Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Razvan Adrian Covache-Busuioc | -- | 3647 | 2023-09-26 12:59:35 | | | |
| 2 | Jason Zhu | Meta information modification | 3647 | 2023-09-27 03:27:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Eva, L.; Brehar, F.; Florian, I.; Covache-Busuioc, R.; Costin, H.P.; Dumitrascu, D.; Bratu, B.; Glavan, L.; Ciurea, A.V. Neuropsychiatric and Neuropsychological Aspects of Alcohol-Related Cognitive Disorders. Encyclopedia. Available online: https://encyclopedia.pub/entry/49668 (accessed on 07 February 2026).
Eva L, Brehar F, Florian I, Covache-Busuioc R, Costin HP, Dumitrascu D, et al. Neuropsychiatric and Neuropsychological Aspects of Alcohol-Related Cognitive Disorders. Encyclopedia. Available at: https://encyclopedia.pub/entry/49668. Accessed February 07, 2026.
Eva, Lucian, Felix-Mircea Brehar, Ioan-Alexandru Florian, Razvan-Adrian Covache-Busuioc, Horia Petre Costin, David-Ioan Dumitrascu, Bogdan-Gabriel Bratu, Luca-Andrei Glavan, Alexandru Vlad Ciurea. "Neuropsychiatric and Neuropsychological Aspects of Alcohol-Related Cognitive Disorders" Encyclopedia, https://encyclopedia.pub/entry/49668 (accessed February 07, 2026).
Eva, L., Brehar, F., Florian, I., Covache-Busuioc, R., Costin, H.P., Dumitrascu, D., Bratu, B., Glavan, L., & Ciurea, A.V. (2023, September 26). Neuropsychiatric and Neuropsychological Aspects of Alcohol-Related Cognitive Disorders. In Encyclopedia. https://encyclopedia.pub/entry/49668
Eva, Lucian, et al. "Neuropsychiatric and Neuropsychological Aspects of Alcohol-Related Cognitive Disorders." Encyclopedia. Web. 26 September, 2023.
Copy Citation
Alcohol-related cognitive disorders have long been an area of study, yet they continue to pose challenges in the diagnosis, treatment, and understanding of underlying neuropsychiatric mechanisms.
Wernicke’s Encephalopathy
Korsakoff’s Syndrome
alcohol-related cognitive disorders
1. Introduction
1.1. Wernicke’s Encephalopathy (WE) and Korsakoff’s Syndrome (KS): Brief History and Linkage
Carl Wernicke first described WE, now named in his honor. This condition featured symptoms like lethargy, ophthalmoplegia, ataxia and cognitive impairment. At roughly the same time, but independently from Wernicke’s, work Sergei Korsakoff presented his doctoral thesis entitled “Alcoholic Paralysis”, discussing a particular form of memory loss found among chronic alcoholism cases known as circumscribed amnesia.
Over fifty years later, it took an unusually long time for researchers to uncover a shared cause between these two disorders: thiamine deficiency was identified as being at the core of both conditions, contributing to their symptoms in both WE and KS and becoming recognized as their link.
Now researchers understand more fully the temporal relationship between WE and KS, with two distinct phases:
-
WE typically presents with acute/phase lesions to areas such as the periventricular nuclei, thalami and components of Papez’s circuit (including mammillary bodies).
-
Korsakoff’s Syndrome progresses into its chronic phase with lesions developing into more permanent bilateral damage that causes global amnesia.
Noting this correlation, the combined condition is often referred to as Wernicke-Korsakoff Syndrome (WKS). Amnesia played an instrumental role in connecting WKS to neuropsychology; further exploration into memory processes as well as identification of distinct neural substrates responsible for different aspects of memory function ensued from this linkage [1].
KS often develops in those who have experienced WE but failed to receive appropriate thiamine replacement therapy promptly and appropriately. The most recognizable feature of KS is an amnesia which may be profound; combined with additional cognitive and behavioral impairments that often appear with more serious cases, its effects can significantly impact an individual’s daily functioning and quality of life [2]. Research endeavors examining the scope, arrangement and essence of episodic memory impairments within KS have significantly advanced understanding of memory as an abstract concept. Furthermore, these studies have shed light on memory being not an all-inclusive function, and have demonstrated its multidimensional nature. Furthermore, examination of KS has highlighted diencephalic structures’ vital importance to memory processes—leading researchers into further exploring distinct brain structures or neural pathways responsible for individual memory processes to create more intricate mnemonic components [3].
1.2. Connection to Alcohol-Related Cognitive Disorders
KS and WE are two distinct neurological conditions; however, they frequently share similarities due to a deficiency of thiamine (vitamin B1) which often stems from chronic alcohol misuse. Current diagnostic frameworks for cognitive impairment related to alcohol consumption can be divided into two primary syndromes: Wernicke-Korsakoff syndrome (WKS) and alcohol/related dementia (ARD) [4].
WE can be identified clinically by three distinct symptoms, including changes to mental status, dysfunction in oculomotor capabilities and issues with cerebellar function. Note, however, that not all patients exhibit all three symptoms at once. According to Western studies, up to 90% of WE cases can be linked to alcohol misuse [5]. Left undiagnosed or untreated, WE can progress into KS, which occurs in 56–84% of those with an alcoholism history and possibly less frequently among cases unrelated to alcohol consumption. The main cognitive manifestation of KS is intense amnesia, characterized by difficulties forming new memories (anterograde) and recalling old ones (retrograde), although it can also produce other cognitive and behavioral symptoms [6]. Alcohol-related dementia (ARD), as an identifiable clinical entity, has generated much discussion, due to the uncertainty regarding its causes, lack of diagnostic criteria and difficulties assessing affected populations. Due to this lack of clarity regarding ARD classification as a distinct clinical entity, some scholars prefer the term ‘alcohol-related brain damage’ which encompasses neurocognitive impairments caused by chronic alcohol consumption such as Wernicke-Korsakoff Syndrome and ARD [4].
Uncertainty remains as to whether alcohol-related dementia (ARD) results directly from neurotoxic effects of alcohol consumption (neurotoxicity hypothesis), alternative pathologies like thiamine deficiency or from multiple interlinked factors. Oslin et al. have developed preliminary diagnostic criteria for ARD. Their five-year period with men drinking an average of 35 standard drinks weekly (30 for women) over this five-year period should be enough to induce this disorder [7].
Diagnosing alcohol-related dementia (ARD) can be complex, due to other related conditions, including head trauma, substance abuse, co-occurring psychiatric disorders, and elevated cardiovascular risk factors. Although neuropsychological characteristics of ARD have received limited study, observations indicate a more comprehensive cognitive decline compared to WKS [4].
2. Neuropsychiatric and Cognitive Sequelae
As previously highlighted, WE and KS are typically perceived as being interrelated, since both originate from a deficiency in thiamine. Consequently, medical professionals should simultaneously assess both conditions. While WE is conventionally associated with a combination of cognitive disturbances, ophthalmoplegia, and ataxia, it is worth noting that less than a fifth of patients will manifest all these symptoms [8].
WE patients frequently exhibit impairments in neuropsychological functioning that become increasingly evident as their physical condition improves. Neuropsychological functioning encompasses various cognitive abilities ranging from fundamental processes like attention and concentration to more intricate ones like memory, executive functioning, and reasoning; all these higher-order cognitive processes play a key role in overall quality of life for an individual, although unfortunately clinicians sometimes overlook this assessment [9].
When delving deeper into KS, in contrast to Wernicke’s encephalopathy, specific cognitive deficiencies surface. These encompass anterograde amnesia, which severely hampers the ability to assimilate new information, and retrograde amnesia, as well as executive function disorders leading to reduced self-restraint and challenges in areas like judgment, strategizing, and problem resolution [10]. A key manifestation of anterograde amnesia is confabulation, where individuals unconsciously fill memory gaps with fabricated details [11].
Assessing memory function—an essential factor for maintaining quality of life (QOL)—was conducted via PGI-BBD evaluation once the physical condition had improved. Korsakoff memory impairments were evident immediately upon assessment, correlating with initial assessments; however, cases where assessments could take place post improvement proved challenging, due to symptoms overlapping globally with memory dysfunction and memory deficits; pinpointing exactly when cognitive confusion recedes, paving the way for memory deficits, was often difficult and required in-depth discussion amongst participants.
Memory dysfunction was so debilitating in these initial cases that these individuals experienced difficulty maintaining an acceptable quality of life (QOL), accompanied by noticeable occupational impairments. Early administration of thiamine led to improvements in ataxia and ophthalmoplegia; however, confusion and neurological dysfunction persisted for an extended period, according to neuropsychological assessments [12], even when confusion eventually resolved itself in later phases; deficits in memory function, as well as the capacity for learning new material, persisted for an extended timeframe.
In KS, while anterograde amnesia stands out as a major symptom, the patient’s remote memory is also impacted. This leads to retrograde amnesia that affects both general knowledge—such as facts from the news—and personal autobiographical events [13]. For those with KS, their memory loss can span numerous years or even multiple decades, though memories from early life, like childhood, tend to remain intact [14]. This pattern of losing more recent memories while retaining distant ones in KS and other conditions has been termed a “temporal gradient”. Théodule Ribot first documented this observation in 1881, leading to its designation as Ribot’s law. While many studies on KS identify a pronounced temporal gradient, some suggest an even memory impairment across all past timelines [15]. These differing findings may arise from methodological challenges or the type of memory being examined: general vs. personal [16]. Autobiographical episodic memories entail personal experiences set in specific places and times, like recalling the exact moment of meeting a significant other on a balmy evening at a poolside bar. Fewer studies focus on these autobiographical memories, due to their complex nature compared to general memories. A recent investigation by Rensen et al. [16] confirmed that the temporal gradient also influences episodic autobiographical memory, supporting earlier research findings.
WE and Korsakoff’s Syndrome can have severely disabling consequences. Impaired memory and executive function make even basic daily tasks daunting for patients. This impairment often results in challenges in sustaining relationships and jobs. The hindered ability to recall new events or acquire new skills drastically affects their independence and life quality. Emotional health deteriorates as patients confront their cognitive restrictions and the loneliness stemming from social disconnect [2].
In essence, both WE and Korsakoff’s Syndrome arise primarily from a thiamine deficiency, frequently linked to prolonged alcohol consumption. These conditions present with psychiatric symptoms and profound cognitive deficits, and significantly hinder daily activities and overall life satisfaction. Prompt identification, thiamine replenishment, and holistic rehabilitation are vital to counteract these conditions’ effects.
3. Neuropathology of Wernicke’s Encephalopathy and Korsakoff’s Syndrome
Thiamine is prevalent in various parts of the body, notably in skeletal muscles, liver, heart, kidney, and brain. The balance of thiamine in the body is maintained through adequate dietary intake, absorption in the intestines, reuptake in the kidneys, and storage and release in the liver, when necessary. The brain has a safety buffer of thiamine, and noticeable neuropsychiatric symptoms only emerge when thiamine levels drop below 20% of the usual amount [17].
The primary active form of thiamine in the central nervous system is thiamine pyrophosphate (TPP). It acts as a critical component or cofactor for three significant enzymes in glucose metabolism: transketolase, pyruvate dehydrogenase, and α-ketoglutarate dehydrogenase. These enzymes participate in crucial metabolic processes like the pentose phosphate pathway, glycolysis, and the citric acid cycle. These processes produce vital molecules for functions in neurons and glial cells, such as nucleic acids, neurotransmitters, myelin, and energy-rich compounds like ATP [18]. Moreover, because of its involvement in cellular energy processes, thiamine aids in defending cells against oxidative stress. In its triphosphorylated form (TTP), thiamine is essential for nerve membrane functionality (Figure 1).
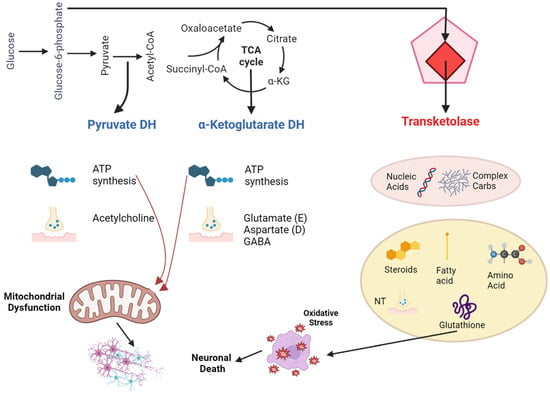
Figure 1. Pathophysiology of thiamine deficiency in Wernicke-Korsakoff syndrome.
Thiamine Deficiency in Relation to Alcohol
Chronic alcohol consumption can lead to thiamine deficiency due to several reasons:
-
Alcoholics often opt for diets high in carbohydrates but low in vitamins [19].
-
Without proper supplementation, thiamine reserves are exhausted within 2–3 weeks.
-
Acute alcohol consumption can hamper thiamine absorption from the gut.
-
Alcohol’s impact on renal epithelial cells results in greater thiamine loss through the kidneys.
-
Chronic alcoholic liver disease can slash the liver’s thiamine storage capability by up to 73%.
-
Alcohol can reduce the enzymatic activity of thiamine pyrophosphokinase (TPK), which in turn lowers the amount of available TPP.
-
Reduced TPK activity further impedes the facilitated diffusion of thiamine into cells.
-
Alcohol can decrease the absorption of thiamine produced by colonic bacteria.
-
Many alcoholics have hypomagnesemia, a deficiency of magnesium, which is a vital cofactor in thiamine utilization.
In essence, alcohol can induce a state of thiamine deficiency through both direct and indirect mechanisms [20].
In situations where Wernicke–Korsakoff Syndrome (WKS) arises without alcohol as a factor, thiamine deficiency can be attributed to one of these four reasons: limited supply, hindered usage, increased consumption, or augmented loss of thiamine. Limited supply often happens during times of starvation, malnourishment, poor absorption, excessive losses, or vomiting. Hindered usage takes place when the body struggles to process thiamine due to diminished enzyme activity or coenzyme deficiencies. Thiamine consumption increases when there is a spike in the body’s glucose metabolism, such as in instances of heightened metabolic rate, enhanced carbohydrate metabolism, or situations with swift cell regeneration. Interestingly, even when on a diet considered sufficient, thiamine deficiency can still occur under these conditions. Specifically, in the context of cancers, particularly the types associated with rapid cellular growth like leukemia and lymphoma, there is a surge in thiamine consumption [21]. Cancer can often lead to a reduced thiamine intake, either due to the disease’s direct effects, loss of appetite, chemotherapy side effects, or vomiting [22]. If patients are started on total parenteral nutrition (TPN) without the necessary thiamine supplementation, they can end up being deficient. Lastly, augmented thiamine loss is observed in hemodialysis, since thiamine is expelled into the dialysate [23].
For an in-depth exploration of the physiological impacts of thiamine deficiency, refer to the work by Sechi and Serra. When the brain experiences a lack of thiamine, it can result in cell-damaging swelling and an increase in astrocyte volume within just 4 days. Between the 7th and 10th day, a drop in transketolase activity brings about dysfunction in endothelial cells, leading to the production of nitrous oxide and the spilling of intracellular glutamate into surrounding spaces. This results in disrupted osmotic balances and the generation of free radicals, further causing swelling and increased permeability in the blood-brain Barrier (BBB). By the time 14 days of thiamine deficiency have passed, the damage to neuronal DNA and increased lactic acid lead to permanent structural harm and neuronal cell death [24]. During the process of alcohol withdrawal, the heightened sensitivity of the NMDA-receptor can compound the neurotoxic effects, releasing even more glutamate. Some brain regions are more susceptible to these damaging effects than others. For instance, in one study, the mammillary bodies displayed signs of damage in every examined case. However, the reasons for the particular vulnerability of areas like the mammillary bodies, the region around the aqueduct, and the tectum are not entirely clear, as factors like the embryonic origin of cells, blood flow patterns, and tissue characteristics do not seem to provide a full explanation. As scientists delve deeper into the molecular mechanisms behind cell damage resulting from thiamine deficiency, the reasons behind the selective harm to specific brain regions, including the mammillary bodies, remain a mystery [25].
4. Somatic Comorbidity and Epidemiology
If WE is suspected, immediate treatment is crucial. On average, symptoms of this condition, such as lethargy, confusion, and difficulty walking that may lead to a fall, manifest around 3 to 4 days prior to a subsequent hospitalization [26]. However, within a hospital setting, WE can be triggered by refeeding syndrome in just 2 to 3 days [27]. This is because most patients with thiamine deficiency may have neglected proper nutrition for months, and in some cases, might not have consumed any food for days or even weeks.
For such patients, it is essential to reintroduce calories slowly and under the guidance of a dietitian. Continuous monitoring is necessary in the initial days of hospitalization, and includes regular checks of blood glucose levels and electrolytes. Refeeding syndrome is typified by imbalances in water and electrolytes, especially low levels of phosphorus, potassium, and magnesium, alongside glucose intolerance, signs of thiamine deficiency, and excessive fluid retention [28]. Administering glucose or reintroducing carbohydrates without supplementing thiamine can pose a risk of triggering Wernicke encephalopathy, particularly in individuals with already low thiamine levels.
Criteria that indicate a high likelihood of developing refeeding syndrome can be found in the recommendations set forth by the American Society for Parenteral and Enteral Nutrition (ASPEN). If no abnormal laboratory results are observed for elements like phosphate, calcium, potassium, magnesium, and glucose, monitoring in relation to refeeding syndrome can cease after 72 h.
Various other risk factors can also play a significant role in leading to thiamine deficiency. These include infections, esophageal narrowing (Barrett’s esophagus), colitis, and, notably, the renal loss of thiamine experienced in conditions like diabetes mellitus or nephropathy [29]. Loss of appetite and vomiting can both result from and exacerbate thiamine deficiency [30].
Examination of Epidemiological Data and Demographics
There is a limited amount of epidemiological information regarding cognitive disorders tied to alcohol. To truly grasp the impact of diseases like Wernicke-Korsakoff syndrome (WKS) and alcohol-related dementia (ARD), it is essential to have updated data on their incidence and mortality rates within populations [31].
The current knowledge about the frequency and occurrence of WE and alcohol-related dementia (ARD) has significant gaps. Most of insights into these conditions are based on research from the 1970s and 1990s, which might now be considered outdated. Autopsy-based data from the past indicated that WE impacted between 0.4% and 2.8% of the overall population, and an alarming 12.5% to 59% of those with alcoholism or deaths related to alcohol consumption [32]. However, modern research is not without its own challenges in methodology and reach. For KS, data from the Netherlands estimated a prevalence of 3–4.8 per 10,000 people, based on healthcare records. Meanwhile, data from Glasgow suggested an annual KS rate of 12.5 to 81.25 per million inhabitants between 1990 and 1995.
The frequency of ARD shows significant variance across studies. Some assessments derive their estimates from associating dementia rates with alcohol use patterns or by examining particular groups. Among various research studies, ARD prevalence varied wildly, from 0.119% in general hospital stays to a staggering 25.6% in elderly patients with alcoholism being treated in substance abuse clinics [33]. Broader population-focused research indicated prevalence rates as low as 0.0066% in those aged 30–64 and as high as 0.7% in US Medicare beneficiaries aged 68 or older.
The future outcomes and prolonged mortality trends of both WKS and ARD remain underexplored. A previous study examining 245 WKS patients reported an immediate mortality rate of 17%, with subsequent deaths primarily attributed to infections, liver diseases, and cancers [34]. Contemporary studies highlight an acute mortality rate of 5.3% to 10% for those diagnosed with WE. Hospital patients diagnosed with alcohol-linked WE or KS exhibited a median survival rate of 8 years and a death rate of 7.4 per 100 person-years. The predominant causes of death were bacterial infections and cancers [33].
5. Pharmacological and Nonpharmacological Treatments
5.1. Effectiveness and Limitations of Nonpharmacological Interventions
Emerging evidence is challenging the long-standing view that Korsakoff’s Syndrome (KS) is an unmodifiable condition resistant to further cognitive recovery. This paradigm shift is bolstered by a growing body of research advocating the efficacy of memory rehabilitation in KS patients [10]. Among various interventions, compensatory memory-enhancement techniques, which encompass traditional aids like agendas and memory cards, as well as digital tools such as smartphones and smartwatches, appear to be particularly promising. A meta-analysis of six studies highlights the fact that these techniques are most effective when (1) the therapeutic objectives are clearly defined, (2) adequate time is committed to individualized patient instruction, and (3) these aids are cohesively integrated into comprehensive learning methodologies.
In this context, errorless learning emerges as a theoretically ideal approach that aligns well with the unique cognitive strengths and vulnerabilities of KS patients. The core principle of this strategy lies in minimizing the opportunity for errors during the acquisition phase of learning. By eliminating the margin for guesswork, errorless learning fortifies the patient’s procedural memory system against the incorporation of maladaptive or erroneous strategies. This is particularly crucial for KS patients whose episodic memory impairments would otherwise be incapable of correcting such errors [35]. Although empirical studies directly comparing errorless learning with traditional trial-and-error methods are sparse and yield mixed results, the technique holds promise, especially within specific learning contexts. Importantly, the utility of errorless learning extends beyond the domain of procedural memory, potentially offering advantages in the realm of semantic memory as well. Given that KS patients often suffer from episodic memory deficits, the errorless learning approach compensates by reinforcing and leveraging more robust procedural and semantic memory systems, thereby providing a more holistic strategy for cognitive rehabilitation.
5.2. Emerging Therapies and Future Directions
5.2.1. Thiamine in the Treatment and Prevention of Wernicke-Korsakoff Syndrome for Alcohol Abusers
While WKS is conventionally treated with thiamine once diagnosed, the efficacy of this treatment, especially regarding cognitive symptoms, remains uncertain. Current guidelines concerning the dosage and treatment duration with thiamine are largely based on best guesses. Researchers aimed to find randomized controlled trials that either contrasted thiamine with placebos or other treatments, or assessed varying thiamine treatments. From search, two studies met the prerequisites for inclusion. However, one did not provide any analyzable data, and the other’s analysis was hampered by design flaws and inadequate data presentation. Consequently, there is an absence of solid evidence from these trials to guide doctors on the optimal thiamine treatment parameters to prevent or address WKS stemming from alcohol misuse [36].
5.2.2. How the Intervention Might Work
This analysis delves into WKS resulting from alcohol abuse, which is the predominant cause in developed nations. Between 30% and 80% of those abusing alcohol exhibit either clinical or biochemical indications of a thiamine deficit. Notably, alcohol seems to amplify the thiamine quantity essential for effective treatment compared to cases where the deficiency stems primarily from nutritional reasons [37]. Even with data pointing towards a significant undetected neuropathological impact due to thiamine shortage, comprehensive studies evaluating thiamine’s therapeutic potency in alcohol abusers remain scant. Rapid improvements in ataxia and eye movement anomalies with thiamine administration have been noted, yet its influence on memory remains ambiguous. One comprehensive look into this area revealed the absence of systematic, placebo-controlled studies exploring the usage of injectable B-complex vitamins (thiamine-inclusive) in alcohol abuse scenarios. Hence, before suggesting varied treatment strategies for those at risk of WE and those currently afflicted by it, more research is imperative [38].
6. Quality of Life and Mental Capacity
Measurements: The QUALIDEM scale was employed to evaluate Quality of Life (QoL). To contrast the QoL of patients with KS against those with dementia, multivariate linear regression analysis was utilized, considering the variables “age”, “gender”, and “nursing home”.
Results: Out of the 147 participants, 72 (48.9%) had a KS diagnosis. KS patients exhibited a higher overall QoL. On the QUALIDEM subscales, KS patients outperformed dementia patients in areas such as “Restless tense behavior”, “Social relations”, and “Having something to do”. There was a noticeable inclination for KS patients to score higher on “Positive affect” and lower on the “Feeling at home” subscale.
Conclusions: KS presents distinct QoL disparities when compared to dementia. Those with KS tend to experience richer social connections and heightened positive emotions than their dementia-afflicted counterparts. While dementia patients exhibit more restless tendencies, KS patients generally feel a lesser sense of belonging in nursing homes. The findings underscore the necessity for tailored nursing homes and care programs to meet the unique requirements of both patient groups.
Individuals diagnosed with KS often exhibit stronger social connections and a higher frequency of positive emotions compared to those with dementia. On the other hand, dementia patients tend to demonstrate increased restless behaviors in contrast to their KS counterparts. The variances between the two patient groups might stem from their distinct cognitive impairments. Specifically, KS is characterized by deep-seated amnesia and often executive dysfunction, necessitating structured and well-organized daily routines.
References
- Sullivan, E.V.; Fama, R. Wernicke’s encephalopathy and Korsakoff’s syndrome revisited. Neuropsychol. Rev. 2012, 22, 69–71.
- Arts, N.J.; Walvoort, S.J.; Kessels, R.P. Korsakoff’s syndrome: A critical review. Neuropsychiatr. Dis. Treat. 2017, 13, 2875–2890.
- Fama, R.; Pitel, A.-L.; Sullivan, E.V. Anterograde Episodic Memory in Korsakoff Syndrome. Neuropsychol. Rev. 2012, 22, 93–104.
- Ridley, N.J.; Draper, B.; Withall, A. Alcohol-related dementia: An update of the evidence. Alzheimer’s Res. Ther. 2013, 5, 3–8.
- Isenberg-Grzeda, E.; Kutner, H.E.; Nicolson, S.E. Wernicke-Korsakoff-Syndrome: Under-Recognized and Under-Treated. Psychosomatics 2012, 53, 507–516.
- Chandrakumar, A.; Bhardwaj, A.; ‘T Jong, G.W. Review of thiamine deficiency disorders: Wernicke encephalopathy and Korsakoff psychosis. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 153–162.
- Oslin, D.; Atkinson, R.M.; Smith, D.M.; Hendrie, H. Alcohol related dementia: Proposed clinical criteria. Int. J. Geriatr. Psychiatry 1998, 13, 203–212.
- Sharp, C.S.; Wilson, M.P.; Nordstrom, K. Psychiatric Emergencies for Clinicians: Emergency Department Management of Wernicke-Korsakoff Syndrome. J. Emerg. Med. 2016, 51, 401–404.
- Swain, S.P.; Behura, S.S. Neuropsychological functioning in Wernicke′s encephalopathy. Ind. Psychiatry J. 2015, 24, 99–103.
- Oudman, E.; Nijboer, T.C.W.; Postma, A.; Wijnia, J.W.; Van der Stigchel, S. Procedural Learning and Memory Rehabilitation in Korsakoff’s Syndrome–a Review of the Literature. Neuropsychol. Rev. 2015, 25, 134–148.
- Johnson, J.M.; Fox, V. Beyond Thiamine: Treatment for Cognitive Impairment in Korsakoff’s Syndrome. Psychosomatics 2018, 59, 311–317.
- Thomson, A.D.; Marshall, E.J. The Treatment of Patients at Risk of Developing Wernicke’s Encephalopathy in the Community. Alcohol Alcohol. 2006, 41, 159–167.
- Kopelman, M. Retrograde amnesia in patients with diencephalic, temporal lobe or frontal lesions. Neuropsychologia 1999, 37, 939–958.
- Moscovitch, M.; Nadel, L.; Winocur, G.; Gilboa, A.; Rosenbaum, R.S. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr. Opin. Neurobiol. 2006, 16, 179–190.
- Race, E.; Verfaellie, M. Remote Memory Function and Dysfunction in Korsakoff’s Syndrome. Neuropsychol. Rev. 2012, 22, 105–116.
- Rensen, Y.C.M.; Kessels, R.P.C.; Migo, E.M.; Wester, A.J.; Eling, P.A.T.M.; Kopelman, M.D. Personal semantic and episodic autobiographical memories in Korsakoff syndrome: A comparison of interview methods. J. Clin. Exp. Neuropsychol. 2017, 39, 534–546.
- Spector, R.; Johanson, C.E. REVIEW: Vitamin transport and homeostasis in mammalian brain: Focus on Vitamins B and E. J. Neurochem. 2007, 103, 425–438.
- Martin, P.R.; Singleton, C.K.; Hiller-Sturmhöfel, S. The Role of Thiamine Deficiency in Alcoholic Brain Disease. Alcohol Res. Health J. Natl. Inst. Alcohol Abus. Alcohol. 2003, 27, 134–142.
- Agabio, R. Thiamine Administration in Alcohol-Dependent Patients. Alcohol Alcohol. 2005, 40, 155–156.
- Thomson, A.D.; Cook, C.C.H.; Touquet, R.; Henry, J.A. The Royal College of Physicians Report on Alcohol: Guidelines for Managing Wernicke’s Encephalopathy in the Accident and Emergency Department. Alcohol Alcohol. 2002, 37, 513–521.
- Yae, S.; Okuno, S.; Onishi, H.; Kawanishi, C. Development of Wernicke encephalopathy in a terminally ill cancer patient consuming an adequate diet: A case report and review of the literature. Palliat. Support. Care 2005, 3, 333–335.
- Kuo, S.-H.; Debnam, J.M.; Fuller, G.N.; de Groot, J. Wernicke’s Encephalopathy: An Underrecognized and Reversible Cause of Confusional State in Cancer Patients. Oncology 2008, 76, 10–18.
- Ueda, K.; Takada, D.; Mii, A.; Tsuzuku, Y.; Saito, S.K.; Kaneko, T.; Utsumi, K.; Iino, Y.; Katayama, Y. Severe thiamine deficiency resulted in Wernicke’s encephalopathy in a chronic dialysis patient. Clin. Exp. Nephrol. 2006, 10, 290–293.
- Sechi, G.; Serra, A. Wernicke’s encephalopathy: New clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007, 6, 442–455.
- Thomson, A.D.; Marshall, E.J. The Natural History and Pathophysiology of Wernicke’s Encephalopathy and Korsakoff’s Psychosis. Alcohol Alcohol. 2006, 41, 151–158.
- Wijnia, J.W.; Oudman, E.; van Gool, W.A.; Wierdsma, A.I.; Bresser, E.L.; Bakker, J.; van de Wiel, A.; Mulder, C.L. Severe Infections are Common in Thiamine Deficiency and May be Related to Cognitive Outcomes: A Cohort Study of 68 Patients with Wernicke-Korsakoff Syndrome. Psychosomatics 2016, 57, 624–633.
- Hershkowitz, E.; Reshef, A.; Munich, O.; Yosefi, B.; Markel, A. Thiamine Deficiency in Self-Induced Refeeding Syndrome, an Undetected and Potentially Lethal Condition. Case Rep. Med. 2014, 2014, 605707.
- Mehanna, H.M.; Moledina, J.; Travis, J. Refeeding syndrome: What it is, and how to prevent and treat it. BMJ 2008, 336, 1495–1498.
- Larkin, J.R.; Zhang, F.; Godfrey, L.; Molostvov, G.; Zehnder, D.; Rabbani, N.; Thornalley, P.J. Glucose-Induced Down Regulation of Thiamine Transporters in the Kidney Proximal Tubular Epithelium Produces Thiamine Insufficiency in Diabetes. PLoS ONE 2012, 7, e53175.
- Oudman, E.; Wijnia, J.W.; Oey, M.; van Dam, M.; Painter, R.C.; Postma, A. Wernicke’s encephalopathy in hyperemesis gravidarum: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 236, 84–93.
- Palm, A.; Vataja, R.; Talaslahti, T.; Ginters, M.; Kautiainen, H.; Elonheimo, H.; Suvisaari, J.; Lindberg, N.; Koponen, H. Incidence and mortality of alcohol-related dementia and Wernicke-Korsakoff syndrome: A nationwide register study. Int. J. Geriatr. Psychiatry 2022, 37, 1–9.
- Naidoo, D.P.; Bramdev, A.; Cooper, K. Wernicke’s encephalopathy and alcohol-related disease. Postgrad. Med. J. 1991, 67, 978–981.
- Cheng, C.; Huang, C.-L.; Tsai, C.-J.; Chou, P.-H.; Lin, C.-C.; Chang, C.-K. Alcohol-Related Dementia: A Systemic Review of Epidemiological Studies. Psychosomatics 2017, 58, 331–342.
- Goodman, R.A.; Lochner, K.A.; Thambisetty, M.; Wingo, T.S.; Posner, S.F.; Ling, S.M. Prevalence of dementia subtypes in United States Medicare fee-for-service beneficiaries, 2011–2013. Alzheimer’s Dement. 2017, 13, 28–37.
- Clare, L.; Jones, R.S.P. Errorless Learning in the Rehabilitation of Memory Impairment: A Critical Review. Neuropsychol. Rev. 2008, 18, 1–23.
- Day, E.; Bentham, P.W.; Callaghan, R.; Kuruvilla, T.; George, S. Thiamine for prevention and treatment of Wernicke-Korsakoff Syndrome in people who abuse alcohol. Cochrane Database Syst. Rev. 2013, 2013, CD004033.
- Thomson, A.D.; Cook, C.C.H.; Guerrini, I.; Sheedy, D.; Harper, C.; Marshall, E.J. Wernicke’s encephalopathy: ‘Plus ca change, plus c’est la meme chose’. Alcohol Alcohol. 2008, 43, 180–186.
- Cook, C.C.H. Prevention and treatment of Wernicke-Korsakoff syndrome. Alcohol Alcohol. 2000, 35 (Suppl. S1), 19–20.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
454
Revisions:
2 times
(View History)
Update Date:
27 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




