
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | SILVIA MARÍA DÍAZ PRADO | -- | 2323 | 2023-09-27 10:09:23 | | | |
| 2 | Lindsay Dong | Meta information modification | 2323 | 2023-09-28 07:13:28 | | |
Video Upload Options
Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) is a biodegradable and biocompatible biopolymer that has gained popularity in the field of biomedicine. PHBV has shown to be a versatile platform for drug delivery, offering controlled release, enhanced therapeutic efficacy, and reduced side effects. The encapsulation of various drugs, such as anticancer agents, antibiotics, and anti-inflammatory drugs, in PHBV nanoparticles or microspheres has been extensively investigated, demonstrating enhanced drug stability, prolonged release kinetics, and increased bioavailability. Additionally, PHBV has been used as a scaffold material for tissue engineering applications, such as bone, cartilage, and skin regeneration. The incorporation of PHBV into scaffolds has been shown to improve mechanical properties, biocompatibility, and cellular interactions, making them suitable for tissue engineering constructs.
1. Introduction
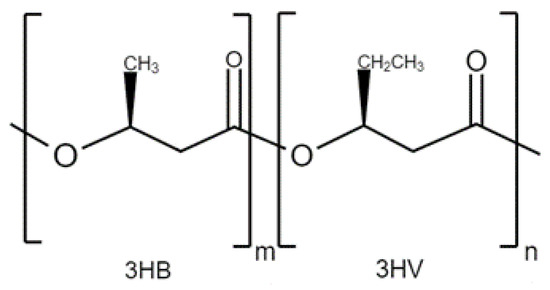
2. Applications
2.1. PHBV Composites for Drug Delivery Applications
2.2. PHBV Composites for Tissue Engineering Applications
3. Conclusions
References
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174.
- Papaneophytou, C.; Katsipis, G.; Halevas, E.; Pantazaki, A.A. Polyhydroxyalkanoates applications in drug carriers. In Biotechnological Applications of Polyhydroxyalkanoates; Springer: Singapore, 2019; pp. 77–124.
- Rivera Briso, A.L.; Serrano Aroca, Á. Métodos de refuerzo mecánico del poli(3-hidroxibutirato-co-3-hidroxivalerato) para aplicaciones industriales avanzadas. Rev. Iberoam. Interdiscip. Métodos Model. Simul. 2018, 10, 79–94.
- Singh, S.; Mohanty, A.K. Wood fiber reinforced bacterial bioplastic composites: Fabrication and performance evaluation. Compos. Sci. Technol. 2007, 67, 1753–1763.
- Silverman, T.; Naffakh, M.; Marco, C.; Ellis, G. Effect of WS2 Inorganic Nanotubes on Isothermal Crystallization Behavior and Kinetics of Poly(3-Hydroxybutyrate-co-3-hydroxyvalerate). Polymers 2018, 10, 166.
- Yu, H.; Qin, Z.; Zhou, Z. Cellulose nanocrystals as green fillers to improve crystallization and hydrophilic property of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Prog. Nat. Sci. Mater. 2011, 21, 478.
- Montanheiro, T.L.; Cristóvan, F.H.; Machado, J.P.; Tada, D.; Durán, N.; Lemes, A.P. Effect of MWCNT functionalization on thermal and electrical properties of PHBV/MWCNT nanocomposites. J. Mater. Res. 2015, 30, 55–65.
- Vidhate, S.; Innocentini-Mei, L.; D’Souza, N.A. Mechanical and electrical multifunctional poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-multiwall carbon nanotube nanocomposites. Polym. Eng. Sci. 2012, 52, 1367–1374.
- Râpă, M.; Stefan, L.M.; Seciu-Grama, A.; Gaspar-Pintiliescu, A.; Matei, E.; Zaharia, C.; Stănescu, P.O.; Predescu, C. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (P(3HB-co-3HV))/Bacterial Cellulose (BC) Biocomposites for Potential Use in Biomedical Applications. Polymers 2022, 14, 5544.
- Gómez-Gaete, C. Nanopartículas poliméricas: Tecnología y aplicaciones farmacéuticas (Polymeric nanoparticles: Technologie and pharmaceutical applications). Rev. Farmacol. Chile 2014, 7, 7–16.
- Göz, E.; Karakeçili, A. Effect of emulsification-diffusion parameters on the formation of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) particles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 226–234.
- Leimann, F.V.; Filho, L.C.; Sayer, C.; Araújo, P.H. Poly(3-hydroxybutyrate-co-3- hydroxyvalerate) nanoparticles prepared by a miniemulsion/solvent evaporation technique: Effect of phbv molar mass and concentration. Braz. J. Chem. Eng. 2013, 30, 369–377.
- Handali, S.; Moghimipour, E.; Rezaei, M.; Ramezani, Z.; Dorkoosh, F.A. PHBV/PLGA nanoparticles for enhanced delivery of 5-fluorouracil as promising treatment of colon cancer. Pharm. Dev. Technol. 2019, 25, 206.
- Handali, S.; Moghimipour, E.; Rezaei, M.; Saremy, S.; Dorkoosh, F.A. Co-delivery of 5-fluorouracil and oxaliplatin in novel poly (3-hydroxybutyrate-co-3-hydroxyvalerate acid)/poly(lactic-co-glycolic acid) nanoparticles for colon cancer therapy. Int. J. Biol. Macromol. 2019, 124, 1299–1311.
- Radu, I.C.; Hudita, A.; Zaharia, C.; Galateanu, B.; Iovu, H.; Tanasa, E.V.; Nitu, S.G.; Ginghina, O.; Negrei, C.; Tsatsakis, A.; et al. Poly(3-hydroxybutyrate-CO-3-hydroxyvalerate) PHBHV biocompatible nanocarriers for 5-FU delivery targeting colorectal cancer. Drug Deliv. 2019, 26, 318.
- Masood, F.; Chen, P.; Yasin, T.; Fatima, N.; Hasan, F.; Hameed, A. Encapsulation of Ellipticine in poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) based nanoparticles and its in vitro application. Mater. Sci. Eng. C 2013, 33, 1054–1060.
- Masood, F.; Chen, P.; Yasin, T.; Hasan, F.; Ahmad, B.; Hameed, A. Synthesis of poly-(3-hydroxybutyrate-co-12 mol % 3-hydroxyvalerate) by Bacillus cereus FB11: Its characterization and application as a drug carrier. J. Mater. Sci. Mater. Med. 2013, 24, 1927–1937.
- Pramual, S.; Lirdprapamongkol, K.; Svasti, J.; Bergkvist, M.; Jouan-Hureaux, V.; Arnoux, P.; Frochot, C.; Barberi-Heyob, M.; Niamsiri, N. Polymer-lipid-PEG hybrid nanoparticles as photosensitizer carrier for photodynamic therapy. J. Photochem. Photobiol. 2017, 173, 12–22.
- Bahari-Javan, N.; Jafary-Omid, N.; Moosavi-Hasab, N.; Rezaie-Shirmard, L.; Rafiee-Tehrani, M.; Dorkoosh, F. Preparation, statistical optimization and in vitro evaluation of pramipexole prolonged delivery system based on poly (3-hydroxybutyrate-co-3-hydroxyvalerate) nanoparticles. J. Drug Deliv. Sci. Technol. 2018, 44, 82–90.
- Eke, G.; Goñi-de-Cerio, F.; Suarez-Merino, B.; Hasirci, N.; Hasirci, V.N. Biocompatibility of Dead Sea Water and retinyl palmitate carrying poly(3-hydroxybutyrate-co-3-hydroxyvalerate) micro/nanoparticles designed for transdermal skin therapy. J. Bioact. Compat. Polym. 2015, 30, 455–471.
- Koller, M. Advances in polyhydroxyalkanoate (PHA) production. Bioengineering 2017, 4, 88.
- Yilgor, P.; Hasirci, N.; Hasirci, V. Sequential BMP-2/BMP-7 delivery from polyester nanocapsules. J. Biomed. Mater. Res. A 2010, 93A, 528–536.
- Diermann, S.H.; Lu, M.; Zhao, Y.; Vandi, L.; Dargusch, M.; Huang, H. Synthesis, microstructure, and mechanical behaviour of a unique porous PHBV scaffold manufactured using selective laser sintering. J. Mech. Behav. Biomed. Mater. 2018, 84, 151–160.
- Diermann, S.H.; Lu, M.; Edwards, G.; Dargusch, M.; Huang, H. In vitro degradation of a unique porous PHBV scaffold manufactured using selective laser sintering. J. Biomed. Mater. Res. 2019, 107, 154–162.
- Gheibi, A.; Khoshnevisan, K.; Ketabchi, N.; Derakhshan, M.A.; Babadi, A. Application of Electrospun Nanofibrous PHBV Scaffold in Neural Graft and Regeneration: A Mini-Review. J. Nanomed. Res. 2016, 1, 107–111.
- Biazar, E.; Heidari-Keshel, S. Development of chitosan-crosslinked nanofibrous PHBV guide for repair of nerve defects. Artif. Cells Nanomed. Biotechnol. 2014, 42, 385–391.
- Zhao, T.; Xu, K.; Wu, Q.; Wang, C.; Xiao, S.; Li, H.; He, T.; Wang, L.; Li, F.; Chen, Q. Duraplasty of PHBV/PLA/Col membranes promotes axonal regeneration by inhibiting NLRP3 complex and M1 macrophage polarization in rats with spinal cord injury. FASEB J. 2020, 34, 12147.
- Meng, W.; Kim, S.Y.; Yuan, J.; Kim, J.C.; Kwon, O.H.; Kawazoe, N.; Chen, G.; Ito, Y.; Kang, I.K. Electrospun PHBV/collagen composite nanofibrous scaffolds for tissue engineering. J. Biomater. Sci. Polym. Ed. 2007, 18, 81–94.
- Xue, K.; Zhang, S.; Ge, J.; Wang, Q.; Qi, L.; Liu, K. Integration of Bioglass Into PHBV-Constructed Tissue-Engineered Cartilages to Improve Chondrogenic Properties of Cartilage Progenitor Cells. Front. Bioeng. Biotechnol. 2022, 10, 868719.
- Dalgic, A.D.; Koman, E.; Karatas, A.; Tezcaner, A.; Keskin, D. Natural origin bilayer pullulan-PHBV scaffold for wound healing applications. Biomater. Adv. 2022, 134, 112554.
- Gong, W.; Cheng, T.; Liu, Q.; Xiao, Q.; Li, J. Surgical repair of abdominal wall defect with biomimetic nano/microfibrous hybrid scaffold. Mater. Sci. Eng. C 2018, 93, 828–837.




