Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paweł Kowalczyk | -- | 2930 | 2023-09-26 12:23:37 | | | |
| 2 | Sirius Huang | Meta information modification | 2930 | 2023-09-27 02:30:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wróbel, M.; Śliwakowski, W.; Kowalczyk, P.; Kramkowski, K.; Dobrzyński, J. Bioremediation of Heavy Metals by the Genus Bacillus. Encyclopedia. Available online: https://encyclopedia.pub/entry/49661 (accessed on 07 February 2026).
Wróbel M, Śliwakowski W, Kowalczyk P, Kramkowski K, Dobrzyński J. Bioremediation of Heavy Metals by the Genus Bacillus. Encyclopedia. Available at: https://encyclopedia.pub/entry/49661. Accessed February 07, 2026.
Wróbel, Monika, Wojciech Śliwakowski, Paweł Kowalczyk, Karol Kramkowski, Jakub Dobrzyński. "Bioremediation of Heavy Metals by the Genus Bacillus" Encyclopedia, https://encyclopedia.pub/entry/49661 (accessed February 07, 2026).
Wróbel, M., Śliwakowski, W., Kowalczyk, P., Kramkowski, K., & Dobrzyński, J. (2023, September 26). Bioremediation of Heavy Metals by the Genus Bacillus. In Encyclopedia. https://encyclopedia.pub/entry/49661
Wróbel, Monika, et al. "Bioremediation of Heavy Metals by the Genus Bacillus." Encyclopedia. Web. 26 September, 2023.
Copy Citation
Bioremediation agents include bacteria of the genus Bacillus, among others. The best-described species in terms of the bioremediation potential of Bacillus spp. Are B. subtilis, B. cereus, or B. thuringiensis. This bacterial genus has several bioremediation strategies, including biosorption, extracellular polymeric substance (EPS)-mediated biosorption, bioaccumulation, or bioprecipitation. Due to the above-mentioned strategies, Bacillus spp. strains can reduce the amounts of metals such as lead, cadmium, mercury, chromium, arsenic or nickel in the environment.
biological removal of heavy metals
spore-forming bacteria
sustainable environmental management
1. Introduction
Microorganisms can use several strategies to remove heavy metals present in the environment (Figure 1) [1][2][3][4]. Biosorption, bioaccumulation, and bioprecipitation are the most common heavy metal removal strategies of the genus Bacillus [1][5].
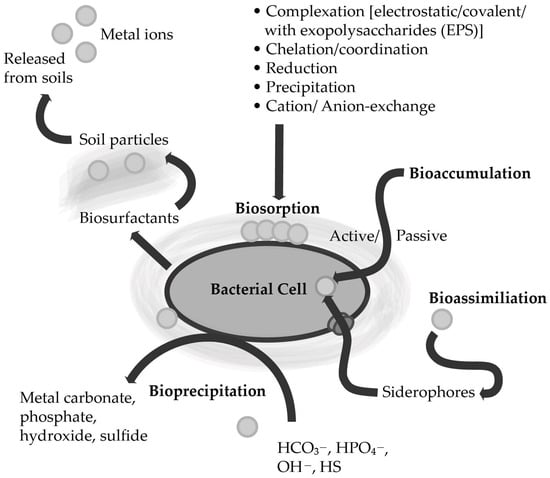
Figure 1. Various types of bacterial interactions with heavy metals accumulated in soils (according to Ahemad [6]; modified).
2. Biosorption
Biosorption is a physicochemical, metabolism-independent heavy metal uptake process based on cell membranes. It functions through compounds with a negative charge that are present in the cell membranes. Importantly, the biomass used for biosorption is usually non-living biomass, as this way, the process proceeds more efficiently than with living microorganisms. The efficiency of this strategy mainly depends on several parameters, including surface properties, e.g., functional groups present on the cell membrane, pH, temperature, or electrostatic interactions [7][8][9]. Understanding the biosorption mechanisms that enable the removal of heavy metals is crucial to optimizing the process. To date, several mechanisms occurring during the sorption process have been discovered, and different mechanisms can proceed at the same time at different rates. Among the biosorption mechanisms, the following mechanisms can be identified: (i) ion change, a reversible chemical reaction involving the exchange of ions for other ions of the same charge; (ii) complexation, heavy metal ions bind to functional groups present in cell membranes; (iii) physical adsorption caused by intermolecular interactions, including Van der Waals forces (Figure 1) [8][10].
To date, several papers on biosorption with Bacillus spp. have been published [11][12][13][14]. Some strains of the Bacillus spp. may also have the ability to bio-sorb a few different heavy metals. B. thuringiensis OSM29, isolated from the rhizosphere of cauliflower grown in soil irrigated with industrial effluent, was capable of remediating Cd, Cu, Cr, Ni, and Pb [15]. The biosorption capacity of B. thuringiensis OSM29 was highest for Ni (94%), while the lowest biosorption by the bacterial biomass was noted for Cd (87,0%). The researchers also observed that the biosorption efficiency was dependent on a few physicochemical parameters, such as pH, initial metal concentration, and contact time. For example, the optimum pH values for copper and lead biosorption efficiency was 6.0, while for Ni and Cr, it was 7.0. Additionally, using FTIR, the authors identified the following chemical functional groups in the studied strain: amino, carboxyl, hydroxyl, and carbonyl groups, involved in the sorption of heavy metals [15]. Nevertheless, most studies concern the biosorption of single heavy metals. Strains of the genus Bacillus are capable of Hg biosorption. For instance, Sinha et al. [16] analyzed a biosorption potential of immobilized B. cereus cells for bioremediation of mercury from synthetic effluent. Importantly, the experiment was conducted under various conditions. The maximum adsorption capacity of B. cereus (immobilized cells) was 104.1 mg g−1 (Hg2+), and was noted for a pH of 7.0 at 30 °C, for a pH of 7.0 after 72 h from contact, and biomass concentration of 0.02 g L−1. Moreover, the average free energy value calculated using the Dubinin–Radushkevich (D–R) model was 15.8 kJ mol−1, indicating that this process was chemically more favorable than the physical adsorption process [16]. Chen et al. [17] conducted a study on Pb(II) biosorption, using the strain B. thuringiensis 016 through batch and microscopic experiments. The authors noted that the highest biosorption potential of Pb for B. thuringiensis 016 was approximately 165 mg g−1 (dry weight). Interestingly, this study showed that pH, amide, carboxyl, and phosphate functional groups of the studied strain (studied by fourier transform infrared (FTIR) analyses and selective passivation experiments) greatly affected the Pb biosorption. Furthermore, the observation by scanning electron microscopy proved that Pb precipitates had accumulated on the surfaces of the bacteria cells [17].
Moreover, Bacillus spp. strains are also capable of biosorption of less toxic metals than those above. For instance, B. cereus AUMC B52 was capable of Zn biosorbing. The maximum adsorption capacity of B. cereus AUMC B52 calculated from the Langmuir adsorption isotherm was 66.6 mg g−1. In addition, the presence of amine, hydroxyl, carboxyl, and carbonyl groups, which are probably responsible for Zn(II) biosorption, was detected in the bacterial biomass using FTIR [18]. There are also examples of As biosorption by B. cereus, which is more often found in the environment in the form of anions. Giri et al. [19] detected an adsorption capacity of approximately 32 mg g−1 for arsenite at pH 7.5, at a biomass dose of 6 g L−1. The ability to bioabsorb arsenic was also noted in B. thuringiensis WS3. The maximum As(III) adsorption capacity was approximately 11 mg g−1, in the optimum As(III) removal conditions: 6 ppm As(III) concentration, pH 7, temperature 37 °C, and biomass dose of 0.50 mg ml−1 [20].
3. Bioremediation by Extracellular Polymeric Substances (EPS)
Another important mechanism for bioremediation of heavy metals that many metal-tolerant bacteria possess is the uptake of metals through the secretion of extracellular polymeric substances (EPS) (Figure 1) [9][21]. The EPS include compounds such as nucleic acids, humic acids, proteins, and polysaccharides, that bind cationic metals with varying degrees of specificity and affinity [9][22]. Their importance in the bioremediation process is based on their participation in the flocculation and the binding of metal ions from solutions [23]. Microorganisms that secrete exopolysaccharides are the most significant in the bioremediation of heavy metals [21]. Factors modulating the removal of metals by EPS include initial metal concentrations and pH [9].
To date, the ability to secrete EPS has been detected in several strains belonging to the genus Bacillus. For instance, multi-metal resistance (Pb, Cd, Cu, and Zn) strain, B. cereus KMS3-1, was able to produce EPS (optimum conditions: pH 7.0, 120 h incubation time, sucrose concentration 5 g L−1, and 10 g L−1 yeast extract) [24]. Furthermore, optimization of EPS production, using a central composite design, revealed that the optimal sucrose and yeast extract concentrations for enhanced EPS production (8.9 g L−1), were 5 g L−1 and 30 g L−1, respectively. In addition, using FTIR, thin layer chromatography (TLC), and high-performance liquid chromatography (HPLC) techniques, the researchers determined studied EPS as heteropolysaccharide, which consisted of glucose, mannose, xylose, and rhamnose [24]. However, there are also studies focusing on bioremediation using EPS against a single heavy metal contamination. Kalpan et al. [25] isolated an exopolysaccharide-producing bacteria, B. cereus VK1. Subsequently, EPS was purified, estimated, and further characterized by FTIR, gas chromatography, mass spectrometry (GC-MS), and thermo gravimetric analysis (TGA). Interestingly, using statistical modeling (response surface methodology, RSM), the researchers carried out media optimization to increase EPS production. The study results showed that B. cereus VK1 cultured in LB was capable of adsorbing up to 80.22 μg Hg2+ in 20 min, while the strain grown in RSM-optimized medium adsorbed up to 295.53 μg Hg2+ [25]. Moreover, the ability to produce EPS has also been detected in a species related to Bacillus spp., Paenibacillus jamilae. EPS showed a notable affinity for Pb in comparison to the other five metals. The bioremediation of lead (303.03 mg g−1) was as much as ten times higher than the removal of the other metals. Alongside that, studied EPS consisted of glucose (the most abundant sugar), rhamnose, galactose, fucose, and mannose [26].
4. Bioaccumulation
In contrast to the biosorption described above, bioaccumulation is a cellular energy-dependent process conducted by active metabolic microorganisms (Figure 1) [27]. Therefore, compared to biosorption, heavy metal uptake takes longer because it depends on the biochemical features, microbial internal structure, genetic and physiological ability, and environmental conditions affected by bioaccumulation activity [3][28]. Moreover, the bioaccumulation process was also found to be influenced by cell surface properties, including changes in charge. In addition, temperature also affects the bioaccumulation process: a higher temperature may significantly disrupt the metabolic activity of a bacterial cell [3][29]. The best-known mechanism of bioaccumulation is likely based on heavy metal binding using metallothioneins. Metallothioneins are cysteine-rich proteins (low molecular weight molecules, can be encoded by the bmtA gene that facilitates the bioaccumulation of heavy metals (e.g., Pb, Hg, Ni, Cd)) inside the cell [30]. Bacterial cells usually produce metallothioneins in reaction to enhanced exposure to metals [31][32]. This mechanism may be transferred by plasmids, facilitating its dispersion from one bacterial cell to another [33]. However, there are other bioaccumulation mechanisms that are often not universal for the bioremediation of all heavy metals. For instance, bioremediation of As in bacteria of the genus Bacillus is mediated by the ars operon through the use of the following genes: arsA, arsB, arsC, arsD, and arsR, where, e.g., the arsA and arsB genes have ATPase activity and arsC encodes an arsenate reductase that converts As(III) to As(V) (less toxic form) [34]. In contrast, Pb bioaccumulation in Bacillus spp. Is based on the pbrD gene [35], while the mechanisms leading to Cu accumulation by bacterial cells are encoded in the cusF gene, which contributes to the binding of copper in the periplasmic space [36]. In the case of mercury, bioaccumulation is related to the merC gene expression [37].
5. Bioprecipitation
Bioprecipitation is another bioremediation strategy that has been found in bacteria. This strategy involves converting the concentration of free metals to insoluble complexes, thereby reducing their bioavailability and toxicity. Microorganisms can facilitate precipitation via catalyzing oxidative and reductive processes, leading to the precipitation of contaminants including Pb, Cd, Cr, Fe, and U. In some microorganisms, it has also been discovered that they can release phosphates and increase the precipitation of metal phosphates, while other bacteria are capable of precipitating hydroxides or carbonates by forming alkanes (Figure 1) [38]. There are not a lot of studies about precipitation carried out by the Bacillus spp. Nevertheless, bacteria of the genus Bacillus can bio-precipitate the most toxic heavy metals, including lead and cadmium. For instance, the lead-resistant strains—B. iodinium GP13 and B. pumilus S3—were found to facilitate the precipitation of lead in the form of lead sulphide (PbS) [39]. Moreover, bacteria capable of precipitating lead into lead phosphate (Pb3(PO4)2) also include B. thuringiensis 016 [17]. Another example of bioprecipitation by the Bacillus spp. is the study by Li et al. [40]: using analyses of energy dispersive spectroscopy, X-ray photoelectron spectroscopy, and select area electron diffraction, the authors showed that B. cereus Cd01 was capable of Cd bioprecipitation into polycrystalline and/or amorphous cadmium phosphate and cadmium sulfide. Furthermore, Molokwane et al. [41] observed Cr(VI) reduction by precipitation after the application was enriched by a mixed culture of bacteria consisting of bacteria of the genus Bacillus, including B. cereus, B. thuringiensis, and related genera, such as Paenibacillus and Oceanobacillus. The highest reduction of Cr(VI) in aerobic cultures was obtained at a high concentration of 200 mg L−1, after incubation for 65 h [41].
To summarize these subsections, it is worth adding that biologically enhanced precipitation may be used to remove metals and metalloids from a range of wastewaters, for example, acid mine drainage, electroplating, and tannery effluents [38].
6. Biological Removal of Heavy Metals Using Plant Growth-Promoting Bacteria
There are still not a lot of studies on the bioremediation by Bacillus spp. in terms of application perspective; most studies focus on bioremediation mechanisms and study bioremediation efficiency in aqueous solutions with heavy metals [17][41][42]. Furthermore, only a few studies present results on the bioremediation activity of Bacillus spp. without the involvement of plants. For instance, for the bioremediation of cadmium, a combination of the bacteria B. megaterium with earthworms (Eisenia fetida) was used. According to the experiment with Cd-contaminated soil (Cd at approximately 2.5 mg kg−1), this combination was more effective than bioremediation using only earthworms [43]. On the other hand, the vast majority of studies on the application of Bacillus spp. as bioremediation agents are also related to phytoremediation [44], indicating that the bioremediation action of Bacillus spp. is not limited to playing a role in the geochemical cycle of heavy metals in soil [45][46]. Moreover, heavy-metal-accumulating plants supported by bacteria of this genus may be used to produce biogas, and the digestate meeting the criteria for heavy metal content can be used as fertilizer. Thus, this type of approach appears to be the most appropriate in the context of bioremediation involving this microbial group [47][48].
Metal-accumulating plants can be enhanced by metal-resistant plant growth-promoting bacteria (PGPB), which can increase the efficiency of bioremediation [6][49][50]. Therefore, the use of PGPB has recently been expanded to include the potential remediation of contaminated soils with crops, energy plants, and hyperaccumulators (plants capable of accumulating extremely large amounts of heavy metals in their aboveground parts, without suffering from phytotoxic effects) [48][51]. Therefore, the application of PGPB has recently been expanded to include remediation of contaminated soils in combination with plant hyperaccumulators, that is, plants capable of accumulating extremely large amounts of heavy metals in their aboveground parts, without suffering from phytotoxic effects. Plant stimulation by PGPB has been observed by many authors [49][52][53][54][55][56]. PGPB may enhance plant growth either directly or indirectly. Mechanisms of direct action include production of various biological substances, for instance, indole-3-acetic acid (IAA), gibberellins, cytokinins, and 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase, and atmospheric nitrogen fixation (nitrogenase production), or phosphorus solubilization [6][57][58][59][60][61]. On the other hand, indirect mechanisms include the production of antibiotics (for example, cyclic lipopeptides), enzymes such as chitinases, cellulases, and glucanases, and siderophore production [54][62][63][64][65].
The abilities that promote PGPB in phytoremediation processes include alleviating harmful effects caused by heavy metal pollution (e.g., reduced chlorophyll level and oxidative stress), boosting heavy metal tolerance of plants, and enhancing the accumulation of heavy metals in plant tissues [52][66][67][68][69][70]. Thus, bacteria can facilitate heavy metal remediation through several mechanisms. For instance, phytohormones, such as IAA, causing root elongation and surface area (enhancing nutrients uptake), lead to an increase in the plants biomass, which results in a larger phytoremediation surface area of plants [47][71][72]. In addition, beneficial microorganisms may help reduce ethylene stress in plants growing in metal-contaminated soil through the deaminase ACC activity, which breaks down the ethylene precursor, ACC. It results in the development of longer roots, thus enabling the phytoremediation process to proceed more efficiently [6][41][73][74]. Plant stress due to the presence of heavy metals can also be alleviated by the secretion of antioxidant enzymes by PGPB [44]. Additionally, PGPB releases siderophores, iron-chelating compounds that enhance iron uptake by plant roots in hostile, metal-contaminated environments [53][75][76][77]. Siderophores can also mobilize heavy metals, increasing metal accumulation by resistant bacteria (Figure 1) [6][78][79][80]. In turn, plant endophytes (e.g., root endosphere endophytes) can also enhance the phytoremediation through bioaccumulation mechanisms [71].
So far, several studies have been reported describing the support of plant phytoremediation by plant growth-promoting bacteria of Bacillus spp. [52][81][82][83]. Most of the research on this topic concerns experiments conducted under controlled conditions. For instance, a study conducted under gnotobiotic conditions showed the possibility of phytoextraction of cadmium- and lead-contaminated soils with bacteria of the genus Bacillus [84]. A heavy-metal-resistant, tomato growth-promoting strain Bacillus sp. RJ16 (Table 1) (which synthesized IAA, siderophores, and ACC deaminase to stimulate tomato root growth) led to an increase in Cd and Pb content in aboveground tissues from 92% to 113% and from 73% to 79%, respectively, in inoculated plants growing in heavy-metal-contaminated soil, compared to the control without inoculation [84]. Similarly, B. subtilis and B. pumilus were also able to facilitate the accumulation of various heavy metals, such as Cu, Cr, Pb, and Zn in tissues of Zea mays and Sorghum bicolor (greenhouse illuminated with natural light; total concentrations of heavy metals in soil: Cu 22,800, Cr 16,865, Pb 1900, Zn 32,500 mg kg−1 dry soil) [85]. Different patterns were noted by Saran et al. [86], who showed that after 2 months, sunflower (Helianthus annuus) seedlings grown on contaminated soil (Cd 0.42, Cu 1.02, Pb 5.48, Zn 12 mg kg−1) and inoculated with a plant growth-promoting strain B. proteolyticus ST89 (Table 1) achieved a 40% higher biomass production than uninoculated control plants, and accumulated 20% less Pb and 40% less Cd in aboveground plant parts, which indicates a reduction in phytotoxicity (controlled conditions, greenhouse) [86].
Table 1. Phytoremediation supporting bacteria of the genus Bacillus, and their effects.
| Strains | Plant | Bioremediated Metal | PGP Traits | PGP Effects | References |
|---|---|---|---|---|---|
| Bacillus sp. RJ16 | Solanum lycopersicum | Cd and Pb | IAA, siderophores and ACC deaminase | Stimulatation of tomato root growth | He et al. [84] |
| Bacillus cereus SRA10 | Brassica juncea | Ni | IAA, siderophores | Overall plant growth promotion | Ma et al. [87] |
| Bacillus sp. Ba32 | Brassica juncea | Cr | Siderophores | Increase in root and shoot length | Rajkumar et al. [88] |
| Bacillus proteolyticus ST89 | Helianthus annuus | Cd and Pb | IAA | Increase in biomass production | Saran et al. [86] |
| Bacillusparamycoides ST9 | Helianthus annuus | Cd and Pb | ⎼ | Increase in shoot biomass production | Saran, et al. [86] |
| Bacillus sp. J119 | Brassica napus Huiyou-50, Zea mays Denhai-11, Sorghum bicolor × Sorghum sudanense, Lycopersicon esculentum Shanghai-906 |
Cd, Pb, Zn and Cu | IAA | Increase in stem length | Sheng et al. [89] |
| Bacillus subtilis SJ-101 | Brassica juncea | Ni | IAA | Increase in growth of above-ground tissue and root | Zaidi et al. [81] |
| Bacillus megaterium BM18-2 | Pennisetum americanum × Pennisetum purpureum Schumach | Cd | IAA | Increase in shoot and root length | Wu et al. [71] |
Regarding the above, the majority of bioremediation experiments involving Bacillus spp. have been carried out under simple or controlled conditions, including growth chamber and greenhouse studies. However, studies on the effectiveness of Bacillus spp. in bioremediation have also been conducted under outdoor conditions.
Importantly, by conducting research on the PGPB application for bioremediation of heavy metals under controlled conditions, researchers are reducing the number of factors affecting their effectiveness. As is well known, the effectiveness of PGPB is influenced by soil properties (including chemical and microbiological properties), which are modulated by a range of factors including meteorological conditions [65]. Thereby, there is still a great need for research conducted under field conditions, which will provide a broader view of the interactions between bacteria, plants, and soil, thus leading to an essential step in the transition from laboratory experiments to practical applications. Unfortunately, field trials using PGPB in phytoremediation are rarely reported [48][71].
References
- Pham, V.H.T.; Kim, J.; Chang, S.; Chung, W. Bacterial biosorbents, an efficient heavy metals green clean-up strategy: Prospects, challenges, and opportunities. Microorganisms 2022, 10, 610.
- Alotaibi, B.S.; Khan, M.; Shamim, S. Unraveling the underlying heavy metal detoxification mechanisms of Bacillus species. Microorganisms 2021, 9, 1628.
- Sharma, B.; Shukla, P. Lead bioaccumulation mediated by Bacillus cereus BPS-9 from an industrial waste contaminated site encoding heavy metal resistant genes and their transporters. J. Hazard. Mater. 2021, 401, 123285.
- Pande, V.; Pandey, S.C.; Sati, D.; Bhatt, P.; Samant, M. Microbial interventions in bioremediation of heavy metal contaminants in agroecosystem. Front. Microbiol. 2022, 13, 824084.
- Shao, W.; Li, M.; Teng, Z.; Qiu, B.; Huo, Y.; Zhang, K. Effects of Pb (II) and Cr (VI) stress on phosphate-solubilizing bacteria (Bacillus sp. strain MRP-3): Oxidative stress and bioaccumulation potential. Int. J. Environ. Res. Public Health. 2019, 16, 2172.
- Ahemad, M. Remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: Paradigms and prospects. Arab. J. Chem. 2019, 12, 1365–1377.
- Shamim, S. Biosorption of Heavy Metals; IntechOpen: London, UK, 2018.
- Zabochnicka-Świątek, M.; Krzywonos, M. Potentials of biosorption and bioaccumulation processes for heavy metal removal. Pol. J. Environ. Stud. 2014, 23, 551–561.
- Tiquia-Arashiro, S.M. Lead absorption mechanisms in bacteria as strategies for lead bioremediation. Appl. Microbiol. Biotechnol. 2018, 102, 5437–5444.
- Babák, L.; Šupinova, P.; Zichova, M.; Burdychova, R.; Vitova, E. Biosorption of Cu, Zn and Pb by thermophilic bacteria–effect of biomass concentration on biosorption capacity. Acta Univ. Agric. Silvic. Mendel. Brun. 2012, 60, 9–18.
- Babar, Z.; Khan, M.; Chotana, G.A.; Murtaza, G.; Shamim, S. Evaluation of the potential role of Bacillus altitudinis MT422188 in nickel bioremediation from contaminated industrial effluents. Sustainability 2021, 13, 7353.
- Das, P.; Sinha, S.; Mukherjee, S.K. Nickel bioremediation potential of Bacillus thuringiensis KUNi1 and some environmental factors in nickel removal. Bioremediat. J. 2014, 18, 169–177.
- Khan, M.; Ijaz, M.; Chotana, G.A.; Murtaza, G.; Malik, A.; Shamim, S. Bacillus altitudinis MT422188: A potential agent for zinc bioremediation. Bioremediat. J. 2022, 26, 228–248.
- Chen, X.; Lin, H.; Dong, Y.; Li, B.; Liu, C.; Yin, T. Mechanisms underlying enhanced bioremediation of sulfamethoxazole and zinc (II) by Bacillus sp. SDB4 immobilized on biochar. J. Clean. Prod. 2020, 370, 133483.
- Oves, M.; Khanm, M.S.; Zaidim, A.; Ahmadm, E. Soil contamination, nutritive value and human health risk assessment of heavy metals: An overview. In Toxicity of Heavy Metals to Legumes and Bioremediation, 1st ed.; Zaidi, A., Wani, P., Khan, M., Eds.; Springer: Vienna, Austria, 2012; pp. 1–27. ISBN 978-3-7091-0729-4.
- Sinha, A.; Pant, K.K.; Khare, S.K. Studies on mercury bioremediation by alginate immobilized mercury tolerant Bacillus cereus cells. Int. Biodeterior. Biodegrad. 2012, 2071, 1–8.
- Chen, Z.; Pan, X.; Chen, H.; Lin, Z.; Guan, X. Investigation of lead (II) uptake by Bacillus thuringiensis 016. World J. Microbiol. Biotechnol. 2015, 31, 1729–1736.
- Joo, J.H.; Hassan, S.H.; Oh, S.E. Comparative study of biosorption of Zn2+ by Pseudomonas aeruginosa and Bacillus cereus. Int. Biodeter. Biodegrad. 2010, 64, 734–741.
- Giri, A.; Patel, R.K.; Mahapatra, S.S.; Mishra, P.C. Biosorption of arsenic (III) from aqueous solution by living cells of Bacillus cereus. Environ. Sci. Pollut. Res. 2013, 20, 1281–1291.
- Altowayti, W.A.H.; Algaifi, H.A.; Bakar, S.A.; Shahir, S. The adsorptive removal of As (III) using biomass of arsenic resistant Bacillus thuringiensis strain WS3: Characteristics and modelling studies. Ecotoxicol. Environ. Saf. 2019, 172, 176–185.
- Kumawat, T.K.; Kumawat, V.; Sharma, S.; Kandwani, N.; Biyani, M. Applications of EPS in environmental bioremediations. In Microbial Exopolysaccharides as Novel and Significant Biomaterials, 1st ed.; Nadda, A.K., Sajna, K.V., Sharma, S., Eds.; Springer: Cham, Switzerland, 2021; pp. 285–302. ISBN 978-3-030-75288-0.
- Pal, A.; Paul, A.K. Microbial extracellular polymeric substances: Central elements in heavy metal bioremediation. Indian J. Microbiol. 2008, 48, 49–64.
- Salehizadeh, H.; Shojaosadati, S.A. Removal of metal ions from aqueous solution by polysaccharide produced from Bacillus firmus. Water Res. 2003, 37, 4231–4235.
- Krishnamurthy, M.; Uthaya, C.J.; Thangavel, M.; Annadurai, V.; Rajendran, R.; Gurusamy, A. Optimization, compositional analysis, and characterization of exopolysaccharides produced by multi-metal resistant Bacillus cereus KMS3-1. Carbohydr. Polym. 2020, 227, 115369.
- Kalpana, R.; Angelaalincy, M.J.; Kamatchirajan, B.V.; Vasantha, V.S.; Ashokkumar, B.; Ganesh, V.; Varalakshmi, P. Exopolysaccharide from Bacillus cereus VK1: Enhancement, characterization and its potential application in heavy metal removal. Colloids Surf. B Biointerfaces 2018, 171, 327–334.
- Morillo Pérez, J.A.; García-Ribera, R.; Quesada, T.; Aguilera, M.; Ramos-Cormenzana, A.; Monteoliva-Sánchez, M. Biosorption of heavy metals by the exopolysaccharide produced by Paenibacillus jamilae. World J. Microbiol. Biotechnol. 2008, 24, 2699–2704.
- Issazadeh, K.; Jahanpour, N.; Pourghorbanali, F.; Raeisi, G.; Faekhondeh, J. Heavy metals resistance by bacterial strains. Ann. Biol. Res. 2013, 4, 60–63.
- Vijayaraghavan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291.
- Srinath, T.; Verma, T.; Ramteke, P.W.; Garg, S.K. Chromium (VI) biosorption and bioaccumulation by chromate resistant bacteria. Chemosphere 2002, 48, 427–435.
- Hamer, D.H. Metallothioneins. Annu. Rev. Biochem. 1986, 55, 913–951.
- Blindauer, C.A.; Harrison, M.D.; Robinson, A.K.; Parkinson, J.A.; Bowness, P.W.; Sadler, P.J.; Robinson, N.J. Multiple bacteria encode metallothione in sand SmtA-like fingers. Mol. Microbiol. 2002, 45, 1421–1432.
- Liu, T.; Nakashima, S.; Hirose, K.; Uemura, Y.; Shibasaka, M.; Katsuhara, M.; Kasamo, K. A metallothionein and CPx-ATPase handle heavy-metal tolerance in the filamentous cyanobacteria Oscillatoria brevis. FEBS Lett. 2003, 542, 159–163.
- Das, S.; Dash, H.R.; Chakraborty, J. Genetic basis and importance of metal resistant genes in bacteria for bioremediation of contaminated environments with toxic metal pollutants. Appl. Microbiol. Biotechnol. 2016, 100, 2967–2984.
- Yang, H.C.; Fu, H.L.; Lin, Y.F.; Rosen, B.P. Pathways of arsenic uptake and efflux. Curr. Top. Membr. 2012, 69, 325–358.
- Borremans, B.; Hobman, J.L.; Provoost, A.; Brown, N.L.; van Der Lelie, D. Cloning and functional analysis of the pbr lead resistance determinant of Ralstonia metallidurans CH34. J. Bacteriol. 2001, 183, 5651–5658.
- Yu, P.; Yuan, J.; Deng, X.; Ma, M.; Zhang, H. Subcellular targeting of bacterial CusF enhances Cu accumulation and alters root to shoot Cu translocation in Arabidopsis. Plant Cell Physiol. 2014, 55, 1568–1581.
- Kiyono, M.; Oka, Y.; Sone, Y.; Nakamura, R.; Sato, M.H.; Sakabe, K.; Pan-Hou, H. Bacterial heavy metal transporter MerC increases mercury accumulation in Arabidopsis thaliana. Biochem. Eng. J. 2013, 71, 19–24.
- Kaksonen, A.H.; Puhakka, J.A. Sulfate reduction-based bioprocesses for the treatment of acid mine drainage and the recovery of metals. Eng. Life Sci. 2007, 7, 541–564.
- De, J.; Ramaiah, N.; Vardanyan, L. Detoxification of toxic heavy metals by marine bacteria highly resistant to mercury. Mar. Biotechnol. 2008, 10, 471–477.
- Li, F.; Wang, W.; Li, C.; Zhu, R.; Ge, F.; Zheng, Y.; Tang, Y. Self-mediated pH changes in culture medium affecting biosorption and biomineralization of Cd(2+) by Bacillus cereus Cd01. J. Hazard. Mater. 2018, 358, 178–186.
- Molokwane, P.E.; Meli, C.K.; Chirwa, E.M. Chromium (VI) reduction in activated sludge bacteria exposed to high chromium loading: Brits culture (South Africa). Water Sci. Technol. 2008, 58, 399–405.
- Naskar, A.; Majumder, R.; Goswami, M. Bioaccumulation of Ni (II) on growing cells of Bacillus sp.: Response surface modeling and mechanistic insight. nviron. Technol. Innov. 2020, 20, 101057.
- Xiao, R.; Liu, X.; Ali, A.; Chen, A.; Zhang, M.; Li, R.; Chang, H.; Zhang, Z. Bioremediation of Cd-spiked soil using earthworms (Eisenia fetida): Enhancement with biochar and bacillus megatherium application. Chemosphere 2021, 264, 128517.
- Li, Q.; Xing, Y.; Huang, B.; Chen, X.; Ji, L.; Fu, X.; Li, T.; Wang, J.; Chen, G.; Zhang, Q. Rhizospheric mechanisms of Bacillus subtilis bioaugmentation-assisted phytostabilization of cadmium-contaminated soil. Sci. Total Environ. 2022, 825, 154136.
- Chen, Z.; Zheng, Y.; Ding, C.; Ren, X.; Yuan, J.; Sun, F.; Li, Y. Integrated metagenomics and molecular ecological network analysis of bacterial community composition during the phytoremediation of cadmium-contaminated soils by bioenergy crops. Ecotoxicol. Environ. Saf. 2017, 145, 111–118.
- Jing, R.; Kjellerup, B.V. Biogeochemical cycling of metals impacting by microbial mobilization and immobilization. J. Environ. Sci. 2018, 66, 146–154.
- Ashraf, M.A.; Hussain, I.; Rasheed, R.; Iqbal, M.; Riaz, M.; Arif, M.S. Advances in microbe-assisted reclamation of heavy metal contaminated soils over the last decade: A review. J. Environ. Manag. 2017, 198, 132–143.
- Ren, X.-M.; Guo, S.-J.; Tian, W.; Chen, Y.; Han, H.; Chen, E.; Li, B.-L.; Li, Y.-Y.; Chen, Z.-J. Effects of Plant Growth-Promoting Bacteria (PGPB) inoculation on the growth, antioxidant activity, Cu uptake, and bacterial community structure of rape (Brassica napus L.) grown in Cu-contaminated agricultural soil. Front. Microbiol. 2019, 10, 1455.
- Pandey, S.; Ghosh, P.K.; Ghosh, S.; De, T.K.; Maiti, T.K. Role of heavy metal resistant Ochrobactrum sp. and Bacillus spp. strains in bioremediation of a rice cultivar and their PGPR like activities. J. Microbiol. 2013, 51, 11–17.
- Pishchik, V.N.; Filippova, P.S.; Mirskaya, G.V.; Khomyakov, Y.V.; Vertebny, V.E.; Dubovitskaya, V.I.; Ostankova, Y.V.; Semenov, A.V.; Chakrabarty, D.; Zuev, E.V.; et al. Epiphytic PGPB Bacillus megaterium AFI1 and Paenibacillus nicotianae AFI2 improve wheat growth and antioxidant status under Ni Stress. Plants 2021, 10, 2334.
- Luo, S.L.; Chen, L.; Chen, J.L.; Xiao, X.; Xu, T.Y.; Wan, Y.; Rao, C.; Liu, C.B.; Liu, Y.T.; Lai, C.; et al. Analysis and characterization of cultivable heavy metal-resistant bacterial endophytes isolated from Cd-hyperaccumulator Solanum nigrum L. and their potential use for phytoremediation. Chemosphere 2011, 85, 1130–1138.
- Wang, L.; Rinklebe, J.; Tack, F.M.; Hou, D. A review of green remediation strategies for heavy metal contaminated soil. Soil Use Manag. 2021, 37, 936–963.
- Schwartz, A.R.; Ortiz, I.; Maymon, M.; Herbold, C.W.; Fujishige, N.A.; Vijanderan, J.A.; Villella, W.; Hanamoto, K.; Diener, A.; Sanders, E.R.; et al. Bacillus simplex—A little known PGPB with anti-fungal activity—Alters pea legume root architecture and nodule morphology when coinoculated with Rhizobium leguminosarum bv. viciae. Agronomy 2013, 3, 595–620.
- Hiller, J.; Napora, A.; Grobelak, A. Growth promotion by PGBP bacteria. In Innovations in Technological Processes , 1st ed.; Bajdur, W.M., Ed.; Wydawnictwo Wydziału Zarządzania Politechniki Częstochowskiej: Częstochowa, Poland, 2016; pp. 74–83. ISBN 978-83-65179-72-2.
- Hong, S.; Kim, T.Y.; Won, S.-J.; Moon, J.-H.; Ajuna, H.B.; Kim, K.Y.; Ahn, Y.S. Control of fungal diseases and fruit yield improvement of strawberry using Bacillus velezensis CE 100. Microorganisms 2022, 10, 365.
- Mirskaya, G.V.; Khomyakov, Y.V.; Rushina, N.A.; Vertebny, V.E.; Chizhevskaya, E.P.; Chebotar, V.K.; Chesnokov, Y.V.; Pishchik, V.N. Plant development of early-maturing spring wheat (Triticum aestivum L.) under inoculation with Bacillus sp. V2026. Plants 2022, 11, 1817.
- Mukhtar, T.; Rehman, S.U.; Smith, D.; Sultan, T.; Seleiman, M.F.; Alsadon, A.A.; Amna; Ali, S.; Chaudhary, H.J.; Solieman, T.H.I.; et al. Mitigation of heat stress in Solanum lycopersicum L. by ACC-deaminase and exopolysaccharide producing Bacillus cereus: Effects on biochemical profiling. Sustainability 2020, 12, 2159.
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant growth enhancement using rhizospheric halotolerant phosphate solubilizing bacterium Bacillus licheniformis QA1 and Enterobacter asburiae QF11 isolated from Chenopodium quinoa Willd. Microorganisms 2020, 8, 948.
- Nithyapriya, S.; Lalitha, S.; Sayyed, R.Z.; Reddy, M.S.; Dailin, D.J.; El Enshasy, H.A.; Luh Suriani, N.; Herlambang, S. Production, purification, and characterization of bacillibactin siderophore of Bacillus subtilis and its application for improvement in plant growth and oil content in sesame. Sustainability 2021, 13, 5394.
- Dobrzyński, J.; Wierzchowski, P.S.; Stępień, W.; Górska, E.B. The reaction of cellulolytic and potentially cellulolytic spore-forming bacteria to various types of crop management and farmyard manure fertilization in bulk soil. Agronomy 2021, 11, 772.
- Dobrzyński, J.; Jakubowska, Z.; Dybek, B. Potential of Bacillus pumilus to directly promote plant growth. Front. Microbiol. 2022, 13, 1069053.
- Khan, N.; Maymon, M.; Hirsch, A.M. Combating Fusarium infection using Bacillus-based antimicrobials. Microorganisms 2017, 5, 75.
- Sibponkrung, S.; Kondo, T.; Tanaka, K.; Tittabutr, P.; Boonkerd, N.; Yoshida, K.-I.; Teaumroong, N. Co-inoculation of Bacillus velezensis strain S141 and Bradyrhizobium strains promotes nodule growth and nitrogen fixation. Microorganisms 2020, 8, 678.
- Kwon, J.-H.; Won, S.-J.; Moon, J.-H.; Lee, U.; Park, Y.-S.; Maung, C.E.H.; Ajuna, H.B.; Ahn, Y.S. Bacillus licheniformis PR2 controls fungal diseases and increases production of jujube fruit under field conditions. Horticulturae 2021, 7, 49.
- Dobrzyński, J.; Wróbel, B.; Górska, E.B. Cellulolytic properties of a potentially lignocellulose-degrading Bacillus sp. 8E1A strain isolated from bulk soil. Agronomy 2022, 12, 665.
- Chiboub, M.; Jebara, S.H.; Saadani, O.; Fatnassi, I.C.; Abdelkerim, S.; Jebara, M. Physiological responses and antioxidant enzyme changes in Sulla coronaria inoculated by cadmium resistant bacteria. J. Plant Res. 2018, 131, 99–110.
- Rizvi, A.; Khan, M.S. Heavy metal-induced oxidative damage and root morphology alterations of maize (Zea mays L.) plants and stress mitigation by metal tolerant nitrogen-fixing Azotobacter chroococcum. Ecotoxicol. Environ. Saf. 2018, 157, 9–20.
- Alka, S.; Shahir, S.; Ibrahim, N.; Chai, T.T.; Mohd Bahari, Z.; Abd Manan, F. The role of plant growth promoting bacteria on arsenic removal: A review of existing perspectives. Environ. Technol. Innov. 2020, 17, 100602.
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation–A review. Earth Sci. Rev. 2017, 171, 621–645.
- Kong, Z.; Glick, B.R. The role of plant growth-promoting bacteria in metal phytoremediation. Adv. Microb. Physiol. 2017, 71, 97–132.
- Wu, J.; Kamal, N.; Hao, H.; Qian, C.; Liu, Z.; Shao, Y.; Zhong, X.; Xu, B. Endophytic Bacillus megaterium BM18-2 mutated for cadmium accumulation and improving plant growth in Hybrid Pennisetum. Biotechnol. Rep. 2019, 24, e00374.
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359.
- Burd, G.I.; Dixon, D.G.; Glick, B.R. A plant growth promoting bacterium that decreases nickel toxicity in seedlings. Appl. Environ. Microbiol. 1998, 64, 3663–3668.
- Glick, B.R.; Penrose, D.M.; Li, J. A model for lowering of plant ethylene concentrations by plant-growth-promoting bacteria. J. Theor. Biol. 1998, 190, 63–68.
- Hussein, K.A.; Joo, J.H. Potential of siderophore production by bacteria isolated from heavy metal: Polluted and rhizosphere soils. Curr. Microbiol. 2014, 68, 717–723.
- Huo, Y.; Kang, J.P.; Ahn, J.C.; Kim, Y.J.; Piao, C.H.; Yang, D.U.; Yang, D.C. Siderophore-producing rhizobacteria reduce heavy metal-induced oxidative stress in Panax ginseng Meyer. J. Ginseng Res. 2021, 45, 218–227.
- Caracciolo, A.B.; Terenzi, V. Rhizosphere microbial communities and heavy metals. Microorganisms 2021, 9, 1462.
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010, 28, 142–149.
- Schalk, I.J.; Hannauer, M.; Braud, A. New roles for bacterial siderophores in metal transport and tolerance. Environ. Microbiol. 2011, 13, 2844–2854.
- Roskova, Z.; Skarohlid, R.; McGachy, L. Siderophores: An alternative bioremediation strategy? Sci. Total Environ. 2022, 819, 153144.
- Zaidi, S.; Usmani, S.; Singh, B.R.; Musarrat, J. Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 2006, 64, 991–997.
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374.
- Sessitsch, A.; Kuffner, M.; Kidd, P.; Vangronsveld, J.; Wenzel, W.W.; Fallmann, K.; Puschenreiter, M. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol. Biochem. 2013, 60, 182–194.
- He, L.Y.; Chen, Z.J.; Ren, G.D.; Zhang, Y.F.; Qian, M.; Sheng, X.F. Increased cadmium and lead uptake of a cadmium hyperaccumulator tomato by cadmium-resistant bacteria. Ecotoxicol. Environ. Saf. 2009, 72, 1343–1348.
- Abou-Shanab, R.A.; Ghanem, K.; Ghanem, N.; Al-Kolaibe, A. The role of bacteria on heavy-metal extraction and uptake by plants growing on multi-metal-contaminated soils. World J. Microbiol. Biotechnol. 2008, 24, 253–262.
- Saran, A.; Imperato, V.; Fernandez, L.; Gkorezis, P.; D′Haen, J.; Merini, L.J.; Vangronsveld, J.; Thijs, S. Phytostabilization of polluted military soil supported by bioaugmentation with PGP-trace element tolerant bacteria isolated from Helianthus petiolaris. Agronomy 2020, 10, 204.
- Ma, Y.; Rajkumar, M.; Freitas, H. Improvement of plant growth and nickel uptake by nickel resistant-plant-growth promoting bacteria. J. Hazard. Mater. 2009, 66, 1154–1161.
- Rajkumar, M.; Nagendran, R.; Lee, K.J.; Lee, W.H.; Kim, S.Z. Influence of plant growth promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere 2006, 62, 741–748.
- Sheng, X.; He, L.; Wang, Q.; Ye, H.; Jiang, C. Effects of inoculation of biosurfactant-producing Bacillus sp. J119 on plant growth and cadmium uptake in a cadmium-amended soil. J. Hazard. Mater. 2008, 155, 17–22.
More
Information
Subjects:
Biochemical Research Methods
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
27 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




