Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Faizan Ahmad | -- | 2618 | 2023-09-28 11:09:54 | | | |
| 2 | Peter Tang | Meta information modification | 2618 | 2023-09-28 11:27:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mondal, A.; Sharma, R.; Abiha, U.; Ahmad, F.; Karan, A.; Jayaraj, R.L.; Sundar, V. Nutritional Supplements for Management of Autism Spectrum Disorder. Encyclopedia. Available online: https://encyclopedia.pub/entry/49765 (accessed on 08 February 2026).
Mondal A, Sharma R, Abiha U, Ahmad F, Karan A, Jayaraj RL, et al. Nutritional Supplements for Management of Autism Spectrum Disorder. Encyclopedia. Available at: https://encyclopedia.pub/entry/49765. Accessed February 08, 2026.
Mondal, Arunima, Rashi Sharma, Umme Abiha, Faizan Ahmad, Anik Karan, Richard L. Jayaraj, Vaishnavi Sundar. "Nutritional Supplements for Management of Autism Spectrum Disorder" Encyclopedia, https://encyclopedia.pub/entry/49765 (accessed February 08, 2026).
Mondal, A., Sharma, R., Abiha, U., Ahmad, F., Karan, A., Jayaraj, R.L., & Sundar, V. (2023, September 28). Nutritional Supplements for Management of Autism Spectrum Disorder. In Encyclopedia. https://encyclopedia.pub/entry/49765
Mondal, Arunima, et al. "Nutritional Supplements for Management of Autism Spectrum Disorder." Encyclopedia. Web. 28 September, 2023.
Copy Citation
Autism spectrum disorder (ASD) is a developmental disorder that causes difficulty while socializing and communicating and the performance of stereotyped behavior. ASD is thought to have a variety of causes when accompanied by genetic disorders and environmental variables together, resulting in abnormalities in the brain. To manage the symptoms of ASD pharmacological interventions are available but long term use show adverse effect on body so, non-pharmacological interventions like different diets or supplements can be used to manage the symptoms without adverse effect.
neurodevelopmental disorder
ASD
neurotherapeutics
nutritional therapy
1. Introduction
In 1908, a Swiss psychiatrist named Eugen Bleuler invented the terminology of “autism”, which originated from the ancient term “autós”, which signifies “self”, to characterize the detachment from reality of patients with schizophrenia [1][2][3]. Leo Kanner used the phrase in 1943 to describe linguistic and social isolation problems in children who did not have psychosis or other psychological illnesses. Such children struggled to engage and communicate with others, had a specific pattern of behavior, and were uninterested in social affairs [4][5][6][7][8]. One out of every 88 children has developmental difficulties, and this percentage seems to be rising. The frequency of autism in males and females is equivalent to approximately 5:1, affecting about 1.5% of the population [9][10][11][12][13][14][15]. The pathophysiology of ASD is not entirely known, and comorbidities, including epilepsy, attention, mood, and language impairments; sleep disturbances; gastrointestinal issues; and intellectual disability, are frequent (70% of cases). ASD is believed to be a developmental defect of brain processes brought on by genetic and neurological reasons, creating social disruption, which results in limited attentiveness and compulsive behaviors [16][17][18][19][20]. An aberrant gene gets “turned on” during the early stages of fetal development, altering the body. Its expression can be changed without modifying the primary DNA sequence of other genes. The pathogenesis of ASD, which appears to be primarily driven by heterogeneous genetic mutations and variants and modulated by diverse gene–environment interactions, including pregnancy-related factors (such as maternal immune activation, maternal toxins, and perinatal trauma), may be a significant factor in the absence of disease-modifying therapies. Currently, there are few accessible pharmacological and non-pharmacological methods for ASD intervention. Different psychiatric drugs are used as pharmacological interventions, whereas specialized foods, herbal supplements, chiropractic adjustments, art therapy, mindfulness practices, and relaxation techniques are a part of non-pharmacological methods. As there are not any particular behaviors that aid in identifying people with ASD, it does not have a single management strategy. In addition to this, the cost of management of an autistic individual for a lifetime, as estimated in a study conducted in the USA, approximately amounts to around USD 3.6 million, which goes up as the case worsens. Apart from this huge cost, the constant care and support required are beyond estimation and are not a treatment that everyone can afford. Many parents have always turned to alternative therapies to help autistic children [21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38]. For different patients of ASD, specific CAM therapies, which include essential fatty acids, vitamins, an oligoantigenic diet, herbal remedies, and amino acids, are found to give favorable results. ASD nutritional dysfunctions should be considered part of the therapy/management process, as managing autism care is a complex condition for individuals and their families [39][40][41][42].
2. Diets for ASD
There is a need for additional management options that can improve outcomes for individuals with ASD. Different dietary supplements for the management of ASD are discussed below.
2.1. Elimination Diet for ASD
As the term signifies, some foods are avoided in the diet on the theory that particular ASD symptoms are related to foods that appear to be impacted by dietary hypersensitivities [43]. Such foods create gastrointestinal issues (GI) issues and raise IgG levels as the individual may be sensitive to the foods or their additives [44]. IgE and IgA antibody types have already been linked to immune dysfunction in people with autism. Diets must be closely controlled because removing foods to which an individual is allergic can cause malnutrition, which can worsen the symptoms of the disease by causing anemia. Findings show that adopting an exclusion diet regimen considerably improved the pathogenic alterations in autistic patients [45]. The popular elimination diet (gluten-free casein-free diet, GFCF) removed the proteins included in milk and cereals. The aforementioned diet calls for a decrease in or total removal of all the above-listed proteins [44]. Cows’ milk, cheese, and other dairy products contain casein, which, when removed, can cause a calcium deficiency since it is a crucial nutrient for bone and tooth health. Alternatives such as goat or sheep milk are frequently recommended but might require the body to confront new allergens [45]. The elimination diet can lead to malnutrition if not carefully monitored, while the specific carbohydrate diet may be challenging to follow and restricts certain foods that are important for overall health. Additionally, some nutritional supplements may interact with medications or have harmful side effects if taken in excessive amounts.
2.2. Casein and Gluten for ASD
Gluten, milk, barley, rye, and wheat include casein, which has anti-inflammatory characteristics that regulate immune responses [46][47]. In particular, in persons with ASD, casein and gluten can promote the production of antibodies against IgA and IgG, worsening their immune dysregulation. The small intestinal mucosa works as a luminal barrier, keeping germs out, and such compounds are not permitted to enter the circulatory system. People with ASD, on the other hand, have higher intestinal permeability to such compounds, resulting in inflammation [48][49][50][51][52]. Casein and gluten products need to be consumed based on a clinician’s advice, as these diets can cause inflammation at high doses, which can lead to other disorders.
2.3. Specific Carbohydrate Diet for ASD
A study by Gottschall, E. (2004) popularized this diet as a method of autism management. This diet’s central premise is to prevent the advancement of pathogenic intestinal microflora’s by alleviating malabsorption [53][54]. This diet recommends consuming monosaccharides, like those found in fruits, vegetables, and honey, rather than complex polysaccharides because polysaccharides take longer to digest [53]. Difficult polysaccharide digestion disrupts gastrointestinal tract function, resulting in absorption difficulty and the accumulation of left-over food. Intestinal pathogenic flora thrives in this food-accumulated environment [54]. This diet aims to help individuals lose weight, restore normal intestine functions, and minimize intestinal cancer formation. Meat, eggs, natural cheese, vegetables (pepper), cauliflower, onions, cabbage, spinach, homemade yogurt, fruits, nuts (walnuts, almonds), beans, and soaked lentils are all excellent protein sources and are recommended. Complex carbohydrates (e.g., sugar) are prohibited in the specific carbohydrate diet [54][55]. Only those foods that require minimal digestion are allowed. In a study conducted by Żarnowska et al. (2018), this diet was followed by people with Crohn’s disease, both colonic and ileocolonic [55]. Symptoms improved after three years of monitoring. These findings may be generalizable across populations of people with ASD. Learning and memory were also highly improved, as were responsive and imaginative language difficulties [55]. Carbohydrate diets need to be consumed based on a clinician’s advice and at the recommended dose as complex carbohydrate diets can cause different complications like inflammatory bowel disease (IBD).
2.4. Ketogenic Diet for ASD
The ketogenic diet is a general term for a low-carbohydrate, moderate-protein, and high-fat diet that encourages our body to use ketones instead of glucose for energy. This results in more ketones in the blood, reduced blood glucose, and better functioning of mitochondria [56][57]. This diet has shown potential in treating patients with refractory epilepsy, which is considerably more typical in persons with ASD than those without ASD and other related nervous system problems [58][59]. A study by Kasprowska-Liśkiewicz D. et al. (2017) revealed that their sample exhibited fewer seizures and superior learning and social abilities [60]. El-Rashidi, O. et al. (2017) studies in people with ASD also revealed that the medication produced moderate improvements [61]. A ketogenic diet produces better responses and fewer complications in comparison to the elimination diet, casein and gluten diet, and carbohydrate diet, but still, the dose needs to be set by a clinician/dietician to avoid complications.
3. Nutritional Supplements for ASD
Numerous studies have suggested that poor behavioral evaluation test scores are continuously connected to low nutritional fulfillment. Hyperactivity, agitation, and irritability decrease when certain nutrient supplements are administered. Impulsivity and the inability to pay attention both improve dramatically [62]. During the day, a diverse mix of vegetarian and animal proteins is consumed to meet the daily need for amino acids. Amino acids (AAs), which have long been the basic building blocks of our bodies, make up proteins. The body may synthesize certain amino acids, but amino acids should be acquired from protein-rich diets [62]. The effect of nutritional therapy on ASD is shown in Figure 1. According to a study, neuroactive amino acids play a vital role in central brain activities. Neuroactive AAs are crucial in etiology and play a part in treating autistic symptoms [63]. It is also essential to watch for changes in their bodily fluid concentrations and see whether they correspond to early signs. Their availability, metabolism, and receptor functionality must all be considered [63]. They have been connected to the causes and therapies of numerous mental illnesses. More research is needed to see if other amino acids are involved. Discussed below are a few nutritional supplements.
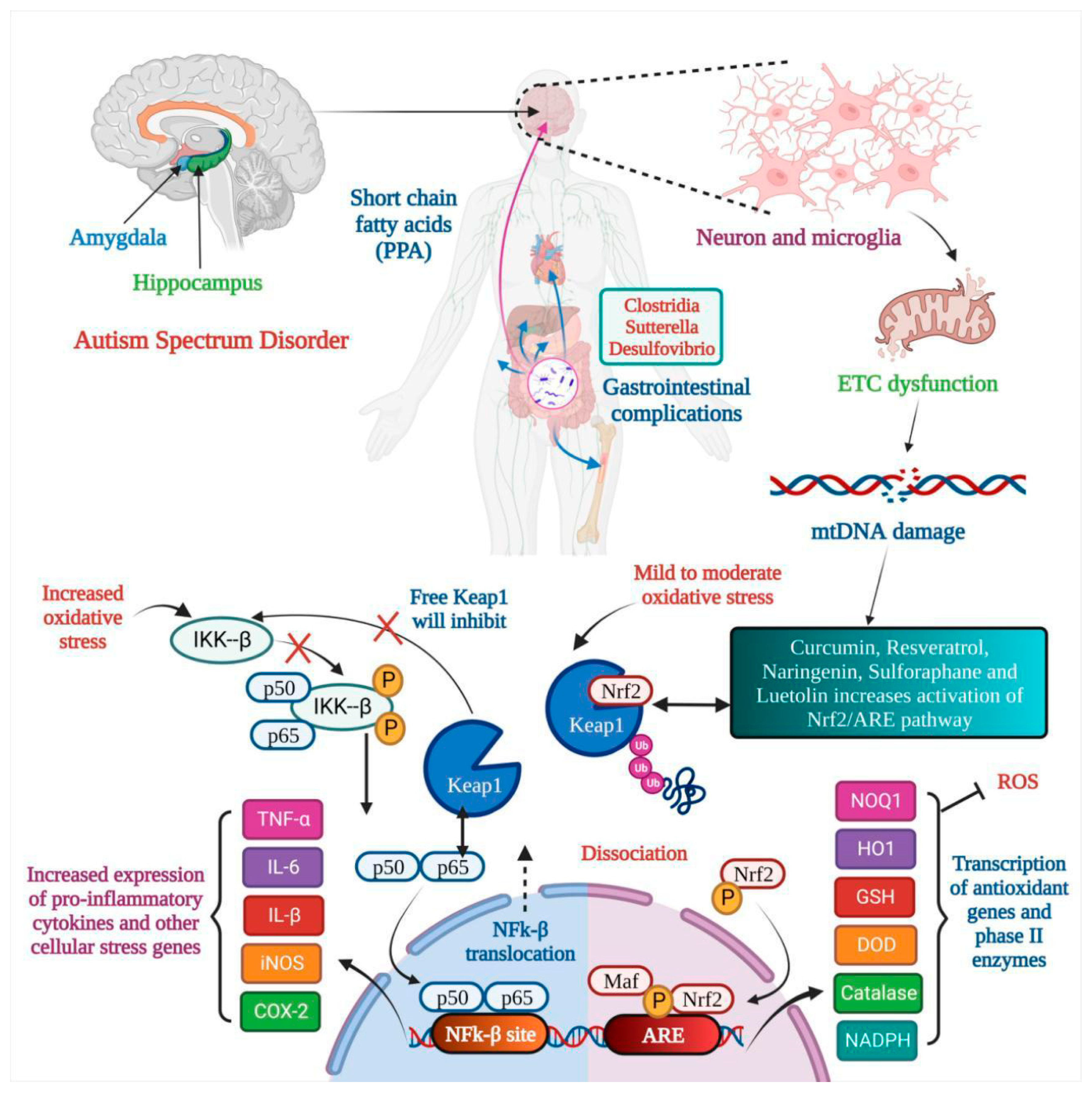
Figure 1. Nutritional therapy attunes mitochondrial dysfunction in Autism Spectrum Disorder. In autism, the amygdala and hippocampus are affected, and gastrointestinal complications are seen. Microglia activation leads to electron transport chain (ETC) dysfunction, which results in mtDNA damage, and oxidative stress leads to dysfunction of the Nrf2 pathway and natural products like curcumin, resveratrol, etc., resulting in inactivation of the Nrf2/ARE pathway. Two outcomes are seen: (a) the transcription of antioxidant genes and (b) the increased expression of pro-inflammatory cytokines.
3.1. Omega-3 Fatty Acids for ASD
Omega-3 fatty acids are polyunsaturated fatty acids (PUFAs) recognized as -3 fatty acids or n-3 fatty acids. Triglycerides and phospholipids are two natural forms of omega-3 fatty acids. Fat is the most common component of brain nerve cells. Human physiology requires three omega-3 fatty acids, i.e., docosahexaenoic acid (DHA), alpha-linolenic acid (ALA) and eicosapentaenoic acid (EPA). Fish, eggs, and flax seeds are their most common natural sources [64]. PUFAs are essential for human health. The brain can generate neuronal signals in response to new experiences and stimuli. Neuronal plasticity, or the learning environment, is critical in long-term learning. DHA and omega-3 fatty acid levels must be balanced to maintain learning ability and enhance neuronal plasticity through membrane fluidity [64][65]. There is not much evidence to back up omega-3 supplementation’s effectiveness in improving the core or linked symptoms of ASD. Three randomized controlled trials (RCTs) comparing omega-3 fatty acids to a placebo revealed no significant differences [64][65]. The placebo group performed significantly better in one trial than the control group. Parent ratings of stereotypy and weariness in children who took omega-3 supplements against those who did not show substantial improvement after six months of treatment compared to the omega-3 group in externalizing behaviors [64][65]. It is advised to consult a physician before consuming omega-3 fatty acids as high doses can cause nausea, loose stools, and stomach upset.
3.2. Zinc for ASD
Zinc, a mood mineral, is vital because it is a cofactor for numerous neurotransmitters that affect mood and learning. Low zinc amounts disrupt dopamine production as this neurotransmitter involves learning and emotions such as motivation and pleasure [66]. A lack of zinc affects normal neural activities, including neurotransmission, brain development, and connection; moreover, it indirectly impacts the brain by impairing the immune system and changing the usual gut–brain link. This metal is essential for the neuropeptide social impact. So, to avoid autism, expecting and new mothers take a zinc supplement in their diets [67][68]. High doses of zinc can cause acute gastrointestinal symptoms like abdominal pain, diarrhea, and vomiting, so it is recommended to consult a physician to determine the dose.
3.3. Vitamins for ASD
Most vitamins must be present in ideal amounts for healthy brain development. Vitamin D supplementation, in particular, has been demonstrated to help the symptoms of people with autism regress. Vitamins are potent antioxidants that help to protect cellular and mitochondrial function from free radical damage [66][68]. They also function as cofactors in a variety of biological processes. They regulate lipid and protein metabolism and are crucial for DNA synthesis. According to a study by Rollett, A. (1909), reduced folate levels during pregnancy are also related to congenital impairments. It has been connected to hyperactivity in youngsters. Autistic youngsters have also been proven to benefit from vitamin B1. Vitamin C has twin benefits, firstly as an antioxidant and secondly in creating some neurotransmitters [66][67]. Researchers have also determined that a specific gene encoding a particular protein is missing, which is the protein necessary to produce vitamin A. Clinical tests showed that vitamin A treatment enhances language and visual abilities in autistic patients. On the other hand, vitamin A supplementation must be carried out under the supervision of a physician as it can cause liver damage [68].
3.4. Iron for ASD
In autistic people, malabsorption of the vitamin inside the gastrointestinal system and their selective eating habits can lead to iron insufficiency. As a result, an iron shortage is reported to negatively affect sleep and neuroprotection. According to specific clinical investigations, cognitive impairment, reduced development, attention issues, and anemia are all related to mood swings in autistic children [69]. Children who have ASD have been found to have a high prevalence of iron deficiency (ID) and anemia that occurs due to iron deficiency (IDA). There are a small number of studies that link autistic clinical symptoms and iron deficiency indicators. The current research compares the levels of HB, hematocrit, Fe, ferritin, mean corpuscular volume, and red cell distribution width in patients with autism and healthy controls to determine the relationship between the numbers and symptoms. Children with ASD had lower HB levels than children without the disorder. Instead of the intensity of autistic symptoms, IDA in children with ASD may be linked to mental retardation [70]. A high dose of iron can cause iron poisoning, which shows multiple symptoms like nausea, abdominal pain, fever, headache, seizures, etc., so the dose needs to be advised by a physician to avoid complications due to a high dose of iron.
3.5. Magnesium (Mg) for ASD
Magnesium (Mg) works synergistically to relieve the clinical signs of autism. When autistic youngsters were given magnesium and vitamin B6, their social interaction and speech increased by 70% [71]. The most recognizable symptoms and indicators of Mg shortage are caused by neuronal and neuromuscular overactivity. In general, the connections between magnesium levels in inverse and direct sites and neurodevelopmental disorders may be a sign of higher excretion of magnesium in children with ASD, which ultimately results in a lower burden of magnesium in the body. The lack of noticeable changes in serum Mg levels may result from homeostatic regulation, which regulates absorption, excretion, and tissue redistribution (particularly in the bones) to maintain circulating Mg levels. Mg has a tremendous impact on neural excitation. Stress-related physical damage is more susceptible to Mg shortage, and Mg supplementation is protective. The neurologic impairment caused by experimental head trauma can be reduced pharmacologically by Mg, probably via the blockage of N-methyl-D-aspartate receptors. Mg salts help around 40% of people with autism when taken with large doses of pyridoxine, perhaps because they impact dopamine metabolism [72]. A high dose of Mg can cause diarrhea, vomiting, depression, low blood pressure, etc., so it is recommended to consult a physician before consuming Mg.
3.6. Selenium for ASD
Numerous vital metabolic processes for life depend on selenium. Countless studies have shown that the neuro-endocrine–immune network plays an important role in the interaction between the intestinal microbiota and the brain that impacts autism, and some animal studies have suggested that the gut microbiota may compete with the host for selenium when its accessibility in the organism becomes limited [73]. Selenium at high doses can cause nausea, bad breath, and fever as well as severe problems in the heart, liver, and kidneys, so it is advised to consult a physician before consuming selenium.
References
- Kanner, L. Autistic disturbances of affective contact. Nerv. Child 1943, 2, 217–250.
- Asperger, H. Die „Autistischen psychopathen” im kindesalter. Arch. Psychiatr. Nervenkrankh. 1944, 117, 76–136.
- Elsabbagh, M.; Divan, G.; Koh, Y.J.; Kim, Y.S.; Kauchali, S.; Marcín, C.; Montiel-Nava, C.; Patel, V.; Paula, C.S.; Wang, C.; et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012, 5, 160–179.
- Wiśniowiecka-Kowalnik, B.; Nowakowska, B.A. Genetics and epigenetics of autism spectrum disorder—Current evidence in the field. J. Appl. Genet. 2019, 60, 37–47.
- Ahmad, F.; Virmani, A.; Irfan, M.; Rankawat, S.; Pathak, U. Critical appraisals on depressions and psychotic symptoms. J. Neurobehav. Sci. 2021, 8, 81–88.
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Rosenberg, C.R.; White, T.; et al. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1.
- Lai, M.C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910.
- Williams, J.G.; Higgins, J.P.; Brayne, C.E. Systematic review of prevalence studies of autism spectrum disorders. Arch. Dis. Child. 2006, 91, 8–15.
- Fombonne, E. Epidemiology of autistic disorder and other pervasive developmental disorders. J. Clin. Psychiatry 2005, 66, 3.
- Shattuck, P.T. The contribution of diagnostic substitution to the growing administrative prevalence of autism in US special education. Pediatrics 2006, 117, 1028–1037.
- Williams, J.; Allison, C.; Scott, F.; Stott, C.; Bolton, P.; Baron-Cohen, S.; Brayne, C. The Childhood Asperger Syndrome Test (CAST): Test-retest reliability. Autism Int. J. Res. Pract. 2006, 10, 415–427.
- Wing, L.; Potter, D. The epidemiology of autistic spectrum disorders: Is the prevalence rising? Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 151–161.
- Bishop, D.V.; Whitehouse, A.J.; Watt, H.J.; Line, E.A. Autism and diagnostic substitution: Evidence from a study of adults with a history of developmental language disorder. Dev. Med. Child Neurol. 2008, 50, 341–345.
- Croen, L.A.; Grether, J.K.; Hoogstrate, J.; Selvin, S. The changing prevalence of autism in California. J. Autism Dev. Disord. 2002, 32, 207–215.
- Barbaresi, W.J.; Katusic, S.K.; Colligan, R.C.; Weaver, A.L.; Jacobsen, S.J. The incidence of autism in Olmsted County, Minnesota, 1976–1997: Results from a population-based study. Arch. Pediatr. Adolesc. Med. 2005, 159, 37–44.
- Hertz-Picciotto, I.; Delwiche, L. The rise in autism and the role of age at diagnosis. Epidemiology 2009, 20, 84.
- Mandell, D.S.; Palmer, R. Differences among states in the identification of autistic spectrum disorders. Arch. Pediatr. Adolesc. Med. 2005, 159, 266–269.
- Parner, E.T.; Schendel, D.E.; Thorsen, P. Autism prevalence trends over time in Denmark: Changes in prevalence and age at diagnosis. Arch. Pediatr. Adolesc. Med. 2008, 162, 1150–1156.
- Loomes, R.; Hull, L.; Mandy, W. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474.
- Asherson, P.J.; Curran, S. Approaches to gene mapping in complex disorders and their application in child psychiatry and psychology. Br. J. Psychiatry 2001, 179, 122–128.
- Zbiciak, A.; Markiewicz, T. A new extraordinary means of appeal in the Polish criminal procedure: The basic principles of a fair trial and a complaint against a cassatory judgment. Access Justice East. Eur. 2023, 6, 1–18.
- Fombonne, E.; Zakarian, R.; Bennett, A.; Meng, L.; McLean-Heywood, D. Pervasive developmental disorders in Montreal, Quebec, Canada: Prevalence and links with immunizations. Pediatrics 2006, 118, e139–e150.
- Jorde, L.B.; Hasstedt, S.J.; Ritvo, E.R.; Mason-Brothers, A.; Freeman, B.J.; Pingree, C.; McMahon, W.M.; Petersen, B.; Jenson, W.R.; Mo, A. Complex segregation analysis of autism. Am. J. Hum. Genet. 1991, 49, 932–938.
- Lauritsen, M.B.; Pedersen, C.B.; Mortensen, P.B. Effects of familial risk factors and place of birth on the risk of autism: A nationwide register-based study. J. Child Psychol. Psychiatry Allied Discip. 2005, 46, 963–971.
- Muhle, R.; Trentacoste, S.V.; Rapin, I. The genetics of autism. Pediatrics 2004, 113, e472–e486.
- Ozonoff, S.; Young, G.S.; Carter, A.; Messinger, D.; Yirmiya, N.; Zwaigenbaum, L.; Bryson, S.; Carver, L.J.; Constantino, J.N.; Dobkins, K.; et al. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics 2011, 128, e488–e495.
- Piven, J.; Gayle, J.; Chase, G.A.; Fink, B.; Landa, R.; Wzorek, M.M.; Folstein, S.E. A family history study of neuropsychiatric disorders in the adult siblings of autistic individuals. J. Am. Acad. Child Adolesc. Psychiatry 1990, 29, 177–183.
- Risch, N.; Spiker, D.; Lotspeich, L.; Nouri, N.; Hinds, D.; Hallmayer, J.; Kalaydjieva, L.; McCague, P.; Dimiceli, S.; Pitts, T.; et al. A genomic screen of autism: Evidence for a multilocus etiology. Am. J. Hum. Genet. 1999, 65, 493–507.
- Schaefer, G.B.; Mendelsohn, N.J.; Professional Practice and Guidelines Committee. Clinical genetics evaluation in identifying the etiology of autism spectrum disorders: 2013 guideline revisions. Genet. Med. Off. J. Am. Coll. Med. Genet. 2013, 15, 399–407.
- Constantino, J.N.; Zhang, Y.; Frazier, T.; Abbacchi, A.M.; Law, P. Sibling recurrence and the genetic epidemiology of autism. Am. J. Psychiatry 2010, 167, 1349–1356.
- Palmer, N.; Beam, A.; Agniel, D.; Eran, A.; Manrai, A.; Spettell, C.; Steinberg, G.; Mandl, K.; Fox, K.; Nelson, S.F.; et al. Association of Sex with Recurrence of Autism Spectrum Disorder Among Siblings. JAMA Pediatr. 2017, 171, 1107–1112.
- Sachdeva, P.; Mehdi, I.; Kaith, R.; Ahmad, F.; Anwar, M.S. Potential natural products for the management of autism spectrum disorder. Ibrain 2022, 8, 365–376.
- Dalton, K.M.; Nacewicz, B.M.; Alexander, A.L.; Davidson, R.J. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biol. Psychiatry 2007, 61, 512–520.
- Gamliel, I.; Yirmiya, N.; Jaffe, D.H.; Manor, O.; Sigman, M. Developmental trajectories in siblings of children with autism: Cognition and language from 4 months to 7 years. J. Autism Dev. Disord. 2009, 39, 1131–1144.
- Gamliel, I.; Yirmiya, N.; Sigman, M. The development of young siblings of children with autism from 4 to 54 months. J. Autism Dev. Disord. 2007, 37, 171–183.
- Piven, J.; Palmer, P.; Jacobi, D.; Childress, D.; Arndt, S. Broader autism phenotype: Evidence from a family history study of multiple-incidence autism families. Am. J. Psychiatry 1997, 154, 185–190.
- Yirmiya, N.; Gamliel, I.; Shaked, M.; Sigman, M. Cognitive and verbal abilities of 24- to 36-month-old siblings of children with autism. J. Autism Dev. Disord. 2007, 37, 218–229.
- Baron-Cohen, S. Two new theories of autism: Hyper-systemising and assortative mating. Arch. Dis. Child. 2006, 91, 2–5.
- Ecker, C.; Bookheimer, S.Y.; Murphy, D.G. Neuroimaging in autism spectrum disorder: Brain structure and function across the lifespan. The Lancet. Neurology 2015, 14, 1121–1134.
- Muhle, R.A.; Reed, H.E.; Stratigos, K.A.; Veenstra-VanderWeele, J. The Emerging Clinical Neuroscience of Autism Spectrum Disorder: A Review. JAMA Psychiatry 2018, 75, 514–523.
- Lopez-Rangel, E.; Lewis, M.E. Loud and clear evidence for gene silencing by epigenetic mechanisms in autism spectrum and related neurodevelopmental disorders. Clin. Genet. 2006, 69, 21–22.
- Samaco, R.C.; Nagarajan, R.P.; Braunschweig, D.; LaSalle, J.M. Multiple pathways regulate MeCP2 expression in normal brain development and exhibit defects in autism-spectrum disorders. Hum. Mol. Genet. 2004, 13, 629–639.
- Christison, G.W.; Ivany, K. Elimination diets in autism spectrum disorders: Any wheat amidst the chaff? J. Dev. Behav. Pediatr. JDBP 2006, 27 (Suppl. 2), S162–S171.
- Buie, T. The relationship of autism and gluten. Clin. Ther. 2013, 35, 578–583.
- Jyonouchi, H.; Sun, S.; Itokazu, N. Innate immunity associated with inflammatory responses and cytokine production against common dietary proteins in patients with autism spectrum disorder. Neuropsychobiology 2002, 46, 76–84.
- Lau, N.M.; Green, P.H.; Taylor, A.K.; Hellberg, D.; Ajamian, M.; Tan, C.Z.; Kosofsky, B.E.; Higgins, J.J.; Rajadhyaksha, A.M.; Alaedini, A. Markers of Celiac Disease and Gluten Sensitivity in Children with Autism. PLoS ONE 2013, 8, e66155.
- Quan, L.; Xu, X.; Cui, Y.; Han, H.; Hendren, R.L.; Zhao, L.; You, X. A systematic review and meta-analysis of the benefits of a gluten-free diet and/or casein-free diet for children with autism spectrum disorder. Nutr. Rev. 2022, 80, 1237–1246.
- Marí-Bauset, S.; Zazpe, I.; Mari-Sanchis, A.; Llopis-González, A.; Morales-Suárez-Varela, M. Evidence of the gluten-free and casein-free diet in autism spectrum disorders: A systematic review. J. Child Neurol. 2014, 29, 1718–1727.
- Geraghty, M.E.; Bates-Wall, J.; Ratliff-Schaub, K.; Lane, A.E. Nutritional interventions and therapies in autism: A spectrum of what we know: Part 2. ICAN Infant Child Adolesc. Nutr. 2010, 2, 120–133.
- Horvath, K.; Perman, J.A. Autism and gastrointestinal symptoms. Curr. Gastroenterol. Rep. 2002, 4, 251–258.
- White, J.F. Intestinal pathophysiology in autism. Exp. Biol. Med. 2003, 228, 639–649.
- Mezzelani, A.; Landini, M.; Facchiano, F.; Raggi, M.E.; Villa, L.; Molteni, M.; De Santis, B.; Brera, C.; Caroli, A.M.; Milanesi, L.; et al. Environment, dysbiosis, immunity and sex-specific susceptibility: A translational hypothesis for regressive autism pathogenesis. Nutr. Neurosci. 2015, 18, 145–161.
- Kawicka, A.; Regulska-Ilow, B. How nutritional status, diet and dietary supplements can affect autism. A review. Rocz. Państwowego Zakładu Hig. 2013, 64, 1–12.
- Gottschall, E. Digestion-gut-autism connection: The specific carbohydrate diet. Med. Veritas 2004, 1, 261–271.
- Żarnowska, I.; Chrapko, B.; Gwizda, G.; Nocuń, A.; Mitosek-Szewczyk, K.; Gasior, M. Therapeutic use of carbohydrate-restricted diets in an autistic child; a case report of clinical and 18FDG PET findings. Metab. Brain Dis. 2018, 33, 1187–1192.
- Ruskin, D.N.; Svedova, J.; Cote, J.L.; Sandau, U.; Rho, J.M.; Kawamura, M., Jr.; Boison, D.; Masino, S.A. Ketogenic diet improves core symptoms of autism in BTBR mice. PLoS ONE 2013, 8, e65021.
- Napoli, E.; Dueñas, N.; Giulivi, C. Potential therapeutic use of the ketogenic diet in autism spectrum disorders. Front. Pediatr. 2014, 2, 69.
- Ruskin, D.N.; Murphy, M.I.; Slade, S.L.; Masino, S.A. Ketogenic diet improves behaviors in a maternal immune activation model of autism spectrum disorder. PLoS ONE 2017, 12, e0171643.
- Dai, Y.; Zhao, Y.; Tomi, M.; Shin, B.C.; Thamotharan, S.; Mazarati, A.; Sankar, R.; Wang, E.A.; Cepeda, C.; Levine, M.S.; et al. Sex-Specific Life Course Changes in the Neuro-Metabolic Phenotype of Glut3 Null Heterozygous Mice: Ketogenic Diet Ameliorates Electroencephalographic Seizures and Improves Sociability. Endocrinology 2017, 158, 936–949.
- Kasprowska-Liśkiewicz, D.; Liśkiewicz, A.D.; Nowacka-Chmielewska, M.M.; Nowicka, J.; Małecki, A.; Barski, J.J. The ketogenic diet affects the social behavior of young male rats. Physiol. Behav. 2017, 179, 168–177.
- El-Rashidy, O.; El-Baz, F.; El-Gendy, Y.; Khalaf, R.; Reda, D.; Saad, K. Ketogenic diet versus gluten free casein free diet in autistic children: A case-control study. Metab. Brain Dis. 2017, 32, 1935–1941.
- Hardy, T.M.; Tollefsbol, T.O. Epigenetic diet: Impact on the epigenome and cancer. Epigenomics 2011, 3, 503–518.
- Meeran, S.M.; Ahmed, A.; Tollefsbol, T.O. Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clin. Epigenet. 2010, 1, 101–116.
- Rollett, A. Zur Kenntnis der Linolensäure und des Leinöls; De Gruyter: Berlin, Germany, 1909.
- Cheng, Y.S.; Tseng, P.T.; Chen, Y.W.; Stubbs, B.; Yang, W.C.; Chen, T.Y.; Wu, C.K.; Lin, P.Y. Supplementation of omega 3 fatty acids may improve hyperactivity, lethargy, and stereotypy in children with autism spectrum disorders: A meta-analysis of randomized controlled trials. Neuropsychiatr. Dis. Treat. 2017, 13, 2531–2543.
- Hagmeyer, S.; Sauer, A.K.; Grabrucker, A.M. Prospects of Zinc Supplementation in Autism Spectrum Disorders and Shankopathies Such as Phelan McDermid Syndrome. Front. Synaptic Neurosci. 2018, 10, 11.
- Parikh, S.; Saneto, R.; Falk, M.J.; Anselm, I.; Cohen, B.H.; Haas, R.; Medicine Society, T.M. A modern approach to the treatment of mitochondrial disease. Curr. Treat. Options Neurol. 2009, 11, 414–430.
- Bou Khalil, R.; Yazbek, J.C. Potential importance of supplementation with zinc for autism spectrum disorder. L’Encephale 2021, 47, 514–517.
- Gunes, S.; Ekinci, O.; Celik, T. Iron deficiency parameters in autism spectrum disorder: Clinical correlates and associated factors. Ital. J. Pediatr. 2017, 43, 86.
- Prakash, P.; Kumari, R.; Sinha, N.; Kumar, S.; Sinha, P. Evaluation of Iron Status in Children with Autism Spectral Disorder: A Case-control Study. J. Clin. Diagn. Res. 2021, 15, BC01–BC04.
- Mousain-Bosc, M.; Roche, M.; Polge, A.; Pradal-Prat, D.; Rapin, J.; Bali, J.P. Improvement of neurobehavioral disorders in children supplemented with magnesium-vitamin B6. Magnes. Res. 2006, 19, 46–52.
- Galland, L. Magnesium, stress and neuropsychiatric disorders. Magnes. Trace Elem. 1993, 10, 287.
- Błażewicz, A.; Szymańska, I.; Dolliver, W.; Suchocki, P.; Turło, J.; Makarewicz, A.; Skórzyńska-Dziduszko, K. Are Obese Patients with Autism Spectrum Disorder More Likely to Be Selenium Deficient? Research Findings on Pre- and Post-Pubertal Children. Nutrients 2020, 12, 3581.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
28 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




