Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | ALESSANDRA VERARDI | -- | 3845 | 2023-09-26 11:25:01 | | | |
| 2 | Jessie Wu | Meta information modification | 3845 | 2023-09-27 03:08:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Verardi, A.; Sangiorgio, P.; Lopresto, C.G.; Casella, P.; Errico, S. Beneficial Effects of Carotenoids. Encyclopedia. Available online: https://encyclopedia.pub/entry/49654 (accessed on 07 February 2026).
Verardi A, Sangiorgio P, Lopresto CG, Casella P, Errico S. Beneficial Effects of Carotenoids. Encyclopedia. Available at: https://encyclopedia.pub/entry/49654. Accessed February 07, 2026.
Verardi, Alessandra, Paola Sangiorgio, Catia Giovanna Lopresto, Patrizia Casella, Simona Errico. "Beneficial Effects of Carotenoids" Encyclopedia, https://encyclopedia.pub/entry/49654 (accessed February 07, 2026).
Verardi, A., Sangiorgio, P., Lopresto, C.G., Casella, P., & Errico, S. (2023, September 26). Beneficial Effects of Carotenoids. In Encyclopedia. https://encyclopedia.pub/entry/49654
Verardi, Alessandra, et al. "Beneficial Effects of Carotenoids." Encyclopedia. Web. 26 September, 2023.
Copy Citation
In humans, the beneficial effects of carotenoids have been widely investigated, including protection against oxidative stress, beneficial properties for eyesight, UV protection for the skin, anticancer properties, the enhancement of cognitive function, and prevention against age-related degenerative diseases, cardiovascular diseases (blood pressure), and obesity. Some carotenoids (i.e., β-carotene, α-carotene, and β-cryptoxanthin) are precursors of vitamin A, a vitamin that, among other things, supports vision, immune function, development and growth, and reproduction.
carotenoids
nutraceuticals
efficacy
1. Physicochemical and Biological Properties
Carotenoids are secondary metabolites found in plants, algae, and some microorganisms, such as bacteria, yeasts, and molds. They are pigments ranging in color from yellow, orange, and red to purple in the case of some carotenoids, such as Rhodobacterioxanthin and Phillipsiaxanthin, present in some microorganisms.
More than 1000 carotenoids are present in nature [1]. In plants, carotenoids perform several essential functions. They are crucial in the photosynthetic process and play a relevant role in the protective mechanism against damage from light and oxygen. Moreover, carotenoids act as signals to attract pollinators and seed dispersers. Finally, they are precursors of relevant apocarotenoids (i.e., carotenoids’ derivatives), such as vitamin A and the phytohormones abscisic acid and strigolactones [2].
Chemically, carotenoids are pigments made up of isoprene units (C5) repeated 6, 8, 9, or 10 times to obtain molecules of 30, 40, 45, and 50 carbon atoms, respectively. Different terminal groups are present at the ends of the skeleton chain, including acyclic, cyclic (e.g., cyclohexane or cyclopentane), or aryl groups formed via the cyclization of the linear precursor [3]. The most abundant carotenoids in nature are C40 (Figure 1).
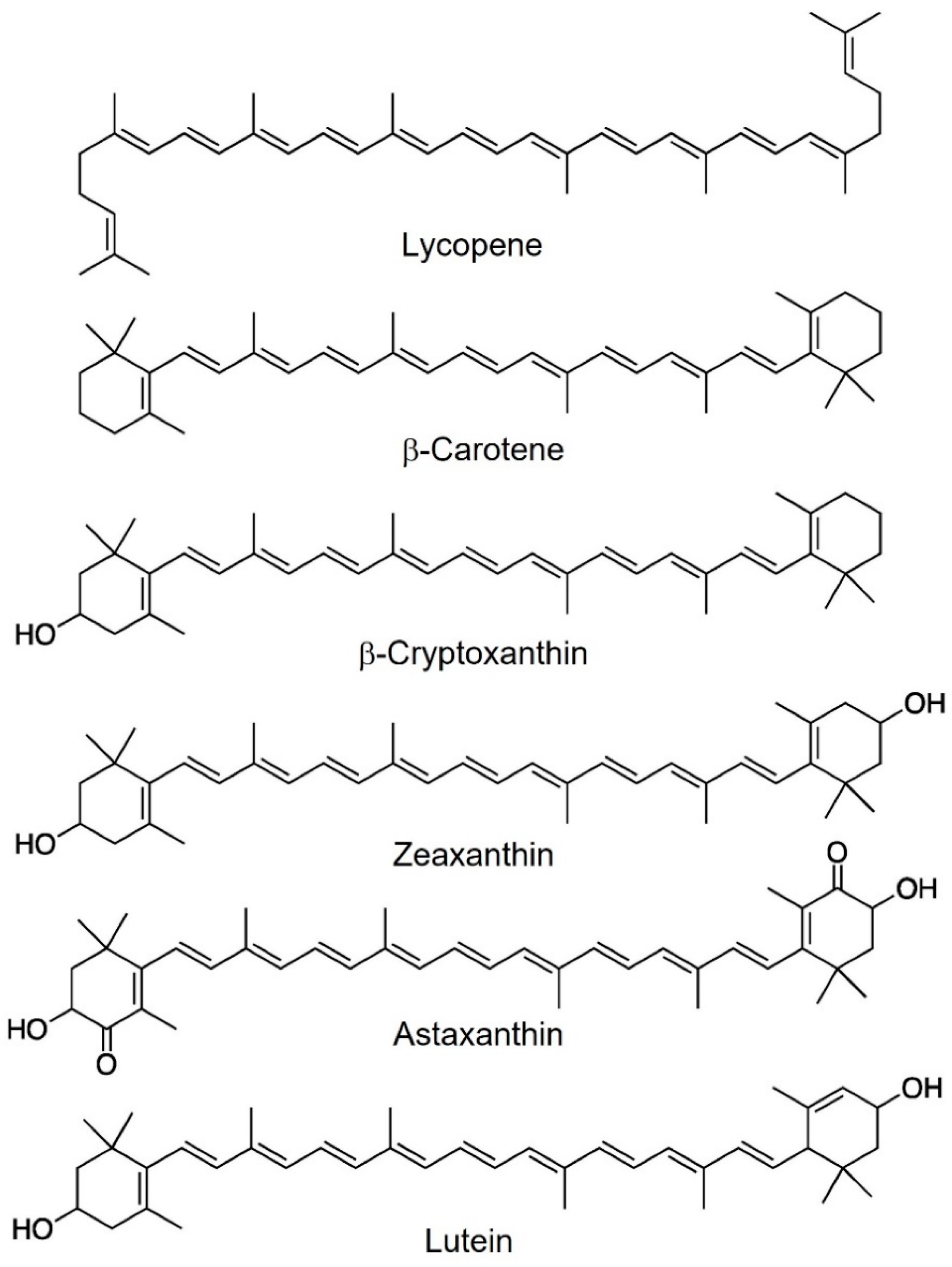
Figure 1. Most abundant carotenoids in human blood.
Carotenoids are divided into two main classes: carotenes, i.e., compounds containing only carbon and hydrogen; and xanthophylls, that is, carotenoids containing oxygen. Carotenes include β-carotene and lycopene, while xanthophylls include zeaxanthin, astaxanthin, and lutein [4].
The presence of substituents containing oxygen (hydroxyl, keto, methoxy, and oxo groups) gives polarity to the xanthophylls and makes them slightly hydrophilic. For this reason, carotenes are lipid-soluble pigments but insoluble in polar solvents. On the contrary, xanthophylls are soluble in polar solvents (like alcohols) and nonpolar solvents (like ether and hexane) [5].
The conjugated double bonds of the isoprenoid structure are the basis of the many functionalities of carotenoids, starting from the colorant capacity and ending with the antioxidant properties of removing free radicals and the singlet oxygen [4][6].
The biological properties of carotenoids depend on many factors beyond the presence of the polyene chain and its length. These factors include the presence of functional groups and their placement (e.g., α and β), acyclic and cyclic structure, and (E)- or (Z)- configuration [7].
Furthermore, the polyunsaturated chain of carotenoids can undergo various reactions mediated by light, oxygen, heat, catalysts, and free radicals, such as oxidation, isomerization, hydrolysis, and degradation. These reactions can lead to the formation of products with diminished or completely absent biological properties [8]. In light of this poor stability, it is necessary to find suitable systems to preserve the beneficial functions of carotenoids when they can come into contact with chemical agents, light, heat, or are extracted from their starting biological matrix.
The physical and chemical properties of carotenoids make them able to function both in plants, where they are produced, and in humans, who intake them through their diet [9].
Over the years, many studies have demonstrated the ability of carotenoids to have various effects on human health. Table 1 provides a summary of these effects.
Table 1. Effects of carotenoids on human health.
| Health Effects | Specific Effects | Carotenoids | References |
|---|---|---|---|
| Prevention and mitigation of age-related macular degeneration (AMD) and cataracts |
Lutein and Zeaxanthin | [10][11] | |
| Delayed onset and mitigation of diabetic retinopathy |
Lutein, Zeaxanthin, Lycopene |
[12][13][14] | |
| Antioxidant activities | Diabetes and osteoporosis | Lycopene | [15] |
| Mitigation of multiple sclerosis and atherosclerosis |
β-Carotene | [4] | |
| Prevention of age-related disease | Astaxanthin | [16] | |
| Protective effect on the skin | β-Carotene, Lutein | [17][18] | |
| Inhibition of retinal impairment | Lutein and Zeaxanthin | [19] | |
| Cancers | Inhibition of lung cancer | Lutein, Astaxanthin and b-Cryptoxanthin | [20][21][22] |
| Inhibition of risk for prostate cancer | Lycopene | [17][21][23] | |
| Mitigation of risk for colon-rectal cancer | Lycopene | [21][24] | |
| Mitigation of non-alcoholic fatty liver disease (associated with hepatocellular carcinoma) | Lycopene | [25] | |
| Mitigation of risk for breast cancer | Lutein and Zeaxanthin Lycopene |
[24][26][27] | |
| Mitigation of risk for non-melanoma cancer | Lutein, β-Carotene, α-Carotene, Lycopene, Zeaxanthin, Astaxanthin, Lutein, Cryptoxanthin, Lycopene, Fucoxanthin |
[28][29] | |
| Mitigation of risk for melanoma | β-Carotene | [30][31] | |
| Cardiovascular diseases | Lycopene | [22][24][32] | |
| Astaxanthin and b-Carotene | [33][34] | ||
| Effects on HDL and LDL cholesterol levels | Phytoene and Phytofluene | [35][36] | |
| Reduction in the severity of cardiovascular disease |
Lutein and Zeaxanthin | [37] | |
| Antihypertensive and anti-aggregative Effect | Lycopene | [38] | |
| Reduction in immune activation (In patients with cardiovascular disease) |
β-Carotene | [39] | |
| Neurological disorders | Regulation of lipid raft formation in neuronal cells (proper membrane fluidity) |
Lutein and Zeaxanthin | [40] |
| Protection for neurodegenerative diseases and For neurological, cognitive, and psycho-behavioral disease conditions |
Lycopene | [41][42] | |
| Bacterial infections | Inhibition of bacteria cells growth | β-Carotene, b-Cryptoxanthin, Lutein, Violaxanthin, Antheraxanthin, Fucoxanthin, Zeaxanthin |
[43] |
| Others | Improvement of sleep quality and duration | Lycopene | [44] |
| Anti-inflammatory properties | Lycopene | [45] | |
| Cognitive performances | β-Carotene and Lutein | [11][46][47] | |
| Bone homeostasis | β-Cryptoxanthin | [48] |
The effects of introducing carotenoids into the human diet on vision and vision-related diseases have long been known and supported by clinical, epidemiological, and interventional studies [49]. Most of the beneficial effects of carotenoids on human health are due to their antioxidant characteristics, which is why they are used in various fields and to hinder the progression or mitigate the effects of numerous diseases [50].
The physical characteristics of carotenoids, namely their tendency to aggregate even in different solvents, affect their antioxidant abilities through interactions with reactive oxygen species and other antioxidants such as tocopherol and vitamin C [9].
These pigments play an important role in counteracting certain types of cancer: the carotenoids’ complex anticancer properties are associated with phosphorylation and activation of the major kinases, modulation of cellular pathways of Nrf2 and NF-κB transcription factors, apoptosis, cell cycle progression, intercellular gap junction communication and angiogenesis [41]. Carotenoids are helpful in this area because, at present, most anticancer drugs have serious side effects, and resistance and toxicity problems. Therefore, new alternatives are always being sought. In addition to having a direct antitumorigenic effect, carotenoids can serve as a natural scaffold for other phytochemicals able to manage and treat certain types of cancer [41].
A key role is played by β-carotene as a precursor of vitamin A. Only carotenoids with an unsubstituted b-ionone ring have pro-vitamin A activity, and, among them, the most abundant in the human diet and tissues is β-carotene. Vitamin A (or retinol) is a potent gene regulator that controls the expression of nearly 700 genes. It is also critical for vision and plays an essential role in lipid metabolism, thus putting obesity under control [50].
Carotenoids and their cleavage products, such as retinoids and apo-carotenoids, positively affect adipocyte differentiation. Through various mechanisms, this eventually leads to a reduction in abdominal and subcutaneous fat. In addition, due to antioxidant activity, carotenoids can reduce the overall oxidative load or decrease its accumulation, thus also exerting beneficial effects on weight management and obesity [17].
The main transcriptionally active form of vitamin A is retinoic acid (or acidic vitamin A), which, under normal conditions, is present only in small amounts in tissues [50]. The importance of vitamin A is evident from the consequences of its deficiency, which, in developing countries, is a major public health problem. In these countries, vitamin A deficiency mainly affects preschool children and pregnant women, and can cause blindnesspoor growth, and sometimes even death [48][51][52]. The beneficial effects of carotenoids on the health of postmenopausal women are manifold and studied, above all, in Eastern countries. Some authors report a positive correlation between β-carotene intake and increased lumbar spine bone mineralization (understood as mineral density) in postmenopausal Korean women [17]. Sugiura et al. (2012) investigated the possible correlation between serum carotenoid levels and bone loss in postmenopausal Japanese women [53]. The research study showed an inverse association, thus suggesting a protective action of carotenoids on bone tissue. Umigai et al. (2020) have shown that the carotenoid intake of paprika suppresses bone resorption, helping to maintain high bone quality in postmenopausal women [54].
Carotenoids, such as lutein and zeaxanthin, are crucial for children’s visual and cognitive development and are the main carotenoids in human milk. Several studies showed that lutein is more bioavailable in human milk than in infant formula. This fact affects postnatal brain development. In total, 75% of the infant’s brain growth occurs in the first year of life, doubling during the third trimester. At these stages, lutein and zeaxanthin are in brain regions specialized for visual processing, memory, learning, and language. In particular, lutein is up to 3–4 times more present than other carotenoids in these brain regions [17][55].
In addition, several carotenoids also have effects on cognitive function in adults. The mechanism is still unclear. It may be related to antioxidant activity. For example, placebo-controlled studies suggest that dietary supplementation of lutein or lutein plus zeaxanthin may positively affect cognitive function in older men and women [17]. On the other hand, lycopene is one of the most potent antioxidants that effectively neutralize reactive oxygen species and is an effective natural neuroprotective agent. Therefore, it is widely studied as a candidate for providing therapeutic benefits and maintaining brain health in neurological, cognitive, and psycho-behavioral diseases. For example, it helps fight neurodegenerative diseases, including Alzheimer’s and Parkinson’s diseases [56]. These diseases (Parkinson’s disease, amyotrophic lateral sclerosis-ASL, stroke, Alzheimer’s disease, and Huntington’s disease—to name only the major ones) are characterized by progressive neuronal depletion and apoptosis, resulting in cognitive, motor, and intellectual dysfunction. However, it is still unclear how biochemical changes lead to neurodegeneration. It is only known that central nervous system inflammation and immune activation are implicated in the pathophysiology of neurodegenerative diseases. Thus, the development of therapies for these diseases is very challenging. Indeed, there are currently no cures for degenerative diseases: available remedies can only slow or delay their onset. In this context, the effects of a molecule such as lycopene, with high neuroprotective abilities, and of carotenoids in general, which promote synaptogenesis and neurogenesis, are very promising [41].
Studies regarding the role of lipophilic compounds, to which carotenoids belong, in cognitive neuroscience and aging are also extremely interesting. Carotenoids have crucial biological effects on proper brain function and brain robustness maintenance and promote healthy aging. Of course, a rigorous and objective assessment of these effects must consider the multifactorial nature of aging and nutrigenetics, geographical, social, and cultural aspects [57].
Another notable aspect is the influence of carotenoids on sleep quality and duration. In particular, the link between sleep quantity/quality and diet, specifically the intake of lycopene-rich fruits/vegetables, has been investigated. In reality, to provide a robust figure regarding the impact of a lycopene-rich diet on sleep, fruit/vegetable-containing foods would have to be disaggregated in the dietary records of the subjects surveyed. Even with this limitation, studies have shed light on the link between dynamic parameters of sleep biology and a lycopene-rich diet, showing a strong correlation that deserves further deeper investigation [56].
A study of the effects of dietary carotenoid supplementation, whatever the desired upshot, must take into account multiple interactions with other (micro)nutrients and involve a broader spectrum of robust biomarkers of antioxidant status and free radical-induced damage [57].
Unfortunately, numerous studies conducted on carotenoids have suggested an overly simplistic idea of their use. There is the belief that because β-carotene is present in healthy diets and has antioxidant effects in vitro, high doses of this carotenoid can only amplify the beneficial effects on health [9]. This assumption does not consider the interaction with other dietary components or their actual bioavailability and bioaccessibility.
2. Dietary Sources and Bioaccessibility/Bioavailability
The human body cannot synthesize carotenoids; therefore, the only way to take advantage of their beneficial effects is to introduce them through the diet [1]. Carotenoid content in the food may vary depending on growing years and areas, cultivars, processing techniques, and storage conditions. The foods richest in β-carotene are certainly sweet potatoes and carrots. According to Arscott (2013), the average β-carotene content in sweet potatoes is 91.8 µg/g FW (fresh weight), while in carrots, it is 88.4 µg/g FW [58]. Other foods with excellent β-carotene content are apricot (66.4 µg/g FW) and spinach (55.9 µg/g FW). The latter, along with broccoli, are the foods with the highest lutein content. Spinach contains an amount of lutein equal to 119.4 µg/g FW and broccoli 34.4 µg/g FW, respectively. On the other hand, the highest amount of lycopene is contained in tomatoes (30.2 µg/g FW), watermelon (23.83 µg/g FW), and grapefruits (17.51 µg/g FW) [59]. Although carotenoid content in foods is essential for human health, their limited absorption is a critical factor in exploiting their countless benefits. Unconventional sources, such as carotenogenic microorganisms and microalgae, have been explored to study the production, content, properties, and bioavailability of carotenoids [59]. The scientific interest in unconventional sources is due to the contribution they could make to the growing demand for healthy, sustainable, and available food for all and an exponentially growing population.
In Table 2, some food sources and unconventional sources were selected as representative of the highest quantity of β-carotene [58].
Table 2. Average concentrations of carotenoids in foods and unconventional sources.
| Carotenoids | Sources | Concentration | References |
|---|---|---|---|
| β-carotene | Sweet potato | 91.8 µg/g FW | [58] |
| Carrot | 88.4 µg/g FW | [58] | |
| Apricots | 66.4 µg/g FW | [58] | |
| Spinach | 55.9 µg/g FW | [58] | |
| Beet greens | 34.4 µg/g FW | [58] | |
| Tomato | 3.4 µg/g FW | [58] | |
| Cassava root, Sweet yellow | 7.27 µg/g FW | [58] | |
| Squash | 3.7 µg/g FW | [58] | |
| Zea mais | 0.17 µg/g DW | [58] | |
| Dunaliella salina (microalgae) | 34,100 µg/g DW | [60] | |
| Blakeslea trispora (fungi) | 59,910 µg/g DW | [61] | |
| Phaffia rhodozyma (yeast) | 42.81 µg/g DW | [62] | |
| Lutein | Spinach | 119.4 µg/g FW | [58] |
| Broccoli | 34.4 µg/g FW | [58] | |
| Tagetes flowers | 2930 µg/g DW | [37] | |
| Scenedesmus almeriensis (microalgae) | 3040 µg/g DW | [63] | |
| Chlorella sp. | 7000 µg/g DW | [64] | |
| Lycopene | Tomato | 30.2 µg/g FW | [58] |
| Grapefruit | 17.51 µg/g FW | [58] | |
| Watermelon | 23.83 µg/g FW | [58] | |
| Astaxanthin | Haematococcus pluvialis (microalgae) | 20,000 µg/g DW | [60] |
| Phaffia rhodozyma (yeast) | 400 µg/g DW | [65] | |
| Xanthophyllomyces dendrorhous (yeast) | 5000 µg/g DW | [66] | |
| Shrimps, prawns, crabs (waste residues) | 57.5 µg/g DW | [67] |
Among the most promising unconventional sources of carotenoids, the microalgae Dunaliella salina was selected for its β-carotene production of 34,100 µg/g DW [60]. Dunaliella salina can accumulate β-carotene up to about 10% DW (dry weight). Blakeslea trispora, on the other hand, is among the most studied microorganisms belonging to the fungi genus for β-carotene production. Some authors produced up to 59,910 µg/g DW through studies on improving β-carotene biosynthesis and optimizing the production process using 35 mM sodium acetate [61].
At the industrial level, the most widely used natural source of lutein is the petals of the flower of the plant Tagetes erecta with an average content of 2930 µg/g DW [64]. New sources of lutein include the microalgae species Scenedesmus almeriensis, which can accumulate about 3040 µg/g DW [63] and whose production has been optimized to achieve 5700 µg/g DW [68]. Other microalgae species belonging to Chlorella species, such as C. sorokiniana, C. minutissima, and C. vulgaris, can produce 5000 to 9000 µg/g DW of lutein through the optimization of autotrophic and heterotrophic growth processes [64].
Haematococcus pluvialis is the microalgae that most accumulates the super antioxidant astaxanthin, up to 5% of its dry weight. Other species can produce astaxanthin as Chlorella zofingiensis (0.7% DW) and Nannochloropsis oculata (2% DW) [69][70] but their content is significantly lower than that of the species Haematoccocus pluvialis.
The red yeast Xanthophyllomyces dendrorhous can accumulate up to 5000 µg/g DW of astaxanthin [66]. Phaffia rhodozyma yeast is recognized by both the FDA and EFSA as a GRAS additive in feed as a source of astaxanthin [71]. Phaffia rhodozyma wild type can produce up to 200–400 µg/g DW of astaxanthin but engineered strains are being experimented with to achieve higher yields for industrial applications [62]. Some authors recently demonstrated a biorefinery process using Phaffia rhodozyma, that produces up to 63.11 µg/g DW of astaxanthin and 42.81 µg/g DW of β-carotene. Other promising sources of astaxanthin are shrimp’s, prawns’, and crabs’ residue/wastes such as heads, shells, tails, and exoskeletons, from which astaxanthin, protein, and chitin can be extracted by using several technologies [72]. Astaxanthin can be recovered by using conventional extraction methodologies, such as solvent extraction, or unconventional technologies, such as microwave, ultrasound, and supercritical fluid extraction, to reach a content of around 100 µg/g DW [67]. As mentioned, although searching for unconventional sources of carotenoids is essential to increase the intake of these substances, the limiting factor remains their absorption in the intestine.
Indeed, due to their apolarity and lipophilic properties (high octanol–water partition coefficients, log Poct > 8), their absorption in the intestinal tract is very poor. Only 5–30% of carotenoids are absorbed. Some key steps, such as micellarization, are necessary for their absorption in the gut [73]. In vitro studies on micellarization and uptake by Caco-2 cells demonstrated that lutein and phytoene incorporate in micelles better than lycopene and β-carotene [24].
Micellarization is crucial for the absorption of fat-soluble compounds such as carotenoids. After their separation from the food matrix, carotenoids are included in the fat fraction of the food, which facilitates biliary and pancreatic secretion. The following gastric emulsification into lipid droplets enables the incorporation of carotenoids into mixed micelles, consisting of bile salts, lysophospholipids, free cholesterol, and mono-acyl-glycerides produced via lipolysis and digestion of their esters and triglycerides. These micelles, which do not have the same apolarity problems as carotenoids, easily pass through the epithelial cells of the gastrointestinal lumen where the carotenoids, once desorbed from the micelles, can be absorbed into the ileum. These mixed micelles are very interesting for researchers because they may be used to enhance the carotenoids’ bioaccessibility and absorption [74]. Chacón-Ordóñez et al. (2019) studied the natural bioaccessibility of carotenoids in some foods using in vivo, ex vivo, and in vitro models. They reported that the highest relative bioaccessibility of β-carotene was from pineapple (96.5%) and kiwi (55%), while, among vegetables, it was from carrots (14%), and broccoli (17%). In the case of lutein, the highest bioaccessibility is from watermelon (49%), spinach (22%), and chicory (27%). The bioaccessibility of lycopene is 2% for tomatoes, while it can reach 33% for the gac fruit [75].
Another significant aspect that can strongly influence the bioavailability of carotenoids is their distribution in foods. Carotenoids in plant foods and colored plant foods are embedded in more complex chloroplast–protein structures than carotenoids in animal foods such as eggs and dairy products, in which they are more bioavailable [76].
Carotenoid separation from the food matrix can be enhanced via various processes of food matrix processing, cooking, and heating that can improve the release of carotenoids from food by weakening the cell walls and making the matrix more attackable by enzymes [53]. Koh et al. (2018) demonstrated that the bioaccessibility of β-carotene in pumpkin and butternut squash increased in fried samples compared to raw matrices, from around 10.56% to 69% in pumpkin and from 2% to 22% in butternut squash [77].
Assessing the bioaccessibility and bioavailability of carotenoids is complicated because of several factors involved, including dietary aspects concerning fat/lipids in meals, dietary fiber content, especially pectin, and the effect of divalent minerals. One solution may be to mimic and accentuate what happens during digestion.
On the effect of fat/lipids on carotenoids’ bioaccessibility, some authors demonstrated via in vivo digestion tests of tomato pulp how the bioaccessibility of lycopene can increase from about 2% to 10% by using the fat of oils such as olive or sunflower [78].
Some authors evaluated the bioaccessibility of β-carotene using a gastrointestinal model study (INFOGEST) testing different concentrations of droplet oils. They observed an increase in the bioaccessibility of β-carotene as the oil concentration increased, achieving up to 93% bioaccessibility with a 10% oil concentration. Finally, the amount of oil did not affect the stability of β-carotene [79]. It has been shown that carotenoids have low bioaccessibility caused by their interaction with other compounds such as dietary fiber [80][81]. Among dietary fiber, pectin has negative effects on lipolysis, thus affecting the bioavailability of carotenoids. The molecular characteristics of pectin, such as molecular weight, degree of methyl esterification, viscosity, and hydrophobicity, influence the lipid digestion process. In particular, the interaction of pectin with bile salts can reduce the micellarization process, thus altering the bioavailability of carotenoids [82]. The intake of bivalent minerals such as calcium, magnesium, and other micronutrients at the dietary recommended doses can inhibit the micelle formation process and carotenoid incorporation. Bivalent minerals may interfere with the absorption processes of carotenoids by causing the precipitation of bile salts and lipids needed in the emulsification processes of carotenoids [83]. Some authors observed that high concentrations of bivalent minerals such as calcium (500 mg/L) and magnesium (around 300 mg/L) negatively affected bile salt precipitation and micelles’ stability [77][84]. The bioaccessibility of carotenoids can also be improved via pasteurization processes. Aschoff et al. (2015) demonstrated an increase in bioaccessibility from about 10.8% β-carotene in orange pieces to about 40% in pasteurized juice [85]. Another process that improves carotenoid bioaccessibility is dehydration, as observed for total carotenoids in a chickpea and amaranthus mixture [86]. Thermal processes can enhance the release of carotenoids from food. However, in most cases, heat-sensitive carotenoids can undergo degradative processes. Astaxanthin, for example, has poor stability, as it is very sensitive to light and temperature and undergoes degradation processes even at prolonged exposure to room temperature up to about 7% thermal degradation [87]. Isomerization processes could also affect bioaccessibility. In vitro digestion model studies show a difference in astaxanthin bioaccessibility between its trans and cis isomers [88].
Another aspect crucial for the potential activity of carotenoids as a drug-like agent concerns their pharmacokinetics, i.e., the processes that condition the achievement and maintenance of an adequate concentration of drugs in the various compartments of the human body. The assessment of pharmacokinetics in carotenoids is complex. A SLAMENGHI-defined systemic approach is followed, which includes the most important factors such as the type of carotenoid, molecular binding, quantity, initial matrix, the implementer of absorption and bioconversion, the physiological state and the genetic factors of the host, and the synergy of all these factors [89]. The mechanisms involved in carotenoid pharmacokinetics are absorption in the gastrointestinal tract, distribution in plasma and tissues, metabolism in the liver and excretion in the kidneys for elimination. Clinical trials showed that β-carotene bioconversion to retinol appears to be dose-dependent with a typical dosage of 15–50 mg/day [90]. Several studies on β-carotene absorption and distribution in mice have shown that β-carotene in its free form does not enter the circulatory system. It is distributed in the stomach or remains predominantly in the chyme. On the contrary, β-carotene transported via nanoemulsion is better distributed and metabolized in the liver [91]. According to some authors, lutein-free, administrated orally to male rats at a dose of 10 mg/kg body weight, reached its maximum concentration in plasma after about 2 h, and it accumulated more in the mesenteric fat deposits than in the liver [92]. The concentration in plasma is also enhanced when lutein is encapsulated in poly(lactic-co-glycolic acid) (PLGA) nanoparticles. Lycopene contained in food is partially released via chewing through mechanical and enzymatic action, then packaged in chylomicrons and transported to the lymphatic system. In the lymph, the chylomicrons are degraded, passively releasing the lycopene before it is eliminated by the liver [93][94]. Pharmacokinetic studies on healthy adult men showed that the bioavailability of astaxanthin administered at 40 mg was around four times higher for lipid-based supplements than that of commercial formulations [95]. Other studies on animal models have shown that astaxanthin administered in different formulations is more distributed in the spleen [96].
Based on the above, carotenoids’ chemical properties and the factors influencing their pharmacokinetics make it necessary to increase the bioaccessibility, stability, and solubility of carotenoids using novel delivery systems such as nanocarriers composed of biopolymers, polysaccharides, and protein- or lipid-based carriers.
References
- Grainger, E.M.; Webb, M.Z.; Simpson, C.M.; Chitchumroonchokchai, C.; Riedl, K.; Moran, N.E.; Clinton, S.K. Assessment of Dietary Carotenoid Intake and Biologic Measurement of Exposure in Humans. Methods Enzymol. 2022, 674, 255–295.
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant Carotenoids: Recent Advances and Future Perspectives. Mol. Hortic. 2022, 2, 3.
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16.
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital Roles of Carotenoids in Plants and Humans to Deteriorate Stress with Its Structure, Biosynthesis, Metabolic Engineering and Functional Aspects. Curr. Plant Biol. 2021, 26, 100203.
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313.
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants 2022, 11, 795.
- Sandmann, G. Antioxidant Protection from UV- and Light-Stress Related to Carotenoid Structures. Antioxidants 2019, 8, 219.
- Semitsoglou-Tsiapou, S.; Meador, T.B.; Peng, B.; Aluwihare, L. Photochemical (UV–Vis/H2O2) Degradation of Carotenoids: Kinetics and Molecular End Products. Chemosphere 2022, 286, 131697.
- Young, A.; Lowe, G. Carotenoids—Antioxidant Properties. Antioxidants 2018, 7, 28.
- Kamoshita, M.; Toda, E.; Osada, H.; Narimatsu, T.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Lutein Acts via Multiple Antioxidant Pathways in the Photo-Stressed Retina. Sci. Rep. 2016, 6, 30226.
- Johnson, E.J.; Mcdonald, K.; Caldarella, S.M.; Chung, H.; Troen, A.M.; Snodderly, D.M. Cognitive Findings of an Exploratory Trial of Docosahexaenoic Acid and Lutein Supplementation in Older Women. Nutr. Neurosci. 2008, 11, 75–83.
- Alabdulmunem, M. Antioxidant Effect of Lycopene on Retinal Pigment Epithelial Cell Line. Med. Res. Arch. 2022, 10, 3441.
- Zhang, P.-C.; Wu, C.-R.; Wang, Z.-L.; Wang, L.-Y.; Han, Y.; Sun, S.-L.; Li, Q.-S.; Ma, L. Effect of Lutein Supplementation on Visual Function in Nonproliferative Diabetic Retinopathy. Asia Pac. J. Clin. Nutr. 2017, 26, 406–411.
- García-Layana, A.; Recalde, S.; Hernandez, M.; Abraldes, M.J.; Nascimento, J.; Hernández-Galilea, E.; Olmedilla-Alonso, B.; Escobar-Barranco, J.J.; Zapata, M.A.; Silva, R.; et al. A Randomized Study of Nutritional Supplementation in Patients with Unilateral Wet Age-Related Macular Degeneration. Nutrients 2021, 13, 1253.
- Ali, S.S.; Ayuob, N.N.; Al Ansary, A.K.; Soluman, E.R. Antioxidants Protect against Increased Risk of Atherosclerosis Induced by Exposure to Cigarette Smoke: Histological and Biochemical Study. Saudi J. Biol. Sci. 2012, 19, 291–301.
- Sztretye, M.; Dienes, B.; Gönczi, M.; Czirják, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pintér, A. Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxid. Med. Cell. Longev. 2019, 2019, 1–14.
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26.
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms—A Comprehensive Review. Antioxidants 2020, 9, 264.
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The Role of Carotenoids in the Prevention of Human Pathologies. Biomed. Pharmacother. 2004, 58, 100–110.
- Zhang, W.; Zhao, Y.; Shi, Z.; Cong, D.; Bai, Y.-S. Lutein Inhibits Cell Growth and Activates Apoptosis via the PI3K/AKT/MTOR Signaling Pathway in A549 Human Non-Small-Cell Lung Cancer Cells. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 341–350.
- Lim, J.Y.; Wang, X.-D. Mechanistic Understanding of β-Cryptoxanthin and Lycopene in Cancer Prevention in Animal Models. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158652.
- Liao, K.-S.; Wei, C.-L.; Chen, J.-C.; Zheng, H.-Y.; Chen, W.-C.; Wu, C.-H.; Wang, T.-J.; Peng, Y.-S.; Chang, P.-Y.; Lin, Y.-W. Astaxanthin Enhances Pemetrexed-Induced Cytotoxicity by Downregulation of Thymidylate Synthase Expression in Human Lung Cancer Cells. Regul. Toxicol. Pharmacol. 2016, 81, 353–361.
- Wan, L.; Tan, H.-L.; Thomas-Ahner, J.M.; Pearl, D.K.; Erdman, J.W.; Moran, N.E.; Clinton, S.K. Dietary Tomato and Lycopene Impact Androgen Signaling- and Carcinogenesis-Related Gene Expression during Early TRAMP Prostate Carcinogenesis. Cancer Prev. Res. 2014, 7, 1228–1239.
- Alhoshani, N.M.; Al-Johani, N.S.; Alkeraishan, N.; Alarifi, S.; Alkahtani, S. Effect of lycopene as an adjuvant therapy with 5-florouracil in human colon cancer. Saudi J. Biol. Sci. 2022, 29, 103392.
- Róvero Costa, M.; Leite Garcia, J.; Cristina Vágula de Almeida Silva, C.; Junio Togneri Ferron, A.; Valentini Francisqueti-Ferron, F.; Kurokawa Hasimoto, F.; Schmitt Gregolin, C.; Henrique Salomé de Campos, D.; Roberto de Andrade, C.; dos Anjos Ferreira, A.L.; et al. Lycopene Modulates Pathophysiological Processes of Non-Alcoholic Fatty Liver Disease in Obese Rats. Antioxidants 2019, 8, 276.
- Arathi, B.P.; Raghavendra-Rao Sowmya, P.; Kuriakose, G.C.; Shilpa, S.; Shwetha, H.J.; Kumar, S.; Raju, M.; Baskaran, V.; Lakshminarayana, R. Fractionation and Characterization of Lycopene-Oxidation Products by LC-MS/MS (ESI)+: Elucidation of the Chemopreventative Potency of Oxidized Lycopene in Breast-Cancer Cell Lines. J. Agric. Food Chem. 2018, 66, 11362–11371.
- Gong, X.; Smith, J.; Swanson, H.; Rubin, L. Carotenoid Lutein Selectively Inhibits Breast Cancer Cell Growth and Potentiates the Effect of Chemotherapeutic Agents through ROS-Mediated Mechanisms. Molecules 2018, 23, 905.
- Dorgan, J.F.; Boakye, N.A.; Fears, T.R.; Schleicher, R.L.; Helsel, W.; Anderson, C.; Robinson, J.; Guin, J.D.; Lessin, S.; Ratnasinghe, L.D.; et al. Serum Carotenoids and α-Tocopherol and Risk of Nonmelanoma Skin Cancer. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1276–1282.
- Žmitek, K.; Žmitek, J.; Rogl Butina, M.; Hristov, H.; Pogačnik, T.; Pravst, I. Dietary Lutein Supplementation Protects against Ultraviolet-Radiation-Induced Erythema: Results of a Randomized Double-Blind Placebo-Controlled Study. J. Funct. Foods 2020, 75, 104265.
- Zhang, Y.-P.; Chu, R.-X.; Liu, H. Vitamin A Intake and Risk of Melanoma: A Meta-Analysis. PLoS ONE 2014, 9, e102527.
- Chen, Y.-T.; Kao, C.-J.; Huang, H.-Y.; Huang, S.-Y.; Chen, C.-Y.; Lin, Y.-S.; Wen, Z.-H.; Wang, H.-M.D. Astaxanthin Reduces MMP Expressions, Suppresses Cancer Cell Migrations, and Triggers Apoptotic Caspases of in Vitro and in Vivo Models in Melanoma. J. Funct. Foods 2017, 31, 20–31.
- Wierzbicki, A.S.; Poston, R.; Ferro, A. The Lipid and Non-Lipid Effects of Statins. Pharmacol. Ther. 2003, 99, 95–112.
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of Natural Astaxanthin Increases Serum HDL-Cholesterol and Adiponectin in Subjects with Mild Hyperlipidemia. Atherosclerosis 2010, 209, 520–523.
- Bechor, S.; Zolberg Relevy, N.; Harari, A.; Almog, T.; Kamari, Y.; Ben-Amotz, A.; Harats, D.; Shaish, A. 9-Cis β-Carotene Increased Cholesterol Efflux to HDL in Macrophages. Nutrients 2016, 8, 435.
- Mapelli-Brahm, P.; Meléndez-Martínez, A.J. The Colourless Carotenoids Phytoene and Phytofluene: Sources, Consumption, Bioavailability and Health Effects. Curr. Opin. Food Sci. 2021, 41, 201–209.
- Maghsoudi, S.; Taghavi Shahraki, B.; Rabiee, N.; Fatahi, Y.; Bagherzadeh, M.; Dinarvand, R.; Ahmadi, S.; Rabiee, M.; Tahriri, M.; Hamblin, M.R.; et al. The Colorful World of Carotenoids: A Profound Insight on Therapeutics and Recent Trends in Nano Delivery Systems. Crit. Rev. Food Sci. Nutr. 2022, 62, 3658–3697.
- Writing Group for the AREDS2 Research Group; Bonds, D.E.; Harrington, M.; Worrall, B.B.; Bertoni, A.G.; Eaton, C.B.; Hsia, J.; Robinson, J.; Clemons, T.E.; Fine, L.J.; et al. Effect of Long-Chain ω-3 Fatty Acids and Lutein + Zeaxanthin Supplements on Cardiovascular Outcomes: Results of the Age-Related Eye Disease Study 2 (AREDS2) Randomized Clinical Trial. JAMA Intern. Med. 2014, 174, 763–771.
- Han, G.-M.; Liu, P. Higher Serum Lycopene Is Associated with Reduced Prevalence of Hypertension in Overweight or Obese Adults. Eur. J. Integr. Med. 2017, 13, 34–40.
- Jonasson, L.; Wikby, A.; Olsson, A.G. Low Serum Beta-Carotene Reflects Immune Activation in Patients with Coronary Artery Disease. Nutr. Metab. Cardiovasc. Dis. 2003, 13, 120–125.
- Lindbergh, C.A.; Renzi-Hammond, L.M.; Hammond, B.R.; Terry, D.P.; Mewborn, C.M.; Puente, A.N.; Miller, L.S. Lutein and Zeaxanthin Influence Brain Function in Older Adults: A Randomized Controlled Trial. J. Int. Neuropsychol. Soc. 2018, 24, 77–90.
- Metibemu, D.S.; Ogungbe, I.V. Carotenoids in Drug Discovery and Medicine: Pathways and Molecular Targets Implicated in Human Diseases. Molecules 2022, 27, 6005.
- Huang, C.; Gan, D.; Fan, C.; Wen, C.; Li, A.; Li, Q.; Zhao, J.; Wang, Z.; Zhu, L.; Lu, D. The Secretion from Neural Stem Cells Pretreated with Lycopene Protects against Tert-Butyl Hydroperoxide-Induced Neuron Oxidative Damage. Oxid. Med. Cell Longev. 2018, 2018, 1–14.
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An Antibacterial Carotenoid. Antioxidants 2019, 8, 239.
- Grandner, M.A.; Jackson, N.; Gerstner, J.R.; Knutson, K.L. Sleep Symptoms Associated with Intake of Specific Dietary Nutrients. J. Sleep Res. 2014, 23, 22–34.
- Moia, V.M.; Leal Portilho, F.; Almeida Pádua, T.; Barbosa Corrêa, L.; Ricci-Junior, E.; Cruz Rosas, E.; Magalhaes Rebelo Alencar, L.; Savio Mendes Sinfronio, F.; Sampson, A.; Hussain Iram, S.; et al. Lycopene Used as Anti-Inflammatory Nanodrug for the Treatment of Rheumathoid Arthritis: Animal Assay, Pharmacokinetics, ABC Transporter and Tissue Deposition. Colloids Surf. B Biointerfaces 2020, 188, 110814.
- Grodstein, F. A randomized trial of beta carotene supplementation and cognitive function in men: The Physicians’ Health Study II. Arch. Intern. Med. 2007, 167, 2184–2190.
- Hammond, B.R.; Miller, L.S.; Bello, M.O.; Lindbergh, C.A.; Mewborn, C.; Renzi-Hammond, L.M. Effects of Lutein/Zeaxanthin Supplementation on the Cognitive Function of Community Dwelling Older Adults: A Randomized, Double-Masked, Placebo-Controlled Trial. Front. Aging Neurosci. 2017, 9, 254.
- Russo, C.; Ferro, Y.; Maurotti, S.; Salvati, M.A.; Mazza, E.; Pujia, R.; Terracciano, R.; Maggisano, G.; Mare, R.; Giannini, S.; et al. Lycopene and Bone: An in Vitro Investigation and a Pilot Prospective Clinical Study. J. Transl. Med. 2020, 18, 43.
- Abdel-Aal, E.-S.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary Sources of Lutein and Zeaxanthin Carotenoids and Their Role in Eye Health. Nutrients 2013, 5, 1169–1185.
- Amengual, J. Bioactive Properties of Carotenoids in Human Health. Nutrients 2019, 11, 2388.
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703.
- Kim, S.Y.; Kim, H.G.; Lee, K.Y.; Yoon, H.J.; Kim, N.J. Effects of Brewer’s Spent Grain (BSG) on Larval Growth of Mealworms, Tenebrio Molitor (Coleoptera: Tenebrionidae). Int. J. Ind. Entomol. 2016, 32, 41–48.
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, Y.; Yano, M. High Serum Carotenoids Associated with Lower Risk for Bone Loss and Osteoporosis in Post-Menopausal Japanese Female Subjects: Prospective Cohort Study. PLoS ONE 2012, 7, e52643.
- Umigai, N.; Kozai, Y.; Saito, T.; Takara, T. Effects of Paprika Carotenoid Supplementation on Bone Turnover in Postmenopausal Women: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Study. Food Nutr. Res. 2020, 64.
- Giordano, E.; Quadro, L. Lutein, Zeaxanthin and Mammalian Development: Metabolism, Functions and Implications for Health. Arch. Biochem. Biophys. 2018, 647, 33–40.
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxid. Med. Cell. Longev. 2021, 2021, 2713511.
- Polidori, M.C.; Stahl, W.; Griffiths, H.R. Nutritional Cognitive Neuroscience of Aging: Focus on Carotenoids and Cognitive Frailty. Redox Biol. 2021, 44, 101996.
- Arscott, S.A. Food Sources of Carotenoids. In Carotenoids and Human Health; Tanumihardjo, S., Ed.; Humana Press: Totowa, NJ, USA, 2013; pp. 3–19.
- Mashurabad, P.C.; Palika, R.; Jyrwa, Y.W.; Bhaskarachary, K.; Pullakhandam, R. Dietary Fat Composition, Food Matrix and Relative Polarity Modulate the Micellarization and Intestinal Uptake of Carotenoids from Vegetables and Fruits. J. Food Sci. Technol. 2017, 54, 333–341.
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae Characterization for Consolidated and New Application in Human Food, Animal Feed and Nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436.
- Jing, K.; He, S.; Chen, T.; Lu, Y.; Ng, I.-S. Enhancing Beta-Carotene Biosynthesis and Gene Transcriptional Regulation in Blakeslea Trispora with Sodium Acetate. Biochem. Eng. J. 2016, 114, 10–17.
- Luna-Flores, C.H.; Wang, A.; von Hellens, J.; Speight, R.E. Towards Commercial Levels of Astaxanthin Production in Phaffia Rhodozyma. J. Biotechnol. 2022, 350, 42–54.
- Casella, P.; Marino, T.; Iovine, A.; Larocca, V.; Balducchi, R.; Musmarra, D.; Molino, A. Optimization of Lutein Extraction from Scenedesmus Almeriensis Using Pressurized Liquid Extraction. Chem. Eng. Trans. 2021, 87, 475–480.
- Zheng, H.; Wang, Y.; Li, S.; Nagarajan, D.; Varjani, S.; Lee, D.-J.; Chang, J.-S. Recent Advances in Lutein Production from Microalgae. Renew. Sustain. Energy Rev. 2022, 153, 111795.
- Zhuang, Y.; Zhu, M.J. Recent Developments in Astaxanthin Production from Phaffia Rhodozyma and Its Applications. In Global Perspectives on Astaxanthin; Academic Press: Cambridge, MA, USA, 2021; pp. 225–251.
- Rodríguez-Sáiz, M.; de la Fuente, J.L.; Barredo, J.L. Xanthophyllomyces Dendrorhous for the Industrial Production of Astaxanthin. Appl. Microbiol. Biotechnol. 2010, 88, 645–658.
- Šimat, V.; Rathod, N.B.; Čagalj, M.; Hamed, I.; Generalić Mekinić, I. Astaxanthin from Crustaceans and Their Byproducts: A Bioactive Metabolite Candidate for Therapeutic Application. Mar. Drugs 2022, 20, 206.
- Marino, T.; Casella, P.; Sangiorgio, P.; Verardi, A.; Ferraro, A.; Hristoforou, E.; Molino, A.; Musmarra, D. Natural Beta-Carotene: A Microalgae Derivate for Nutraceutical Applications. Chem. Eng. Trans. 2020, 79, 103–108.
- Villaró, S.; Ciardi, M.; Morillas-España, A.; Sánchez-Zurano, A.; Acién-Fernández, G.; Lafarga, T. Microalgae Derived Astaxanthin: Research and Consumer Trends and Industrial Use as Food. Foods 2021, 10, 2303.
- Elbahnaswy, S.; Elshopakey, G.E. Recent Progress in Practical Applications of a Potential Carotenoid Astaxanthin in Aquaculture Industry: A Review. Fish. Physiol. Biochem. 2023; online ahead of print.
- Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Dusemund, B.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and Efficacy of a Feed Additive Consisting of Astaxanthin-rich Phaffia Rhodozyma for Salmon and Trout (Igene Biotechnology, Inc.). EFSA J. 2022, 20, 7161.
- Mussagy, C.U.; Remonatto, D.; Picheli, F.P.; Paula, A.V.; Herculano, R.D.; Santos-Ebinuma, V.C.; Farias, R.L.; Onishi, B.S.D.; Ribeiro, S.J.L.; Pereira, J.F.B.; et al. A Look into Phaffia Rhodozyma Biorefinery: From the Recovery and Fractionation of Carotenoids, Lipids and Proteins to the Sustainable Manufacturing of Biologically Active Bioplastics. Bioresour. Technol. 2022, 362, 127785.
- Bohn, T. 65 Food Chemistry, Function and Analysis No. 5 Non-Extractable Polyphenols and Carotenoids: Importance in Human Nutrition and Health; Saura-Calixto, F., Pérez-Jiménez, P., Eds.; Royal Society of Chemistry: London, UK, 2018; ISBN 9781788011068.
- Boonlao, N.; Ruktanonchai, U.R.; Anal, A.K. Enhancing bioaccessibility and bioavailability of carotenoids using emulsion-based delivery systems. Colloids Surf. B 2022, 209, 112211.
- Chacón-Ordóñez, T.; Carle, R.; Schweiggert, R. Bioaccessibility of Carotenoids from Plant and Animal Foods. J. Sci. Food Agric. 2019, 99, 3220–3239.
- Schweiggert, R.M.; Carle, R. Carotenoid Deposition in Plant And Animal Foods and Its Impact on Bioavailability. Crit. Rev. Food Sci. Nutr. 2015, 13, 1807–1830.
- Koh, S.; Loh, S. In Vitro Bioaccessibility of Â-Carotene in Pumpkin and Butternut Squash Subjected to Different Cooking Methods. Int. Food Res. J. 2018, 25, 188–195.
- Colle, I.J.P.; Van Buggenhout, S.; Lemmens, L.; Van Loey, A.M.; Hendrickx, M.E. The Type and Quantity of Lipids Present during Digestion Influence the in Vitro Bioaccessibility of Lycopene from Raw Tomato Pulp. Food Res. Int. 2012, 45, 250–255.
- Tan, Y.; Zhang, Z.; Zhou, H.; Xiao, H.; McClements, D.J. Factors Impacting Lipid Digestion and β-Carotene Bioaccessibility Assessed by Standardized Gastrointestinal Model (INFOGEST): Oil Droplet Concentration. Food Funct. 2020, 11, 7126–7137.
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability and Bioaccessibility of Food Bioactive Compounds; Overview and Assessment by In Vitro Methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2862–2884.
- Ochoa Becerra, M.; Mojica Contreras, L.; Hsieh Lo, M.; Mateos Díaz, J.; Castillo Herrera, G. Lutein as a Functional Food Ingredient: Stability and Bioavailability. J. Funct. Foods 2020, 66, 103771.
- Cervantes-Paz, B.; Ornelas-Paz, J.d.J.; Ruiz-Cruz, S.; Rios-Velasco, C.; Ibarra-Junquera, V.; Yahia, E.M.; Gardea-Béjar, A.A. Effects of Pectin on Lipid Digestion and Possible Implications for Carotenoid Bioavailability during Pre-Absorptive Stages: A Review. Food Res. Int. 2017, 99, 917–927.
- Corte-Real, J.; Bohn, T. Interaction of Divalent Minerals with Liposoluble Nutrients and Phytochemicals during Digestion and Influences on Their Bioavailability—A Review. Food Chem. 2018, 252, 285–293.
- Corte-Real, J.; Bertucci, M.; Soukoulis, C.; Desmarchelier, C.; Borel, P.; Richling, E.; Hoffmann, L.; Bohn, T. Negative Effects of Divalent Mineral Cations on the Bioaccessibility of Carotenoids from Plant Food Matrices and Related Physical Properties of Gastro-Intestinal Fluids. Food Funct. 2017, 8, 1008–1019.
- Aschoff, J.K.; Kaufmann, S.; Kalkan, O.; Neidhart, S.; Carle, R.; Schweiggert, R.M. In Vitro Bioaccessibility of Carotenoids, Flavonoids, and Vitamin C from Differently Processed Oranges and Orange Juices . J. Agric. Food Chem. 2015, 63, 578–587.
- Oghbaei, M.; Prakash, J. Antioxidant Components and Their in Vitro Bioaccessibility in Processed and Stored Chick Pea and Amaranth Greens Mix. Croat. J. Food Technol. Biotechnol. Nutr. 2015, 10, 44–49.
- Casella, P.; Iovine, A.; Mehariya, S.; Marino, T.; Musmarra, D.; Molino, A. Smart Method for Carotenoids Characterization in Haematococcus Pluvialis Red Phase and Evaluation of Astaxanthin Thermal Stability. Antioxidants 2020, 9, 422.
- Yang, C.; Zhang, H.; Liu, R.; Zhu, H.; Zhang, L.; Tsao, R. Bioaccessibility, Cellular Uptake, and Transport of Astaxanthin Isomers and Their Antioxidative Effects in Human Intestinal Epithelial Caco-2 Cells. J. Agric. Food Chem. 2017, 65, 10223–10232.
- Schwedhelm, E.; Maas, R.; Troost, R.; Böger, R.H. Clinical Pharmacokinetics of Antioxidants and Their Impact on Systemic Oxidative Stress. Clin. Pharmacokinet. 2003, 42, 437–459.
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, Pharmacology and Treatment. Br. J. Pharmacol. 2017, 174, 1290–1324.
- Chen, L.; Yokoyama, W.; Alves, P.; Tan, Y.; Pan, J.; Zhong, F. Effect of Encapsulation on β-Carotene Absorption and Metabolism in Mice. Food Hydrocoll. 2021, 121, 107009.
- Kamil, A.; Smith, D.E.; Blumberg, J.B.; Astete, C.; Sabliov, C.; Oliver Chen, C.-Y. Bioavailability and Biodistribution of Nanodelivered Lutein. Food Chem. 2016, 192, 915–923.
- Arballo, J.; Amengual, J.; Erdman, J.W. Lycopene: A Critical Review of Digestion, Absorption, Metabolism, and Excretion. Antioxidants 2021, 10, 342.
- Donoso, A.; González-Durán, J.; Muñoz, A.A.; González, P.A.; Agurto-Muñoz, C. Therapeutic Uses of Natural Astaxanthin: An Evidence-Based Review Focused on Human Clinical Trials. Pharmacol. Res. 2021, 166, 105479.
- Mercke Odeberg, J.; Lignell, Å.; Pettersson, A.; Höglund, P. Oral Bioavailability of the Antioxidant Astaxanthin in Humans Is Enhanced by Incorporation of Lipid Based Formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304.
- Singh, G.K.S.; Ismail, M.A.; Zulkefli, N.A.A.; Mohd Affandi, M.M.R.M. Tissue Distribution of Astaxanthin Formulation in Rats. Curr. Nutr. Food Sci. 2018, 14, 329–334.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
773
Revisions:
2 times
(View History)
Update Date:
27 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




