIM-binding protein/Aplip1 interaction prevents the formation of ectopic axonal active zones. Elife 2015, 4, e06935.
- Maeder, C.I.; San-Miguel, A.; Wu, E.Y.; Lu, H.; Shen, K. In vivo neuron-wide analysis of synaptic vesicle precursor trafficking. Traffic 2014, 15, 273–291.
- Bury, L.A.; Sabo, S.L. Coordinated trafficking of synaptic vesicle and active zone proteins prior to synapse formation. Neural Dev. 2011, 6, 24.
- Wu, Y.E.; Huo, L.; Maeder, C.I.; Feng, W.; Shen, K. The balance between capture and dissociation of presynaptic proteins controls the spatial distribution of synapses. Neuron 2013, 78, 994–1011.
- Kalla, S.; Stern, M.; Basu, J.; Varoqueaux, F.; Reim, K.; Rosenmund, C.; Ziv, N.E.; Brose, N. Molecular dynamics of a presynaptic active zone protein studied in Munc13-1-enhanced yellow fluorescent protein knock-in mutant mice. J. Neurosci. 2006, 26, 13054–13066.
- Tsuriel, S.; Fisher, A.; Wittenmayer, N.; Dresbach, T.; Garner, C.C.; Ziv, N.E. Exchange and redistribution dynamics of the cytoskeleton of the active zone molecule bassoon. J. Neurosci. 2009, 29, 351–358.
- Tsuriel, S.; Geva, R.; Zamorano, P.; Dresbach, T.; Boeckers, T.; Gundelfinger, E.D.; Garner, C.C.; Ziv, N.E. Local sharing as a predominant determinant of synaptic matrix molecular dynamics. PLOS Biol. 2006, 4, e271.
- Graf, E.R.; Daniels, R.W.; Burgess, R.W.; Schwarz, T.L.; DiAntonio, A. Rab3 dynamically controls protein composition at active zones. Neuron 2009, 64, 663–677.
- Dresbach, T.; Torres, V.; Wittenmayer, N.; Altrock, W.D.; Zamorano, P.; Zuschratter, W.; Nawrotzki, R.; Ziv, N.E.; Garner, C.C.; Gundelfinger, E.D.; et al. Assembly of active zone precursor vesicles: Obligatory trafficking of presynaptic cytomatrix proteins Bassoon and Piccolo via a trans-Golgi compartment. J. Biol. Chem. 2006, 281, 6038–6047.
- Griffiths, G.; Simons, K. The trans Golgi network: Sorting at the exit site of the Golgi complex. Science 1986, 234, 438–443.
- De Matteis, M.A.; Luini, A. Exiting the Golgi complex. Nat. Rev. Mol. Cell Biol. 2008, 9, 273–284.
- Park, K.; Ju, S.; Kim, N.; Park, S.Y. The Golgi complex: A hub of the secretory pathway. BMB Rep. 2021, 54, 246–252.
- Hirschberg, K.; Miller, C.M.; Ellenberg, J.; Presley, J.F.; Siggia, E.D.; Phair, R.D.; Lippincott-Schwartz, J. Kinetic analysis of secretory protein traffic and characterization of golgi to plasma membrane transport intermediates in living cells. J. Cell Biol. 1998, 143, 1485–1503.
- Dolphin, A.C.; Lee, A. Presynaptic calcium channels: Specialized control of synaptic neurotransmitter release. Nat. Rev. Neurosci. 2020, 21, 213–229.
- Macabuag, N.; Dolphin, A.C. Alternative Splicing in Ca(V)2.2 Regulates Neuronal Trafficking via Adaptor Protein Complex-1 Adaptor Protein Motifs. J. Neurosci. 2015, 35, 14636–14652.
- Gotz, T.W.B.; Puchkov, D.; Lysiuk, V.; Lutzkendorf, J.; Nikonenko, A.G.; Quentin, C.; Lehmann, M.; Sigrist, S.J.; Petzoldt, A.G. Rab2 regulates presynaptic precursor vesicle biogenesis at the trans-Golgi. J. Cell Biol. 2021, 220, e202006040.
- Stenmark, H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525.
- Homma, Y.; Hiragi, S.; Fukuda, M. Rab family of small GTPases: An updated view on their regulation and functions. FEBS J. 2021, 288, 36–55.
- Liu, S.; Storrie, B. Are Rab proteins the link between Golgi organization and membrane trafficking? Cell Mol. Life Sci. 2012, 69, 4093–4106.
- Saraste, J. Spatial and Functional Aspects of ER-Golgi Rabs and Tethers. Front. Cell Dev. Biol. 2016, 4, 28.
- Tisdale, E.J.; Balch, W.E. Rab2 is essential for the maturation of pre-Golgi intermediates. J. Biol. Chem. 1996, 271, 29372–29379.
- Tooze, S.A.; Martens, G.J.; Huttner, W.B. Secretory granule biogenesis: Rafting to the SNARE. Trends Cell Biol. 2001, 11, 116–122.
- Borgonovo, B.; Ouwendijk, J.; Solimena, M. Biogenesis of secretory granules. Curr. Opin. Cell Biol. 2006, 18, 365–370.
- Gondré-Lewis, M.C.; Park, J.J.; Loh, Y.P. Cellular mechanisms for the biogenesis and transport of synaptic and dense-core vesicles. Int. Rev. Cell Mol. Biol. 2012, 299, 27–115.
- Sumakovic, M.; Hegermann, J.; Luo, L.; Husson, S.J.; Schwarze, K.; Olendrowitz, C.; Schoofs, L.; Richmond, J.; Eimer, S. UNC-108/RAB-2 and its effector RIC-19 are involved in dense core vesicle maturation in Caenorhabditis elegans. J. Cell Biol. 2009, 186, 897–914.
- Edwards, S.L.; Charlie, N.K.; Richmond, J.E.; Hegermann, J.; Eimer, S.; Miller, K.G. Impaired dense core vesicle maturation in Caenorhabditis elegans mutants lacking Rab2. J. Cell Biol. 2009, 186, 881–895.
- Ailion, M.; Hannemann, M.; Dalton, S.; Pappas, A.; Watanabe, S.; Hegermann, J.; Liu, Q.; Han, H.F.; Gu, M.; Goulding, M.Q.; et al. Two Rab2 interactors regulate dense-core vesicle maturation. Neuron 2014, 82, 167–180.
- Lund, V.K.; Lycas, M.D.; Schack, A.; Andersen, R.C.; Gether, U.; Kjaerulff, O. Rab2 drives axonal transport of dense core vesicles and lysosomal organelles. Cell Rep. 2021, 35, 108973.
- Hannemann, M.; Sasidharan, N.; Hegermann, J.; Kutscher, L.M.; Koenig, S.; Eimer, S. TBC-8, a putative RAB-2 GAP, regulates dense core vesicle maturation in Caenorhabditis elegans. PLOS Genet. 2012, 8, e1002722.
- Lorincz, P.; Toth, S.; Benko, P.; Lakatos, Z.; Boda, A.; Glatz, G.; Zobel, M.; Bisi, S.; Hegedus, K.; Takats, S.; et al. Rab2 promotes autophagic and endocytic lysosomal degradation. J. Cell Biol. 2017, 216, 1937–1947.
- Lund, V.K.; Madsen, K.L.; Kjaerulff, O. Drosophila Rab2 controls endosome-lysosome fusion and LAMP delivery to late endosomes. Autophagy 2018, 14, 1520–1542.
- Klassen, M.P.; Wu, Y.E.; Maeder, C.I.; Nakae, I.; Cueva, J.G.; Lehrman, E.K.; Tada, M.; Gengyo-Ando, K.; Wang, G.J.; Goodman, M.; et al. An Arf-like small G protein, ARL-8, promotes the axonal transport of presynaptic cargoes by suppressing vesicle aggregation. Neuron 2010, 66, 710–723.
More
Information
Subjects:
Cell Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
09 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




