Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tsvetelina Doncheva | -- | 1663 | 2023-09-28 13:45:24 | | | |
| 2 | Jessie Wu | Meta information modification | 1663 | 2023-10-09 09:23:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kostova, N.; Doncheva, T. Chemistry of Hypecoum Alkaloids. Encyclopedia. Available online: https://encyclopedia.pub/entry/49772 (accessed on 07 February 2026).
Kostova N, Doncheva T. Chemistry of Hypecoum Alkaloids. Encyclopedia. Available at: https://encyclopedia.pub/entry/49772. Accessed February 07, 2026.
Kostova, Nadezhda, Tsvetelina Doncheva. "Chemistry of Hypecoum Alkaloids" Encyclopedia, https://encyclopedia.pub/entry/49772 (accessed February 07, 2026).
Kostova, N., & Doncheva, T. (2023, September 28). Chemistry of Hypecoum Alkaloids. In Encyclopedia. https://encyclopedia.pub/entry/49772
Kostova, Nadezhda and Tsvetelina Doncheva. "Chemistry of Hypecoum Alkaloids." Encyclopedia. Web. 28 September, 2023.
Copy Citation
Genus Hypecoum Tourn. ex L. belongs to the poppy family Papaveraceae and comprises about 19 species occurring in Europe, Northern Africa and Asia. Hypecoum species have been widely used in traditional medicine as antipyretic, analgesic and anti-inflammatory remedies. The effects are associated with the biologically and pharmacologically active isoquinoline alkaloids in them such as protopines, protoberberines, benzophenanthridines, aporphines, simple isoquinolines, secoberbines, spirobenzylisoquinolines and others.
isoquinoline alkaloids
Hypecoum
1. Introduction
The flowering plants of the genus Hypecoum, belonging to the family Papaveraceae, subfamily Fumarioideae, are widely distributed in temperate areas from Europe to Africa to Southwestern Asia. It is represented by 19 species (23 synonyms), with 2 accepted subspecies and 1 ambiguous name [1]. These plants have recognized medicinal value and are used in different forms of traditional medicine in the treatment and prevention of many diseases—colds, pneumonia, pharyngitis, inflamed red eyes, hepatitis, cholecystitis and other [2][3][4][5][6]. Scientific investigations of the Hypecoum species show that many of the therapeutic effects of the plants are primarily related to the bioactive isoquinoline alkaloids found in them. The known alkaloids in the genus belong to the various classes of isoquinolines—protopines, protoberberines, benzophenanthridines, aporphines, simple isoquinolines, secoberbines, spirobenzylisoquinolines and others. Protopine is a predominant alkaloid isolated from all investigated plants of the genus [2][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24]. Besides being biologically active compounds, isoquinoline alkaloids are characteristic secondary metabolites in the family Papaveraceae and can be used for chemotaxonomic purposes [25].
In recent years, more than 40 alkaloids have been identified in Hypecoum species, and new alkaloid structures, along with their pharmacological activities, are reported every year, enriching the diversity of natural compounds. Some of them have promising properties for the treatment of important diseases such as inflammation, cancer and microbial infections [18][19][22][23][26][27][28][29].
2. Hypecoum alkaloids
Alkaloids are a large group of N-containing natural compounds with diverse structures and a wide spectrum of pharmacological effects on human health. The Hypecoum species are recognized as important medicinal plants, biosynthesizing isoquinoline alkaloids as major active ingredients.
This section summarizes the alkaloid compounds that have been identified in the Hypecoum spp. since 1972 (Table 1) [2][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][27][30][31][32][33][34][35][36][37][38][39][40]. So far, a total of 86 naturally occurring alkaloids have been reported for ten plant species—H. procumbens, H. procumbens var. glaucescens, H. imberbe, H. lactiflorum, H. pendulum, H. trilobum, H. leptocarpum, H. erectum, H. ponticum and H. parviflorum. Based on their structures, the Hypecoum alkaloids can be divided into eight major groups: protopines (1–11), protoberberines (12–17), benzophenantridines (18–28), aporphines (29–33), simple isoquinolines (34–38), secoberbines (39–66), spirobenzilisoquinolines (67–73) and others (74–86). Two chemical structures have been detected in the literature with the name leptocarpine—protopine alkaloid 8 and alkaloid 82. Both compounds have been identified in H. leptocarpum [10][22][32]. On the other hand, the structure of alkaloid 41 has been published in the literature under two different names, dihydroleptopine and coryximine, isolated from H. leptocarpum [22][23][33]. Similarly, the newly found in H. erectum 2-(1,3-dioxolo [4,5-h]isoquinolin-7-yl)-4,5-dimethoxy-N-methyl-benzenethanamine has the same structure as the alkaloid leptocarmine (62), isolated from H. leptocarpum [19][23][24].
Table 1. Alkaloid structures reported for Hypecoum spp.
| Alkaloids/Types | Structure | Species | References |
|---|---|---|---|
| Protopines | |||
| Protopine (1) | 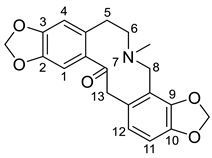 |
H. procumbens H. procumbens var. glaucescens H. imberbe H. lactiflorum H. pendulum H. trilobum H. leptocarpum H. erectum H. ponticum H. parviflorum |
[2][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24] |
| Dihydroprotopine (2) | 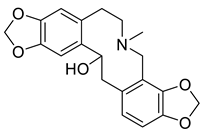 |
H. procumbens H. imberbe H. leptocarpum |
[17][24] |
| 13-Oxoprotopine (3) | 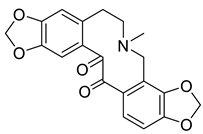 |
H. procumbens H. erectum |
[15][19] |
| Cryptopine (4) | 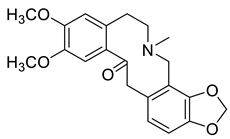 |
H. procumbens var. glaucescens H. erectum H. leptocarpum |
[2][11][13][19][20][21][22][23][30] |
| Allocryptopine (5) | 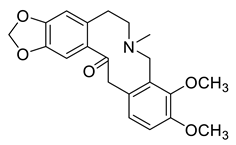 |
H. lactiflorum H. leptocarpum H. procumbens H. procumbens var. glaucescens H. erectum |
[7][8][10][11][13][14][19][21][30][31] |
| 13-Oxocryptopine (6) | 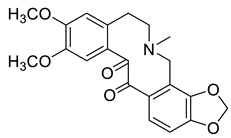 |
H. erectum | [19] |
| 13-Oxoallocryptopine (7) | 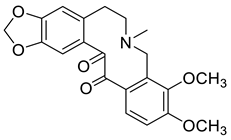 |
H. erectum | [19] |
| Leptocarpine (8) * | 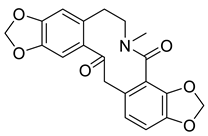 |
H. leptocarpum | [32] |
| Hunnemanine (9) | 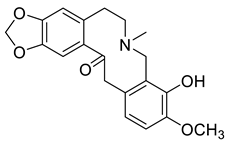 |
H. procumbens | [15] |
| Bis[1,3]benzodioxolo[4,5-c:5′,6′-g]azecin-14(4H)-one, 5,6,7,13-tetrahydro-5-methyl-one (10) | 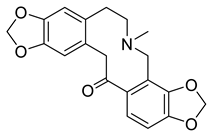 |
H. erectum | [19] |
| 6,7,8,14-Tetrahydro-3,4-dimethoxy-6-methylbenzo [c][1,3]benzodioxolo [5,6-g]azecin-15(5H)-one (11) |
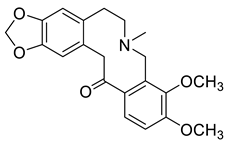 |
H. erectum | [19] |
| Protoberberines | |||
| (S)-Stylopine (12) | 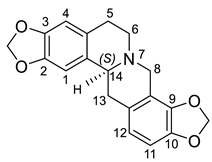 |
H. procumbens | [17] |
| (S)-Scoulerine (13) | 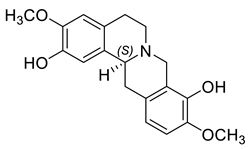 |
H. procumbens | [8][15] |
| Coptisine (14) | 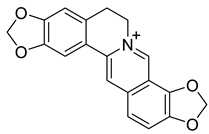 |
H. procumbens H. leptocarpum H. erectum |
[8][14][20][23][30][31] |
| (S)-N-Methylstylopine (15) | 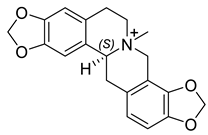 |
H. lactiflorum H. procumbens H. ponticum |
[7][17][18] |
| (S)-N-Methylcanadine (16) | 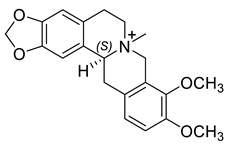 |
H. lactiflorum H. erectum H. procumbens H. ponticum H. leptocarpum |
[7][13][17][18][21] |
| Hydroprotopine (17) | 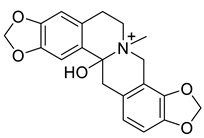 |
H. leptocarpum | [27] |
| Benzophenanthridines | |||
| Chelerythrine (18) | 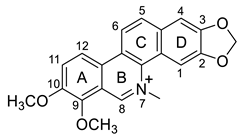 |
H. procumbens H. leptocarpum |
[8][14][30] |
| Chelirubine (19) | 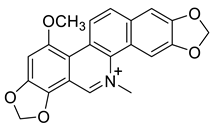 |
H. procumbens | [8][14] |
| Dihydrochelirubine (20) | 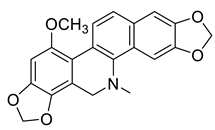 |
H. procumbens | [17] |
| Sanguinarine (21) | 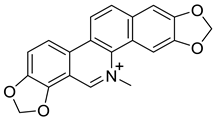 |
H. procumbens H. leptocarpum H. trilobum H. ponticum , H. imberbe |
[14][17][18][21][30] |
| Dihydrosanguinarine (22) | 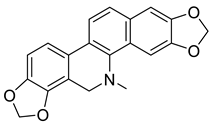 |
H. imberbe H. leptocarpum H. procumbens |
[9][10][15] |
| (R,S)-8-Methoxydihydrosanguinarine (23) | 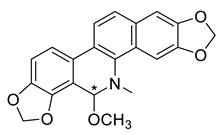 |
H. procumbens H. leptocarpum H. imberbe |
[8][9][10] |
| (S)-8-Hydroxymethyldihydrosanguinarine (24) | 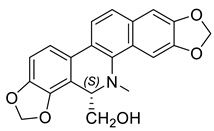 |
H. erectum | [19] |
| 8-Acetonyldihydrosanguinarine (25) | 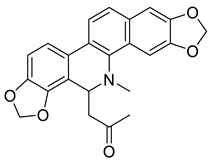 |
H. procumbens H. leptocarpum |
[8][10] |
| (R,S)-Nitrotyrasanguinarine (26) | 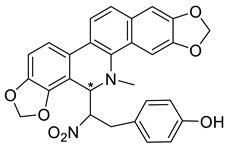 |
H. imberbe H. pendulum H. procumbens |
[9] |
| Oxysanguinarine (27) | 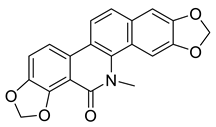 |
H. procumbens | [8][15] |
| Norsanguinarine (28) | 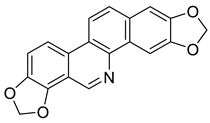 |
H. procumbens H. leptocarpum |
[8][15][23] |
| Aporphines | |||
| (S)-Corydine (29) | 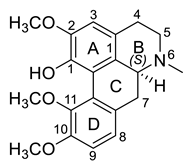 |
H. procumbens H. leptocarpum |
[30] |
| (S)-Isocorydine (30) | 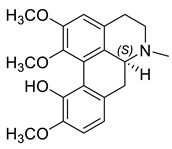 |
H. procumbens H. procumbens var. glaucescens H. leptocarpum |
[8][11][30] |
| (S)-Bulbocapnine (31) | 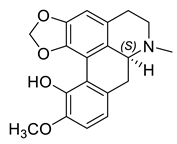 |
H. imberbe | [9] |
| (S)-Glaucine (32) | 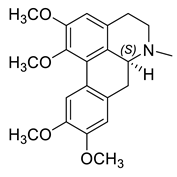 |
H. procumbens | [8] |
| (S)-Magnoflorine (33) | 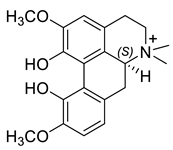 |
H. procumbens H. leptocarpum |
[30] |
| Simple isoquinolines | |||
| Oxohydrastinine (34) | 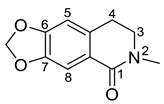 |
H. imberbe H. leptocarpum H. erectum |
[2][9][10][12][13][19][21][22][23][24][27] |
| Doryanine (35) | 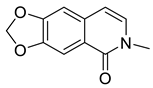 |
H. erectum | [19] |
| N-Methylcorydaldine (36) | 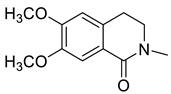 |
H. erectum H. leptocarpum |
[13][19][23] |
| 2-Methyl-1,2,3,4-tetrahydro isoquinoline-7-carboxylic acid methyl ester (37) |
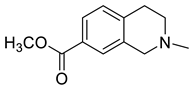 |
H. erectum | [19] |
| 1,3,6,6-Tetramethyl-5,6,7,8- tetrahydroisoquiolin-8-one (38) |
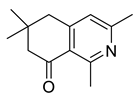 |
H. erectum | [19] |
| Secoberbines | |||
| (S)-Corydalisol (39) | 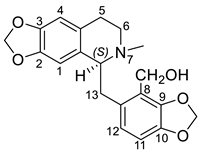 |
H. procumbens | [8] |
| (S)-N-Methylcorydalisol (40) |  |
H. lactiflorum | [7] |
| (S)-Dihydroleptopine (41) (Coryximine) |
 |
H. leptocarpum | [22][23][33] |
| Hypecocarpinine (42) |  |
H. leptocarpum | [24] |
| Hypecocarpine (43) |  |
H. leptocarpum | [27] |
| Leptopinine (44) |  |
H. leptocarpum | [12] |
| Leptopine (45) |  |
H. leptocarpum | [12][21] |
| Leptopidine (46) |  |
H. leptocarpum | [12][21][22][23][24][27] |
| Hypepontine (47) |  |
H. ponticum | [17][18] |
| N-Methylcoryximine (48) |  |
H. pendulum | [34] |
| Hendersine B (49) |  |
H. leptocarpum | [23] |
| Procumbine (50) |  |
H. procumbens H. leptocarpum |
[30][35] |
| Leptopidinine (51) |  |
H. leptocarpum | [12][21][22] |
| Torulosine (52) |  |
H. leptocarpum | [23][33] |
| 8-Oxohypecorinine N-oxide (53) |  |
H. leptocarpum | [33] |
| N-Methyldemethyltorulosine (54) |  |
H. leptocarpum | [23][33] |
| (R,S)-Hypecorinine (55) |  |
H. procumbens H. procumbens var. glaucescens H. erectum |
[8][11][13][14][19][31][36] |
| (R,S)-8-Oxohypecorinine (56) |  |
H. procumbens var. glaucescens | [11] |
| Hypecorine (57) |  |
H. erectum | [14][31][36] |
| 2,3-Dimethoxyhypecorinine (58) |  |
H. erectum | [19] |
| (S)-Peshawarine (59) |  |
H. parviflorum | [8][16] |
| Corydamine (60) |  |
H. leptocarpum H. erectum |
[10][19][22][23][24][31] |
| Corydamine acid (61) |  |
H. leptocarpum | [23] |
| Leptocaramine (62) (2-(1,3-dioxolo [4,5-h] isoquinolin-7-yl)-4,5-dimethoxy-N-methyl-benzeneethanamine) |
 |
H. leptocarpum H. erectum |
[19][23][24] |
| N-Formylcorydamine (63) |  |
H. erectum H. procumbens |
[15][19] |
| 2,3-Dimethoxy-N-formyl corydamine (64) |
 |
H. erectum | [19] |
| Hypecumine (65) |  |
H. procumbens | [15] |
| 7-N-Methyl-7-N-oxide-2,3-di- methoxycorydamine (66) |
 |
H. erectum | [19] |
| Spirobenzylisoquinolines | |||
| (S,S)-Hyperectumine B (67) |  |
H. erectum | [19] |
| (S,S)-2,3-Dimethoxyhyperectumine B (68) |  |
H. erectum | [19] |
| (R,S) and (S,R)-2,3-Dimethoxyhyperectine (69) |  |
H. erectum | [19] |
| (R,S) and (S,R)-Hyperectine (70) |  |
H. leptocarpum H. erectum |
[10][19][31][36][37] |
| (S,S) and (R,R)-Isohyperectine (71) |  |
H. leptocarpum H. erectum |
[10][12][37] |
| (R,R)-Sibiricine (72) |  |
H. erectum | [19] |
| (R,S)-Hypecoleptopine (73) |  |
H. erectum H. leptocarpum |
[21][33][38] |
| Other alkaloids | |||
| (S)-N-Methylcoclaurine (74) |  |
H. procumbens | [17] |
| N-Methylsecoglaucine (75) |  |
H. ponticum | [17][18] |
| (R)-Turkiyenine (76) |  |
H. procumbens H. pendulum H. imberbe |
[9][15][39][40] |
| (S)-Oxoturkiyenine (77) |  |
H. pendulum | [39] |
| Leptocarpinine (78) |  |
H. leptocarpum | [12] |
| Leptocarpinine B (79) |  |
H. leptocarpum | [23] |
| (S,S)-Trans-benzindenoazepines (80) |  |
H. erectum | [19] |
| (S,S)-O-Methylfumarostelline (81) |  |
H. erectum | [19] |
| Leptocarpine (82) * |  |
H. leptocarpum | [10][22] |
| Hypecoumine (83) |  |
H. procumbens | [2] |
| Hyperectumine (84) (8S, 9R, 15R) (8R, 9S, 15S) |
 |
H. erectum | [25] |
| Dibenzooxazacycloundecine (85) |  |
H. erectum | [19] |
| Hypeisoxazole A (86) (6S, 8R, 14R) (6R, 8S, 14S) |
 |
H. erectum | [38] |
* Two chemical structures with the name leptocarpine (8, 82).
2.1. Protopine Alkaloids
The protopine alkaloids (1–11) represent the major class of alkaloids in the Hypecoum species (Table 1). Their structure includes a tricyclic frame with a carbonyl group at C-14 and methoxy- or methylendioxy- groups at C-2, C-3, C-9 and C-10. Alkaloids 3, 6 and 7 are further oxygenated at C-13. Although only eleven protopines have been identified in the genus, protopine (1) has been found in all studied Hypecoum species [2][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24]. Alkaloid 1 together with cryptopine (4) and allocryptopine (5) are the most pharmacologically studied and biologically active protopines in the genus. It has been established that these alkaloids possess potent anti-inflammatory, analgesic and antibacterial effect [13][19].
2.2. Protoberberine Alkaloids
The protoberberine alkaloids (12–17) are a small group of Hypecoum isoquinolines containing only six representatives (Table 1). Most of them are of the tetrahydroprotoberberine type (12, 13, 15–17), and only coptisine (14) is a typical protoberberine with a three aromatic ring system. Alkaloid 14 has shown significant cytotoxic activity against different cancer cell lines, which is probably related to the positive N-ion in its molecule [23].
2.3. Benzophenantridine Alkaloids
Eleven benzophenantridine alkaloids (18–28) have been identified in six Hypecoum species—H. procumbens, H. leptocarpum, H. trilobum, H. ponticum, H. imberbe and H. erectum (Table 1). Their structure includes a tetracyclic skeleton with an aromatic (18, 19, 21 and 28) or nonaromatic (20, 22–27) ring B. The alkaloids with three aromatic ring systems are usually in the form of quaternary ammonium salts (18, 19, 21) and those with non-aromatic ring B are usually C-8-substituted (23–27). Benzophenanthridine alkaloids are known to have diverse and significant biological properties, but so far, only 8-hydroxymethyldihydrosanguinarine (24), isolated from H. erectum, has been found to possess in vitro anti-inflammatory activity [19][41].
2.4. Aporphine Alkaloids
The alkaloids in this group (29–33) contain an aporphine skeleton with two aromatic rings and N-methyl function. Five aporphines have been isolated from four plant species—H. procumbens, H. procumbens var. glaucescens, H. leptocarpum and H. imberbe (Table 1). Alkaloids 29–32 are tertiary and only one—magnoflorine—(33) is a quaternary ammonium salt. Although alkaloids of this type are of great research interest due to their important biological properties such as cytotoxicity, antioxidation, immunoregulation, etc., the isolated Hypecoum aporphines have not been studied pharmacologically [42].
2.5. Simple Isoquinoline Alkaloids
The simple isoquinoline group includes five alkaloids (34–38) present in three Hypecoum species—H. imberbe, H. leptocarpum and H. erectum (Table 1). These alkaloids have the simplest structure, involving an isoquinoline nucleus which is C-6- and/or C-7-substituted. Compound 38 is, unusually, 1,3,6,6-tetramethylated and oxygenated at C-8. Several biological activities have been established for alkaloids 34, 36 and 38, including anti-inflammatory, antibacterial, anticancer and analgesic effects [13][19][27].
2.6. Secoberbine Alkaloids
The secoberbine alkaloids (39–66) are the largest group of isoquinoline alkaloids, consisting of 28 members, which have been identified in seven Hypecoum species—H. procumbens, H. procumbens var. glaucescens, H. leptocarpum, H. erectum, H. lactiflorum, H. erectum, H. ponticum and H. parviflorum (Table 1).
According to the chemical structure, the alkaloids are divided into three subgroups: 7,8-secoberbines—tricyclic (39–49) and tetracyclic (50–58); 7,8-14,7-bissecoberbine—peshawarine (59) and 6,7-secoberbines (60–66) [43]. All tricyclic 7,8-secoberbines in the Hypecoum species have substitutions at C-8 with -CH2OH or -COOH groups. Structure–activity relationship studies on these compounds have shown that the presence of the carboxyl group in coryximine (41), leptopidine (46) and hendersine B (49) is the probable reason for the low cytotoxicity against different tumor cell lines [23][27]. Furthermore, the quaternary alkaloid hypepontine 47, and -CH2OH substituted at C-8, has demonstrated good in vitro antibacterial and antifungal activity [18]. Among the tetracyclic 7,8-secoberbines, hypecorinine (55) and 2,3-dimethoxyhypecorinine (58) have been found to possess good antimicrobial and anti-inflammatory properties, respectively [13][19]. The alkaloids 60 and 62 of the 6,7-secoberbine subgroup have moderate cytotoxicity against different cancer cell lines, probably due to possessing the most electron-donating groups in their structures [23][24]. Moreover, 60 possesses potent anticancer activity, and its acidified form can be used as a potential regulator of diabetes mellitus [28][44].
2.7. Spirobenzylisoquinoline Alkaloids
Spirobenzylisoquinolines (67–73) are a small group isoquinolines with a “spiro” structure identified in two Hypecoum species—H. leptocarpum and H. erectum (Table 1). In these species, most of the spirobenzylisoquinoline alkaloids are C-8 linked via a C–C bond to an amide carbonyl group (67, 68) or 3-aminomaleimide group (69–71). Alkaloid 73 is spirobenzylisoquinoline with a unique 6/6/5/6/6 skeleton. Some of the isolated alkaloids, such as 68–70 and 72, have shown good anti-inflammatory activity via selective inhibition of inflammatory mediators [19].
2.8. Other Alkaloids
This group summarizes the Hypecoum alkaloids of various structural types (74–86) such as the benzylisoquinoline—N-methylcoclaurine (74), the phenantrene—N-methylsecoglaucine (75), the indenobenzazepines (80, 81), the phthalideisoquinoline—hypecoumine (83), the C19-benzylisoquinoline—hyperectumine (84) and alkaloids with unusual structures that cannot be assigned to any of the established groups (76–79, 82, 85, 86) (Table 1). The alkaloids 80, 81 and 84 have shown significant anti-inflammatory properties in different in vitro models [19].
References
- WFO. World Flora Online. 2023. Available online: http://www.worldfloraonline.org/search?query=Hypecoum (accessed on 17 July 2023).
- Zhang, R.F.; Zha, S.; Yin, X.; Bai, R.F.; Li, Y.; Tu, P.F.; Chai, X.Y. Phytochemical and pharmacological progress on Tibetan medicine Hypecoi Erecti Herba and plants of Hypecoum L. Chin. Tradit. Herb. Drugs 2016, 47, 1217–1224.
- Oyuntsetseg, N.; Khasnatinov, M.A.; Molor-Erdene, P.; Oyunbileg, J.; Liapunov, A.V.; Danchinova, G.A.; Oldokh, S.; Baigalmaa, J.; Chimedragchaa, C. Evaluation of direct antiviral activity of the Deva-5 herb formulation and extracts of five Asian plants against influenza A virus H3N8. BMC Complement. Altern. Med. 2014, 14, 235.
- Song, P.; Xia, J.; Rezeng, C.; Tong, L.; Tang, W. Traditional, complementary, and alternative medicine: Focusing on research into traditional Tibetan medicine in China. BioSci. Trends 2016, 10, 163–170.
- Zhou, J.; Xie, G.; Yan, X. Encyclopedia of Traditional Chinese Medicines—Molecular Structures, Pharmacological Activities, Natural Sources and Applications; Springer: Berlin/Heidelberg, Germany, 2011; Volume 5, p. 468.
- Hao, D.; Xu, L.; Zheng, Y.; Lyu, H.; Xiao, P. Mining therapeutic efficacy from treasure chest of biodiversity and chemodiversity: Pharmacophylogeny of Ranunculales medicinal plants. Chin. J. Integr. Med. 2022, 28, 1111–1126.
- Philipov, S.; Istatkova, R.; Denkova, P.; Dangaa, S.; Samdan, J.; Krosnova, M.; Munkh-Amgalan, C. Alkaloids from Mongolian species Hypecoum lactiflorum Kar. et Kir. Pazij. Nat. Prod. Res. 2009, 23, 982–987.
- Gözler, T.; Önür, M.A.; Minard, R.D.; Shamma, M. (−)-Corydalisol: A new secoberbine alkaloid. J. Nat. Prod. 1983, 46, 414–416.
- Pabuççuoglu, V.; Arar, G.; Gözler, T.; Freyer, A.J.; Shamma, M. Nitrotyrasanguinarine: An unusual nitrated benzophenanthridine alkaloid from Hypecoum species. J. Nat. Prod. 1989, 52, 716–719.
- Zhang, G.; Rücker, G.; Breitmaier, E.; Mayer, R. Alkaloids from Hypecoum leptocarpum. Phytochemistry 1995, 40, 1813–1816.
- Saad, H.E.A. (±)-8-Oxohypecorinine from Hypecoum procumbens var. glaucescens. Phytochemistry 1995, 38, 1049–1051.
- Zhou, Y.; Zhang, G.; Li, B. Five alkaloids from Hypecoum leptocarpum. Phytochemistry 1999, 50, 339–343.
- Su, Y.F.; Li, S.K.; Li, N.; Chen, L.L.; Zhang, J.W.; Wang, J.R. Seven alkaloids and their antibacterial activity from Hypecoum erectum L. J. Med. Plants Res. 2011, 5, 5428–5432.
- Yakhontova, L.D.; Komarova, M.N.; Perel’son, M.E.; Blinova, K.F.; Tolkachev, O.N. Alkaloids of Hypecoum erectum structure of hypecorine and hypecorinine. Chem. Nat. Comp. 1972, 8, 592–595.
- Önür, M.A.; Abu Zarga, M.H.; Gözler, T. Hypecumine: A new 3-arylisoquinoline Hypecoum procumbens. Planta Med. 1986, 52, 70–71.
- Shamma, M.; Rothenberg, A.S.; Jayatilak, G.S.; Hussain, S.F. A new group of isoquinoline alkaloids: The secoberbines. Tetrahedron 1978, 34, 635–640.
- Doncheva, T.; Kostova, N.; Vutov, V.; Aneva, I.; Philipov, S. Comparative study of the alkaloid composition in some Bulgarian species of genus Hypecoum. Compt. Rend. Acad. Bulg. Sci. 2019, 72, 727–731.
- Doncheva, T.; Kostova, N.; Valcheva, V.; Toshkovska, R.; Vutov, V.; Philipov, S. Hypepontine, a new quaternary alkaloid with antimicrobial properties. Nat. Prod. Res. 2020, 34, 668–674.
- Yuan, H.L.; Zhao, Y.L.; Qin, X.J.; Liu, Y.P.; Yang, X.W.; Luo, X.D. Diverse isoquinolines with anti-inflammatory and analgesic bioactivities from Hypecoum erectum. J. Ethnopharmacol. 2021, 270, 113811.
- Xu, N.; Bao, W.; Xin, J.; Xiao, H.; Yu, J.; Xu, L. A new 1, 3-Benzodioxole compound from Hypecoum erectum and its antioxidant activity. Molecules 2022, 27, 6657.
- Zhang, Q.; Ma, T.; Hu, N.; Ding, C.; Liang, Y.; Li, W.; Suo, Y.; Ding, C. One step to separate five alkaloids from Hypecoum leptocarpum by High-Speed Counter-Current Chromatography. J. Chromatogr. Sci. 2016, 54, 466–471.
- Wen, H.X.; Jiang, L.; Zhang, D.; Yuan, X.; Dang, J.; Mei, L.; Shao, Y.; Tao, Y. Anti-inflammatory activity of total alkaloids from Hypecoum leptocarpum Hook. f. et Thoms. Pharmacogn. Mag. 2018, 14, 397–403.
- Sun, B.; Assani, I.; Wang, C.G.; Wang, M.X.; Liu, L.F.; Li, Y.; Yan, G.; Yang, Y.R.; Chen, Z.; Liao, Z.X. Two new alkaloids from Tibetan medicine of Hypecoum leptocarpum. Nat. Prod. Res. 2022, 36, 5304–5310.
- Wen, H.; Yuan, X.; Li, C.; Li, J.; Yue, H. Two new isoquinoline alkaloids from Hypecoum leptocarpum Hook. f. et Thoms. Nat. Prod. Res. 2022; in press.
- Preininger, V. Chemotaxonomy of Papaveraceae and Fumariaceae. In The Alkaloids; Brossi, A., Ed.; Academic Press: London, UK, 1986; Volume 29, pp. 1–98.
- Yuan, H.L.; Zhao, Y.L.; Hu, K.; He, Y.J.; Yang, X.W.; Luo, X.D. C19 Benzylisoquinoline alkaloid with unprecedented architecture from Hypecoum erectum. J. Org. Chem. 2021, 86, 16764–16769.
- Zhang, Q.; Luan, G.; Ma, T.; Hu, N.; Suo, Y.; Wang, X.; Ma, X.; Ding, C. Application of chromatography technology in the separation of active alkaloids from Hypecoum leptocarpum and their inhibitory effect on fatty acid synthase. J. Sep. Sci. 2015, 38, 4063–4070.
- Luo, T.; Deng, X.; Li, Z.; Liu, D.; Wen, H.; Wang, Z. Synthesis and anti-hepatocellular carcinoma effects of corydamine from Hypecoum leptocarpum. Phytochem. Lett. 2022, 51, 119–126.
- Baskan, S.; Bulbul, A.S. Investigation of antimicrobial properties of endemic Hypecoum trullatum Å.E.Dahl plant in in-vitro conditions. Res. J. Biol. Sci. 2022, 15, 143–148.
- Taborska, E.; Mikesova, M.; Veznik, F.; Slavik, J. Alkaloids of the two Hypecoum L. species. Coll. Czech. Chem. Commun. 1987, 52, 508–513.
- Yakhontova, L.D.; Tolkachev, O.N.; Komarova, M.N.; Shreter, A.I. The alkaloid compositions of Hypecoum erectum and H. lactiflorum. Chem. Nat. Comp. 1984, 20, 645–646.
- Taborska, E.; Bochorakova, H.; Sedmera, P.; Valka, I.; Simanek, V. Leptocarpine, a new protopine alkaloid. Heterocycles 1995, 41, 799–805.
- Li, B.G.; Zhou, M.; Zhang, G. Four alkaloids from Hypecoum leptocarpum Hook. f. et Thoms. Indian J. Chem. 2001, 40B, 1215–1218.
- Mete, E.; Kayalar, H. A novel quaternary alkaloid N-methylcoryximine from Hypecoum pendulum L. Univ. J. Pharm. Res. 2022, 7, 40–47.
- Taborska, E.; Veznik, F.; Slavik, J.; Sedmera, P.; Simanek, V. Procumbine, a new secoberbine alkaloid. Heterocycles 1988, 27, 39–44.
- Perel’son, M.E.; Aleksandrov, G.G.; Yakhontova, L.D.; Tolkachev, O.N.; Fesenko, D.A.; Komarova, M.N.; Esipov, S.E. Alkaloids of Hypecoum erectum, the structure of hyperectine. Chem. Nat. Comp. 1984, 20, 592–598.
- Yakhontova, L.D.; Yartseva, I.V.; Klyuev, N.A.; Tolkachev, O.N. Structure of isohyperectine—An alkaloid from Hypecoum erectum. Chem. Nat. Comp. 1993, 29, 744–747.
- Sun, P.T.; Cao, Y.G.; Xue, G.M.; Li, M.; Zhang, C.L.; Zhao, F.; Cao, Z.Y.; Wang, D.; Gustafson, K.R.; Zheng, X.K.; et al. Hypeisoxazole A, a racemic pair of tetrahydroisoxazole-fused benzylisoquinoline alkaloids from Hypecoum erectum and structural revision of hypecoleptopine. Org. Lett. 2022, 24, 1476–1480.
- Mete, I.E.; Gözler, T. (+)-Oxoturkiyenine: An isoquinoline-derived alkaloid from Hypecoum pendulum. J. Nat. Prod. 1988, 51, 272–274.
- Gözler, T.; Gözler, B.; Weiss, I.; Freyer, A.J.; Shamma, M. (+)-Turkiyenine: An unusual extension of the biogenetic sequence for isoquinoline alkaloids. J. Am. Chem. Soc. 1984, 106, 6101–6102.
- Han, N.; Yang, Z.; Liu, Z.; Liu, H.; Yin, J. Research progress on natural benzophenanthridine alkaloids and their pharmacological functions: A Review. Nat. Prod. Commun. 2016, 11, 1181–1188.
- Chen, J.; Gao, K.; Liu, T.; Zhao, H.; Wang, J.; Wu, H.; Wang, W. Aporphine alkaloids: A kind of alkaloids’ extract source, chemical constitution and pharmacological actions in different botany. Asian J. Chem. 2013, 18, 10015–10027.
- Rozwadowska, M.D. Secoisoquinoline alkaloids. In The Alkaloids; Brossi, A., Ed.; Academic Press: New York, NY, USA, 1988; Volume 33, pp. 231–306.
- Gong, J.N.; Zhao, L.; Chen, G.; Chen, X.; Chen, Z.D.; Chen, C.Y.C. A novel artificial intelligence protocol to investigate potential leads for diabetes mellitus. Mol. Divers. 2021, 25, 1375–1393.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
600
Revisions:
2 times
(View History)
Update Date:
09 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




