
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tahir Ahmad | -- | 4070 | 2023-09-26 12:04:11 | | | |
| 2 | Lindsay Dong | Meta information modification | 4070 | 2023-09-28 02:44:29 | | |
Video Upload Options
Hepatitis E virus (HEV) is a single-stranded, positive RNA virus. The HEV is the causing agent of hepatitis, with a high prevalence rate in low-income countries due to poor sanitary conditions. It can exhibit acute, continuous, or extrahepatic consequences in immunocompromised individuals such as those undergoing organ transplantation and having HIV infection. HEV infection is either self-limiting (silent), meaning the patient will possibly recover on his own, or symptomatic, causing acute liver injury or fulminant hepatitis, and may eventually cause death. It can also cause chronic hepatitis that can progress to cirrhosis or recovery. Pregnancy-related HEV infection has an incidence rate of 30%. HEV escape from innate immunity, hormonal imbalances, defective monocyte–macrophage function, downregulation of the T-cell-mediated immune system, high cytokine production, nutritional factors, and socioeconomic conditions may play fundamental roles in the prevalence of HEV infection. It is necessary to take particular measures to reduce the incidence burden of HEV infection in high endemic locations.
1. Introduction
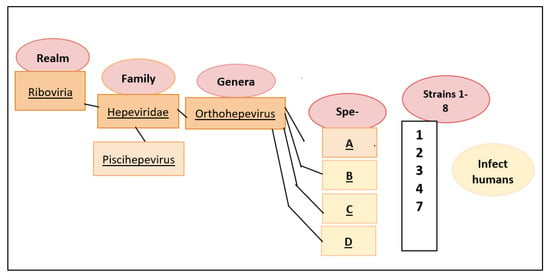
2. Classification and Molecular Characteristics of HEV
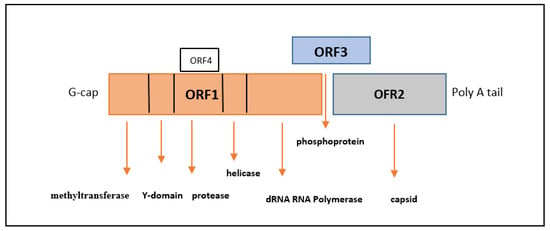
3. Manifestations of HEV Infection
-
Myalgia;
-
Arthralgia;
-
Anorexia;
-
Hepatomegaly;
-
Fever;
-
Weakness;
-
Vomiting;
-
Jaundice.
4. Incidence Rate of HEV in Developing and Developed Nations
5. Incidence Rate of HEV in Pregnant Females
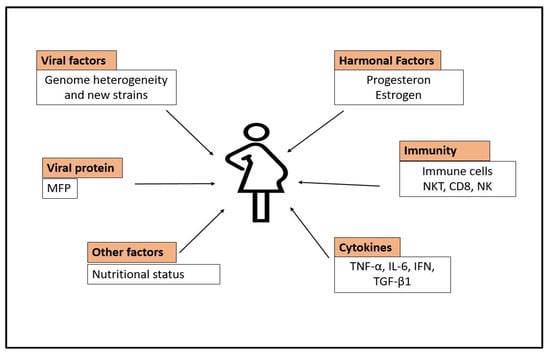
6. Relation of HEV with the Immune System during Pregnancy
6.1. T-Cell-Mediated Immune System
6.2. Defective Monocyte-Macrophage Function
6.3. HEV-Induced Acute Liver Injury
6.4. High Level of Cytokines
6.5. Hormonal Imbalance in HEV-Positive Pregnancy
6.6. Antibody-Mediated Hepatitis E Severity in Pregnancy
7. Extrahepatic Manifestations of HEV
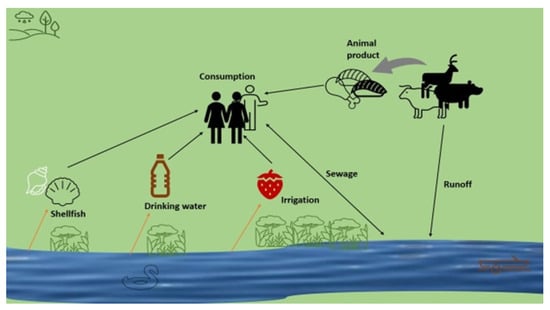
8. Transmission Route of HEV
9. Clinical Diagnosis of HEV
10. Treatment Strategies for HEV Infection
10.1. Vaccination Therapy
10.2. Drug Development
11. Conclusions
References
- Nishiyama, T.; Kobayashi, T.; Jirintai, S.; Kii, I.; Nagashima, S.; Primadharsini, P.P.; Nishizawa, T.; Okamoto, H. Screening of novel drugs for inhibiting hepatitis E virus replication. J. Virol. Methods 2019, 270, 1–11.
- Nimgaonkar, I.; Ding, Q.; Schwartz, R.E.; Ploss, A. Hepatitis E virus: Advances and challenges. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 96–110.
- Smith, D.B.; Purdy, M.A.; Simmonds, P. Genetic variability and the classification of hepatitis E virus. J. Virol. 2013, 87, 4161–4169.
- Sridhar, S.; Teng, J.L.L.; Chiu, T.-H.; Lau, S.K.P.; Woo, P.C.Y. Hepatitis E Virus Genotypes and Evolution: Emergence of Camel Hepatitis E Variants. Int. J. Mol. Sci. 2017, 18, 869.
- Kenney, S.P.; Meng, X.-J. Hepatitis E Virus Genome Structure and Replication Strategy. Cold Spring Harb. Perspect. Med. 2018, 9, a031724.
- Nair, V.P.; Anang, S.; Subramani, C.; Madhvi, A.; Bakshi, K.; Srivastava, A.; Shalimar; Nayak, B.; Ct, R.K.; Surjit, M. Endoplasmic Reticulum Stress Induced Synthesis of a Novel Viral Factor Mediates Efficient Replication of Genotype-1 Hepatitis E Virus. PLoS Pathog. 2016, 12, e1005521.
- Aslan, A.T.; Balaban, H.Y. Hepatitis E virus: Epidemiology, diagnosis, clinical manifestations, and treatment. World J. Gastroenterol. 2020, 26, 5543.
- Aggarwal, R.; Krawczynski, K. Hepatitis E: An overview and recent advances in clinical and laboratory research. J. Gastroenterol. Hepatol. 2000, 15, 9–20.
- Pérez-Gracia, M.T.; Suay, B.; Mateos-Lindemann, M.L. Hepatitis E: An emerging disease. Infect. Genet. Evol. 2014, 22, 40–59.
- World Health Organization (WHO). Hepatitis E. Fact Sheet. Geneva: WHO. 2018. Available online: http://www.who.int/news-room/fact-sheets/detail/hepatitis-e (accessed on 11 June 2023).
- Lin, S.; Zhang, Y.-J. Advances in Hepatitis E Virus Biology and Pathogenesis. Viruses 2021, 13, 267.
- Nelson, K.E.; Labrique, A.B.; Kmush, B.L. Epidemiology of Genotype 1 and 2 Hepatitis E Virus Infections. Cold Spring Harb. Perspect. Med. 2018, 9, a031732.
- Van der Poel, W.H. Food and environmental routes of Hepatitis E virus transmission. Curr. Opin. Virol. 2014, 4, 91–96.
- Yazaki, Y.; Mizuo, H.; Takahashi, M.; Nishizawa, T.; Sasaki, N.; Gotanda, Y.; Okamoto, H. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J. Gen. Virol. 2003, 84, 2351–2357.
- Lee, G.-H.; Tan, B.-H.; Teo, E.C.-Y.; Lim, S.-G.; Dan, Y.-Y.; Wee, A.; Aw, P.P.K.; Zhu, Y.; Hibberd, M.L.; Tan, C.-K.; et al. Chronic Infection With Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology 2016, 150, 355–357.e3.
- der Honing, R.W.H.-V.; van Coillie, E.; Antonis, A.F.G.; van der Poel, W.H.M. First Isolation of Hepatitis E Virus Genotype 4 in Europe through Swine Surveillance in the Netherlands and Belgium. PLoS ONE 2011, 6, e22673.
- Garbuglia, A.R.; Scognamiglio, P.; Petrosillo, N.; Mastroianni, C.M.; Sordillo, P.; Gentile, D.; La Scala, P.; Girardi, E.; Capobianchi, M.R. Hepatitis E Virus Genotype 4 Outbreak, Italy, 2011. Emerg. Infect. Dis. 2013, 19, 110–114.
- Kar, P.; Sengupta, A. A guide to the management of hepatitis E infection during pregnancy. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 205–211.
- Patra, S.; Kumar, A.; Trivedi, S.S.; Puri, M.; Sarin, S.K. Maternal and Fetal Outcomes in Pregnant Women with Acute Hepatitis E Virus Infection. Ann. Intern. Med. 2007, 147, 28–33.
- Wu, C.; Wu, X.; Xia, J. Hepatitis E virus infection during pregnancy. Virol. J. 2020, 17, 1–11.
- Fiore, S.; Savasi, V. Treatment of viral hepatitis in pregnancy. Expert Opin. Pharmacother. 2009, 10, 2801–2809.
- Chibber, R.M.; Usmani, M.A.; Al-Sibai, M.H. Should HEV infected mothers breast feed? Arch. Gynecol. Obstet. 2003, 270.
- Kraus, T.A.; Engel, S.M.; Sperling, R.S.; Kellerman, L.; Lo, Y.; Wallenstein, S.; Escribese, M.M.; Garrido, J.L.; Singh, T.; Loubeau, M.; et al. Characterizing the Pregnancy Immune Phenotype: Results of the Viral Immunity and Pregnancy (VIP) Study. J. Clin. Immunol. 2011, 32, 300–311.
- Zumla, A.; Bates, M.; Maeurer, M. Pregnancy and infection. New Engl. J. Med. 2014, 371, 1076.
- Sehgal, R.; Patra, S.; David, P.; Vyas, A.; Khanam, A.; Hissar, S.; Gupta, E.; Kumar, G.; Kottilil, S.; Maiwall, R.; et al. Impaired monocyte-macrophage functions and defective toll-like receptor signaling in hepatitis E virus-infected pregnant women with acute liver failure. Hepatology 2015, 62, 1683–1696.
- Prusty, B.K.; Hedau, S.; Singh, A.; Kar, P.; Das, B.C. Selective suppression of NF-kBp65 in hepatitis virus-infected pregnant women manifesting severe liver damage and high mortality. Mol. Med. 2007, 13, 518–526.
- Milan, S.; Varshney, B.; Lal, S.K. The ORF2 glycoprotein of hepatitis E virus inhibits cellular NF-κB activity by blocking ubiquitination mediated proteasomal degradation of IκBα in human hepatoma cells. BMC Biochem. 2012, 13, 7.
- Jilani, N.; Das, B.C.; Husain, S.A.; Baweja, U.K.; Chattopadhya, D.; Gupta, R.K.; Sardana, S.; Kar, P. Hepatitis E virus infection and fulminant hepatic failure during pregnancy. J. Gastroenterol. Hepatol. 2007, 22, 676–682.
- Swati, S.; Daga, M.K.; Kumar, A.; Husain, S.A.; Kar, P. Role of oestrogen and its receptors in HEV-associated feto-maternal outcomes. Liver Int. 2019, 39, 633–639.
- Nan, Y.; Yu, Y.; Ma, Z.; Khattar, S.K.; Fredericksen, B.; Zhang, Y.-J. Hepatitis E Virus Inhibits Type I Interferon Induction by ORF1 Products. J. Virol. 2014, 88, 11924–11932.
- Lhomme, S.; Marion, O.; Abravanel, F.; Chapuy-Regaud, S.; Kamar, N.; Izopet, J. Hepatitis E Pathogenesis. Viruses 2016, 8, 212.
- Dalton, H.R.; Kamar, N.; van Eijk, J.J.J.; Mclean, B.N.; Cintas, P.; Bendall, R.P.; Jacobs, B.C. Hepatitis E virus and neurological injury. Nat. Rev. Neurol. 2015, 12, 77–85.
- Awan, S.; Siddiqi, A.I.; Asif, A.; Ahmed, N.; Brohi, H.; Jalbani, S.; Wasay, M. Spectrum of neurological disorders in neurology outpatients clinics in urban and rural Sindh, Pakistan: A cross sectional study. BMC Neurol. 2019, 19, 1–6.
- Gourie-Devi, M.; Gururaj, G.; Satishchandra, P.; Subbakrishna, D. Prevalence of Neurological Disorders in Bangalore, India: A Community-Based Study with a Comparison between Urban and Rural Areas. Neuroepidemiology 2004, 23, 261–268.
- Kamar, N.; Weclawiak, H.; Guilbeau-Frugier, C.; Legrand-Abravanel, F.; Cointault, O.; Ribes, D.; Esposito, L.; Cardeau-Desangles, I.; Guitard, J.; Sallusto, F.; et al. Hepatitis E Virus and the Kidney in Solid-Organ Transplant Patients. Transplantation 2012, 93, 617–623.
- Bi, H.; Yang, R.; Wu, C.; Xia, J. Hepatitis E virus and blood transfusion safety. Epidemiology Infect. 2020, 148, 1–16.
- Ayoola, E.; Want, M.; Gadour, M.; Al-Hazmi, M.; Hamza, M. Hepatitis E virus infection in haemodialysis patients: A case-control study in Saudi Arabia. J. Med. Virol. 2002, 66, 329–334.
- Kamar, N.; Izopet, J.; Dalton, H.R. Chronic Hepatitis E Virus Infection and Treatment. J. Clin. Exp. Hepatol. 2013, 3, 134–140.
- Ouji, M.; Taherkhani, R.; Farshadpour, F. High prevalence of hepatitis E among regular hemodialysis patients in South of Iran. Int. J. Artif. Organs 2021, 44, 658–663.
- Al-Sadeq, D.W.; Majdalawieh, A.F.; Mesleh, A.G.; Abdalla, O.M.; Nasrallah, G.K. Laboratory challenges in the diagnosis of hepatitis E virus. J. Med. Microbiol. 2018, 67, 466–480.
- Aggarwal, R. Diagnosis of hepatitis E. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 24–33.
- Shrestha, A.C.; Flower, R.L.P.; Seed, C.R.; Stramer, S.L.; Faddy, H.M. A Comparative Study of Assay Performance of Commercial Hepatitis E Virus Enzyme-Linked Immunosorbent Assay Kits in Australian Blood Donor Samples. J. Blood Transfus. 2016, 2016, 9647675.
- Todt, D.; Meister, T.L.; Steinmann, E. Hepatitis E virus treatment and ribavirin therapy: Viral mechanisms of nonresponse. Curr. Opin. Virol. 2018, 32, 80–87.
- Kinast, V.; Burkard, T.L.; Todt, D.; Steinmann, E. Hepatitis E Virus Drug Development. Viruses 2019, 11, 485.
- Kamar, N.; Izopet, J.; Tripon, S.; Bismuth, M.; Hillaire, S.; Dumortier, J.; Radenne, S.; Coilly, A.; Garrigue, V.; D’Alteroche, L.; et al. Ribavirin for chronic hepatitis E virus infection in transplant recipients. New Engl. J. Med. 2014, 370, 1111–1120.
- Cameron, C.E.; Castro, C. The mechanism of action of ribavirin: Lethal mutagenesis of RNA virus genomes mediated by the viral RNA-dependent RNA polymerase. Curr. Opin. Infect. Dis. 2001, 14, 757–764.
- Choi, Y.; Zhang, X.; Skinner, B. Analysis of IgG Anti-HEV Antibody Protective Levels During Hepatitis E Virus Reinfection in Experimentally Infected Rhesus Macaques. J. Infect. Dis. 2019, 219, 916–924.
- Wang, Y.; Zhou, X.; Debing, Y.; Chen, K.; Van Der Laan, L.J.; Neyts, J.; Janssen, H.L.; Metselaar, H.J.; Peppelenbosch, M.P.; Pan, Q. Calcineurin inhibitors stimulate and mycophenolic acid inhibits replication of hepatitis E virus. Gastroenterology 2014, 146, 1775–1783.
- Zhou, X.; Wang, Y.; Metselaar, H.J.; Janssen, H.L.; Peppelenbosch, M.P.; Pan, Q. Rapamycin and everolimus facilitate hepatitis E virus replication: Revealing a basal defense mechanism of PI3K-PKB-mTOR pathway. J. Hepatol. 2014, 61, 746–754.
- Foster, T.L.; Thompson, G.S.; Kalverda, A.P.; Kankanala, J.; Bentham, M.; Wetherill, L.F.; Thompson, J.; Barker, A.M.; Clarke, D.; Noerenberg, M.; et al. Structure-guided design affirms inhibitors of hepatitis C virus p7 as a viable class of antivirals targeting virion release. Hepatology 2014, 59, 408–422.




