Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pan, S.; Zhu, C.; Wu, Y.; Tao, L. Chitosan-Based Self-Healing Hydrogel. Encyclopedia. Available online: https://encyclopedia.pub/entry/49615 (accessed on 08 February 2026).
Pan S, Zhu C, Wu Y, Tao L. Chitosan-Based Self-Healing Hydrogel. Encyclopedia. Available at: https://encyclopedia.pub/entry/49615. Accessed February 08, 2026.
Pan, Siyu, Chongyu Zhu, Yuwei Wu, Lei Tao. "Chitosan-Based Self-Healing Hydrogel" Encyclopedia, https://encyclopedia.pub/entry/49615 (accessed February 08, 2026).
Pan, S., Zhu, C., Wu, Y., & Tao, L. (2023, September 26). Chitosan-Based Self-Healing Hydrogel. In Encyclopedia. https://encyclopedia.pub/entry/49615
Pan, Siyu, et al. "Chitosan-Based Self-Healing Hydrogel." Encyclopedia. Web. 26 September, 2023.
Copy Citation
Biocompatible self-healing hydrogels are new-generation smart soft materials that hold great promise in biomedical fields. Chitosan-based self-healing hydrogels, mainly prepared via dynamic imine bonds, have attracted broad attention due to their mild preparation conditions, excellent biocompatibility, and self-recovery ability under a physiological environment.
chitosan
self-healing
hydrogel
fabrication
tissue engineering
1. Introduction
Hydrogels are soft materials that have 3D networks holding a high water content. Their unique structures have similarity to the extracellular matrix (ECM), and are beneficial for cell metabolism and tissue growth, demonstrating their potential in biomedical applications [1][2][3]. Inspired by the spontaneous wound repair of organisms, hydrogels with self-healing properties, known as self-healing hydrogels, have been developed over recent decades [4][5][6][7]. Upon being mechanically fractured, these hydrogels will reassemble their fragments, and restore their integrity and mechanical properties. Hence, they can be formulated into injectable form for various biomedical applications, such as cell or drug therapy. Compared to traditional two-component injectable hydrogels that require a gelling process during injection, these self-healing hydrogels can be injected after gel formation. Therefore, there are no concerns about unreacted precursors, needle blocking due to the fast gelling process, or leakage of cargoes during injection. More importantly, these self-healing hydrogels can transform into new geometric shapes to adapt to their surroundings at the injection site [8][9][10].
Chitosan is a cationic polymer obtained by partial deacetylation of chitin, which is a natural polysaccharide in fungi, the exoskeleton of crustaceans and insects [11]. Possessing excellent biocompatibility, biodegradability, antibacterial ability and cell affinity, chitosan is an ideal candidate for the fabrication of a self-healing hydrogel for biomedical applications [12]. The key to installing a spontaneous self-healing property onto chitosan-based hydrogels is to construct a dynamic physical or chemical crosslinked 3D network. Thanks to the abundant hydroxyl and amine groups on the chitosan chain, both noncovalent interactions (such as hydrogen bonding [13], electrostatic interactions [14][15], hydrophobic interactions [16], etc.) and dynamic covalent bonds (i.e., borate ester [17], acyhydrazone [18], imine [19] and disulfide bonds [20]) have been introduced to generate dynamic 3D frameworks in chitosan-based hydrogels with a self-healing property. Among these, imine bonds can be formed and are reversible under a physiological environment, leading to facile preparation, as well as broad application in biomedical fields.
2. Biomedical Applications
Self-healing hydrogels are excellent carriers for delivery of various cargoes (cells, bioactive molecules, drugs, et al.) for different requirements [21]. The self-healing hydrogels based on chitosan and aldehyde crosslinkers can achieve adjustable mechanical properties and high biocompatibility, which is important in maintaining the functions of encapsulated cargoes. Chitosan-based self-healing hydrogels with imine bonds are relatively stable in normal physiological conditions (pH~7); however, several stimuli such as changes in pH values [22][23], temperature [24][25] and presence of enzymes [26][27] can disrupt the hydrogel. This property enables controlled release of cargoes, which is valuable in drug delivery and tissue engineering applications. Moreover, chitosan stands out as a promising material for biological sensors owing to its biocompatibility and possible electrical conductivity. The injectability and self-healing ability of chitosan-based hydrogel make it an ideal material for 3D/4D printing.
2.1. Tissue Regeneration
Tissue regeneration is a complex biological process that involves the proliferation and differentiation of cells [28]. For tissue regeneration, methods are required to repair and replace the injured tissues. Cell therapy has served as a direct method to treat diseases by replacing the physiological functions of defected tissues. The key to cell therapy is how to transplant cells to the defected sites and maintain their bioactivity and functions. One possible strategy is to incorporate cells into protective biomaterials. Injectable self-healing chitosan-based hydrogels can encapsulate various cargoes (i.e., cells and bioactive molecules) and deliver them in order to repair tissues at a high concentration without impairing the bioactivity of the cargoes [29]. Thus, chitosan self-healing hydrogels are ideal candidates for tissue regeneration, such as in nerves and skin.
For better cell encapsulation, hydrogels need to be biocompatible and have a suitable gelation rate for cells to evenly distribute. Early in 2012, Yang et al. prepared a self-healing hydrogel using DF-PEG and GC to embed cells (Figure 1a) [30]. Before cell encapsulation, the biosafety of gel precursors was evaluated by cell experiments. The cell viability was greater than 80%, and even the concentration of DF-PEG reached 9.0 mg/mL. Thus, the GC/PEG hydrogel had excellent biocompatibility considering that GC is a well-known biocompatible biopolymer. After mixing cell-suspended GC solution with DF-PEG solution in a mild condition, the GC/PEG hydrogel with uniformly-distributed cells was formed, which reached ~97% cell viability after 24 h and still ~87% cell viability after 72 h (Figure 1b). These results indicated that the GC/PEG hydrogel was safe for cell growth as an ECM-like carrier and oxygen supplier. After injection via a needle, there were ~87% live cells in GC/PEG hydrogel and this remained ~85% for 24 h, indicating that most of the cells could tolerate the injection process. Thus, the chitosan-based self-healing hydrogel proved to be a potential cell carrier.

Figure 1. (a) Preparation of cells loaded in GC/PEG hydrogels under physiological conditions. (b) 3D (A,B) and z-axis maximum projection (A’,B’) views of confocal microscopy images. Cell viability (viable cells: green, dead cells: red) and spatial distribution of HeLa cells encapsulated in the hydrogels after 24 h (A) and 72 h (B). (c) Injection of cell-embedded GC/PEG hydrogel and cell proliferation behavior in the hydrogel. (d) Cell numbers in a series of hydrogels with different modulus at denoted time after injection. Data represent mean ± SD (n = 3, *: p < 0.05).
The modulus of hydrogels can affect the behavior of cell proliferation. Li et al. prepared a series of GC/PEG self-healing hydrogels with different storage moduli by simply verifying the amount of DF-PEG [31]. They further investigated the cell proliferation of GC/PEG hydrogels by injecting cell-loaded hydrogels through a syringe to a peri-dish and post-culturing them (Figure 1c). After injection and post-culture, the cell numbers in GC/PEG hydrogels with different moduli increased, indicating cell proliferation behavior in the hydrogels. Moreover, the cell number increased by 70%, 88% and 110% in the soft, medium and stiff hydrogels, which showed the significant positive correlation between the proliferation rate of the loaded cells and the moduli of the hydrogels (Figure 1d).
Besides cell proliferation, the mechanical performance of the hydrogel can influence other functions, such as cell differentiation, which is important for tissue regeneration. Neural stem cells (NSCs) are prone to glial differentiation on slightly stiffer materials (≈7–10 kPa) and neuronal differentiation in relatively soft materials (≈0.1–1 kPa) [32]. Tseng et al. used chitosan-based self-healing hydrogels (GC-PEG hydrogel) with appropriate stiffness (≈1.5 kPa) to heal the impaired central nervous system [33]. According to the RT-PCR tests, cells encapsulated in self-healing hydrogels expressed more neuronal marker genes (β-tubulin and Map2) than those on alginate hydrogel and traditional culture plates at 3 d and 7 d. The expression of β-tubulin gene (an early neuronal marker) was observed to be down-regulated from 3 d to 7 d, while the expression of Map2 gene (a marker of mature neurons) showed an up-regulation from 3 d to 7 d, particularly in the self-healing hydrogel (Figure 2a). These results indicated that the chitosan-based self-healing hydrogel provided favorable environments for the growth of neuronal cells. Zebrafish embryos after ethanol exposure were used to evaluate the rescue of the neural deficits by different treatments (Figure 2b). Several groups of damaged embryos were injected with the NSCs (dispersed cells or spheroids)-encapsulated GC-PEG hydrogels, dispersed NSC cells and GC-PEG hydrogels only. Among these, the embryos with injection of NSC spheroid-loaded self-healing hydrogels had the highest total of coiling contractions and hatching rates. This indicated that the chitosan-based self-healing hydrogels with embedded NSC spheroid might heal the damaged central nervous system and rescue the impaired nervous functions. The appropriate modulus (≈1.5 kPa) provided adequate porosity for cell metabolism and migration, as well as convenience for the location of the NSC spheroid. The authors made a point that the amino groups on the chitosan backbone might interact with NSCs to induce neuronal differentiation.

Figure 2. (a) The expressions of specific neuronal-related genes (Nestin, β-tubulin, Map2) in the TCPS, alginate hydrogel and GC-PEG self-healing hydrogel after 3 d and 7 d. (b) Treatment procedures for the central nervous system rescue in zebrafish by the GC-PEG self-healing hydrogel. The zebrafish embryos were exposed to ethanol, resulting in central nervous system deficits. Spontaneous side-to-side contraction of the tail was examined at the 18 somite stage. (c) Preparation of Hyaluronan-included GC-PEG hydrogel (CH hydrogel). (d) Schematic diagram of the zebrafish model. (e) Swimming track of adult zebrafish after the implantation of the hydrogel. * and **** each represent p < 0.05 and p < 0.0001 between the groups treated with CS and CH hydrogels (n = 9).
In order to enhance the spreading, migration, proliferation and differentiation of embedded NSCs, hyaluronan (HA) was introduced into the chitosan-based self-healing hydrogels. In the research of Liu et al. [34], the HA was incorporated into NSC-embedded self-healing hydrogels to prepare a semi-interpenetrating polymer network (SIPN) (Figure 2c). The SIPN proved to provide a denser nanostructure and a looser microstructure, which promoted the growth of embedded NSCs. The zebrafish traumatic brain injury model was employed to verify the functional recovery (Figure 2d). The swimming behavior of these zebrafish was then recorded. The zebrafish which were injected with HA-containing GC-PEG hydrogels (CH0.1) demonstrated the best functional recovery with the largest distance of forward swimming. Injection of GC-PEG hydrogels (CS in Figure 2e) alone exhibited a significant short-term effect, but its impact diminished after 4 days compared to the PBS group, with only minor effects observed (Figure 2e). These results indicated that optimization of the 3D network of chitosan-based hydrogels can probably improve tissue regeneration by changing the cell microenvironment.
Apart from encapsulating cells, chitosan-based self-healing hydrogels can also embed bioactive molecules to promote tissue regeneration, such as wound healing. Compared with traditional hydrogels, self-healing hydrogels can adapt to the shape of wounds so that the hydrogel dressing accompanying the medicines can keep complete contact with the wound for better healing. Li et al. reported this kind of self-adapting chitosan-based hydrogel (Figure 3a) [35]. This hydrogel exhibited excellent biocompatibility, self-healing ability and tissue adhesive ability. Moreover, the chitosan-based hydrogel had an adequate degradation rate and thrombin release rate in vitro, which was suitable for long-lasting treatment of wound healing. In in vivo wound-healing tests, a cruciate incision was made using a surgical scalpel on the right lobe of a rat liver, resulting in a greater VI liver laceration (Figure 3b). Then, different treatments were employed including chitosan-based hydrogel containing thrombin (CPT-2.50), thrombin aqueous solution (Thr·H2O), Plurionic (F127) hydrogel containing thrombin (FT-hydrogel) and chitosan-based hydrogel without thrombin (CP-2.50). During 7 days observation, the damaged liver under the treatment of thrombin-loaded chitosan-based hydrogel was completely healed with excellent tissue regeneration (Figure 3(bA,B)). There was no obvious inflammatory cell infiltration in the regenerated tissue (Figure 3(bB′)) in histological analyses. However, scars on the liver of the other groups were visible (Figure 3(bC1–C4)). Inflammatory cells could still be seen (Figure 3(bC1′–C4′)) and the Thr·H2O treated group showed relatively slight infection. These results indicated that chitosan-based self-healing hydrogel was an ideal candidate as a carrier for wound-healing because of its physical adhesion to the wound and by using the natural anti-inflammatory chitosan as a gelator. The thrombin can perform better as a bioactive factor when encapsulated in the chitosan-based self-healing hydrogel than in direct use.

Figure 3. (a) Schematic illustration of self-adapting chitosan-based hydrogel. (b) Photographs and microscopic evaluation of rat liver laceration with different treatments. (A) Cruciate incision wound; wound-healing after 7 days with treatment by (B,B′) CPT hydrogel; (C1,C1′) no treatment; (C2,C2′) thrombin aqueous solution (Thr·H2O); (C3,C3′) plurionic hydrogel containing thrombin (FT-hydrogel); (C4,C4′) chitosan-based hydrogel without thrombin (CP-2.50) (n = 6).
Several bioactive molecules exhibit poor stability, which inhibit their biological activity in the body. For example, curcumin is a kind of bioactive molecule with antimicrobial, anti-inflammatory and antioxidant abilities, but its poor water solubility leads to low bioavailability [36]. Encapsulating it in hydrogel can prolong its metabolism and further promote its bioavailability. Qu et al. reported a curcumin-loaded chitosan-based self-healing hydrogel which showed excellent antibacterial ability as wound dressing for joints and skin wound healing [37]. Platelet-rich plasma (PRP) which contains various bioactive molecules, such as exosomes, nerve growth factor, and platelet-derived growth factor, can promote wound healing [38]. However, the rapid degradation of these growth factors limits their application in the treatment of chronic wounds. To deal with this, Qian et al. reported a PRP-loaded chitosan-based self-healing hydrogel (CBPGCTS) with the addition of biocompatible and enzymatic hydrolysis-resistant silk fibroin (SF) (CBPGCTS-SF@PRP) (Figure 4a) [39]. Tests of enzymatic degradation in vitro and angiogenesis in vivo were carried out. The bioactive molecules, such as PDGF-BB, NGF and EVs, from PRP gels were monitored in enzyme solutions at 0, 24, 48, and 72 h. The expression of PDGF-BB and NGF in PRP gel proved negative within 24 h and EVs could be barely seen after 48 h, which indicated that bioactive molecules in PRP gels degraded within 48 h (Figure 4b, left). However, the expression of PDGF-BB, NGF and EVs in CBPGCTS-SF@PRP proved positive during 72 h, which suggested that the chitosan-based self-healing hydrogel prolonged the bioactivity of these molecules (Figure 4b, right). Moreover, the histological sections of the PRP gel-implanted group exhibited fewer blood vessels in 7 d and 14 d than the CBPGCTS-SF@PRP group (Figure 4c,d). Quantitative data agreed well with the qualitative results (Figure 4e,f). Therefore, chitosan-based self-healing hydrogel can promote the bioactivity of growth factors and controlled release, making it suitable for chronic diabetic wound healing.

Figure 4. (a) Fabrication of PRP-loaded chitosan-based self-healing hydrogel (CBPGCTS) with the addition of biocompatible and enzymatic hydrolysis-resistant silk fibroin (SF) (CBPGCTS-SF@PRP). (b) PDGF-BB, NGF, and EVs contents in the PRP gel (left) and CBPGCTS−SF@PRP (right) during degradation by enzymes at 0, 24, 48, and 72 h. (c,d) Angiogenesis induced by (c) PRP gel and (d) CBPGCTS−SF@PRP implantation into subcutaneous tissue and muscle at 7 d and 14 d. (e,f) Quantitative data of angiogenesis per field in the PRP gel and CBPGCTS−SF@PRP groups at (e) 7 d and (f) 14 d (** p < 0.01).
Smart release of bioactive molecules needs more than physical encapsulation. The dynamic network provides free amino/aldehyde groups, allowing other dynamic connections between additive bioactive molecules and hydrogel networks. Therefore, the bioactive molecules can be controllably released due to stimuli responsive interactions. Huang et al. reported a tobramycin (TOB) smart release self-healing hydrogel for burn wound healing [40]. One of the gel precursors was quaternized chitosan (QCS) with the ability to resist drug-resistant bacteria. Oxidized dextran (OD) with aldehyde groups was selected to form a self-healing hydrogel with QCS via imine bonds. TOB, known as an aminoglycoside antibiotic with rich amino groups, was introduced to the OCS/OD hydrogel through imine bonds to realize the sustained release of TOB. Polydopamine-coated poly-pyrrole (PPY@PDA) nanowires were incorporated to enhance the wound-healing ability of the hydrogel (Figure 5a). The versatility of gel precursors and the tolerance of hydrogels to the embedding of various functional ingredients promote the development of chitosan-based self-healing hydrogels for wound healing. In the agar diffusion test, both QCS/OD/TOB (1 in Figure 5b) and QCS/OD/TOB/PPY@PDA (2 in Figure 5b) exhibited great antibacterial activity for pseudomonas aeruginosa (PA). The release behavior of TOB in vitro was also evaluated. A larger amount of TOB was released in a slightly acidic microenvironment (PBS, pH = 5.5) compared with that in a physiological microenvironment (PBS, pH = 7.4), because imine bonds were fragile to acid. Despite the continuous release of TPB in the physiological microenvironment over 9 days, the amount of TOB release was only 31% (55% in the slightly acidic microenvironment); the slow release of TOB in QCS/OD/TOB/PPY@PDA hydrogels could be attributed to the cross-linking reaction between TOB and OD in the hydrogels (Figure 5c). Then, the wound healing test in vivo using a PA-infected burn wound model was investigated. The QCS/OD/TOB/PPY@PDA hydrogel showed better effect of wound contraction (Figure 5d,e) and a higher amount of collagen deposition (Figure 5f) than the other groups, which indicated excellent ability in promoting the healing of infected burn wounds.
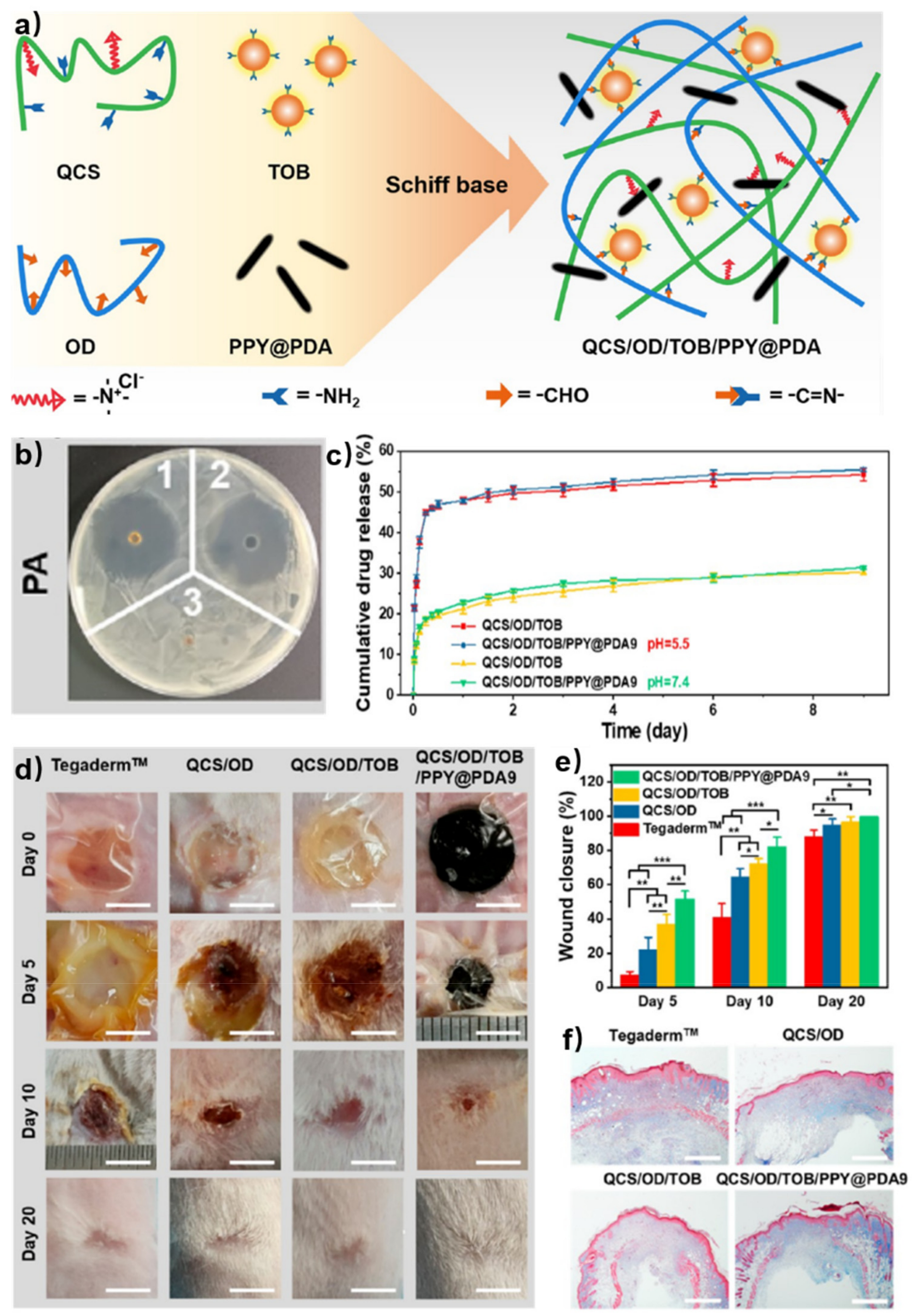
Figure 5. (a) Schematic illustration of self-healing hydrogels based on QCS and OD. (b) Inhibition zone diameters of PA. (1 for QCS/OD/TOB, 2 for QCS/OD/TOB/PPY@PDA9, and 3 for QCS/OD). (c) The release behavior of in QCS/OD/TOB hydrogel and QCS/OD/TOB/PPY@PDA9 hydrogel at pH = 7.4 and pH = 5.5. (d) Wound photographs at 0, 5, 10, 20 d for different treatments. Scale bar: 5 mm. (e) Wound contraction at 5, 10, 20 d after treatments of different hydrogels. *, **, and *** each represent p < 0.05, p < 0.01 and p < 0.001. (f) The collagen deposition at 10 d after treatments of different hydrogels. Scale bar: 800 μm.
2.2. Customized Drug Delivery
Customized drug delivery has been widely used in cancer therapy. Chemotherapy is considered as the primary choice for cancer treatment, where chemotherapy drugs are delivered to the whole body through the blood circulation. However, the lack of drug concentration in lesion sites after circulation needs continuous drug uptake; most chemotherapy drugs carry the risks of both short-term and long-term toxic effects, which cause pain to patients [41]. Chitosan-based self-healing hydrogels are excellent candidates for drug delivery due to the above-mentioned bioactivity of chitosan [42]. Injectability and self-healing ability allow for targeted drug delivery with high concentration, leading to improved utilization efficiency and decreased toxicity to normal tissues.
Taxol is a well-known natural antitumor drug with poor water solubility [43][44]. Yang et al. encapsulated Taxol into the chitosan-based self-healing hydrogel formed by DF-PEG and GC [45]. The drug-loaded hydrogel was then injected to in vivo intra-tumors of nude mice (Figure 6a). A series of images of the tumor-bearing mice showed that the tumor volumes of the mice under treatment with Taxol solution (TAX·H2O) and Taxol-embedded Pluronic F127 hydrogel (TAX·F127) were smaller than those in blank groups. This indicated that Taxol had distinct antitumor ability while F127 hydrogel did not improve the therapy efficacy. However, the mice under treatment with Taxol-loaded chitosan-based self-healing hydrogel (TAX·CP) showed the smallest tumor volume among all groups, demonstrating that the CP hydrogel not only realized targeted drug delivery, but also promoted the antitumor efficacy of Taxol (Figure 6b,d). The quantitative data agreed well with the qualitative results (Figure 6c). Doxorubicin (DOX) is another antitumor drug with a broad spectrum. However, DOX has high cardiotoxicity and drug resistance [46]. In order to diminish the toxicity of DOX and improve therapy efficacy, Qu et al. reported a DOX-loaded chitosan-based self-healing hydrogel which exhibited in vitro pH-responsive gel degradation and DOX release [47]. These results suggested that chitosan-based self-healing hydrogel had great potential to be a drug carrier for hepatocellular carcinoma therapy.

Figure 6. (a) Schematic illustration of the intra-tumor injection of Taxol-loaded chitosan-based hydrogel injection (TAX·CP). (b) Pictures of nude mice from different groups. (c) Tumor volume of mice under different treatments. (d) Tumors of the four groups on the 18th day.
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121.
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267.
- Li, J.; Mo, L.; Lu, C.-H.; Fu, T.; Yang, H.-H.; Tan, W. Functional nucleic acid-based hydrogels for bioanalytical and biomedical applications. Chem. Soc. Rev. 2016, 45, 1410–1431.
- Taylor, D.L.; In Het Panhuis, M. Self-Healing Hydrogels. Adv. Mater. 2016, 28, 9060–9093.
- Deng, C.C.; Brooks, W.L.A.; Abboud, K.A.; Sumerlin, B.S. Boronic Acid-Based Hydrogels Undergo Self-Healing at Neutral and Acidic pH. ACS Macro Lett. 2015, 4, 220–224.
- Li, C.; Guo, H.; Wu, Z.; Wang, P.; Zhang, D.; Sun, Y. Self-Healable Triboelectric Nanogenerators: Marriage between Self-Healing Polymer Chemistry and Triboelectric Devices. Adv. Funct. Mater. 2022, 33, 2208372.
- Wang, C.; Liu, Y.; Qu, X.; Shi, B.; Zheng, Q.; Lin, X.; Chao, S.; Wang, C.; Zhou, J.; Sun, Y.; et al. Ultra-Stretchable and Fast Self-Healing Ionic Hydrogel in Cryogenic Environments for Artificial Nerve Fiber. Adv. Mater. 2022, 34, 2105416.
- Gupta, D.; Tator, C.H.; Shoichet, M.S. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials 2006, 27, 2370–2379.
- Ko, D.Y.; Shinde, U.P.; Yeon, B.; Jeong, B. Recent progress of in situ formed gels for biomedical applications. Prog. Polym. Sci. 2013, 38, 672–701.
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481.
- Arteche Pujana, M.; Perez-Alvarez, L.; Cesteros Iturbe, L.C.; Katime, I. Biodegradable chitosan nanogels crosslinked with genipin. Carbohydr. Polym. 2013, 94, 836–842.
- Pella, M.C.G.; Lima-Tenorio, M.K.; Tenorio-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245.
- Baghaie, S.; Khorasani, M.T.; Zarrabi, A.; Moshtaghian, J. Wound healing properties of PVA/starch/chitosan hydrogel membranes with nano Zinc oxide as antibacterial wound dressing material. J. Biomater. Sci. Polym. Ed. 2017, 28, 2220–2241.
- Zhang, L.; Ma, Y.; Pan, X.; Chen, S.; Zhuang, H.; Wang, S. A composite hydrogel of chitosan/heparin/poly (gamma-glutamic acid) loaded with superoxide dismutase for wound healing. Carbohydr. Polym. 2018, 180, 168–174.
- Zhang, J.; Chen, L.; Shen, B.; Wang, Y.; Peng, P.; Tang, F.; Feng, J. Highly transparent, self-healing, injectable and self-adhesive chitosan/polyzwitterion-based double network hydrogel for potential 3D printing wearable strain sensor. Mater. Sci. Eng. C 2020, 117, 111298.
- Hao, X.; Liu, H.; Xie, Y.; Fang, C.; Yang, H. Thermal-responsive self-healing hydrogel based on hydrophobically modified chitosan and vesicle. Colloid. Polym. Sci. 2013, 291, 1749–1758.
- Liu, Y.; Mao, J.; Guo, Z.; Hu, Y.; Wang, S. Polyvinyl alcohol/carboxymethyl chitosan hydrogel loaded with silver nanoparticles exhibited antibacterial and self-healing properties. Int. J. Biol. Macromol. 2022, 220, 211–222.
- Wei, Z.; Yang, J.H.; Liu, Z.Q.; Xu, F.; Zhou, J.X.; Zrínyi, M.; Osada, Y.; Chen, Y.M. Novel Biocompatible Polysaccharide-Based Self-Healing Hydrogel. Adv. Funct. Mater. 2015, 25, 1352–1359.
- Nisar, S.; Pandit, A.H.; Wang, L.-F.; Rattan, S. Strategy to design a smart photocleavable and pH sensitive chitosan based hydrogel through a novel crosslinker: A potential vehicle for controlled drug delivery. RSC Adv. 2020, 10, 14694–14704.
- Fu, B.; Wang, X.; Chen, Z.; Jiang, N.; Guo, Z.; Zhang, Y.; Zhang, S.; Liu, X.; Liu, L. Improved myocardial performance in infarcted rat heart by injection of disulfide-cross-linked chitosan hydrogels loaded with basic fibroblast growth factor. J. Mater. Chem. B 2022, 10, 656–665.
- Yang, Y.; Xu, L.; Wang, J.; Meng, Q.; Zhong, S.; Gao, Y.; Cui, X. Recent advances in polysaccharide-based self-healing hydrogels for biomedical applications. Carbohydr. Polym. 2022, 283, 119161.
- Liang, Y.; Li, M.; Yang, Y.; Qiao, L.; Xu, H.; Guo, B. pH/Glucose Dual Responsive Metformin Release Hydrogel Dressings with Adhesion and Self-Healing via Dual-Dynamic Bonding for Athletic Diabetic Foot Wound Healing. ACS Nano 2022, 16, 3194–3207.
- Gao, J.; Xu, Y.; Zheng, Y.; Wang, X.; Li, S.; Yan, G.; Wang, J.; Tang, R. pH-sensitive carboxymethyl chitosan hydrogels via acid-labile ortho ester linkage as an implantable drug delivery system. Carbohydr. Polym. 2019, 225, 115237.
- Do, N.H.N.; Truong, Q.T.; Le, P.K.; Ha, A.C. Recent developments in chitosan hydrogels carrying natural bioactive compounds. Carbohydr. Polym. 2022, 294, 119726.
- Li, Z.; Shim, H.; Cho, M.O.; Cho, I.S.; Lee, J.H.; Kang, S.-W.; Kwon, B.; Huh, K.M. Thermo-sensitive injectable glycol chitosan-based hydrogel for treatment of degenerative disc disease. Carbohydr. Polym. 2018, 184, 342–353.
- Zhang, Y.; Tao, L.; Li, S.; Wei, Y. Synthesis of Multiresponsive and Dynamic Chitosan-Based Hydrogels for Controlled Release of Bioactive Molecules. Biomacromolecules 2011, 12, 2894–2901.
- Wang, T.; Chen, L.; Shen, T.; Wu, D. Preparation and properties of a novel thermo-sensitive hydrogel based on chitosan/hydroxypropyl methylcellulose/glycerol. Int. J. Biol. Macromol. 2016, 93, 775–782.
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020, 5, 686–705.
- Feng, Q.; Wei, K.; Lin, S.; Xu, Z.; Sun, Y.; Shi, P.; Li, G.; Bian, L. Mechanically resilient, injectable, and bioadhesive supramolecular gelatin hydrogels crosslinked by weak host-guest interactions assist cell infiltration and in situ tissue regeneration. Biomaterials 2016, 101, 217–228.
- Yang, B.; Zhang, Y.; Zhang, X.; Tao, L.; Li, S.; Wei, Y. Facilely prepared inexpensive and biocompatible self-healing hydrogel: A new injectable cell therapy carrier. Polym. Chem. 2012, 3, 3235–3238.
- Li, Y.; Zhang, Y.; Shi, F.; Tao, L.; Wei, Y.; Wang, X. Modulus-regulated 3D-cell proliferation in an injectable self-healing hydrogel. Colloids Surf. B. Biointerfaces 2017, 149, 168–173.
- Leipzig, N.D.; Shoichet, M.S. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 2009, 30, 6867–6878.
- Tseng, T.C.; Tao, L.; Hsieh, F.Y.; Wei, Y.; Chiu, I.M.; Hsu, S.H. An Injectable, Self-Healing Hydrogel to Repair the Central Nervous System. Adv. Mater. 2015, 27, 3518–3524.
- Liu, Y.; Hsu, Y.-H.; Huang, A.P.-H.; Hsu, S.-h. Semi-Interpenetrating Polymer Network of Hyaluronan and Chitosan Self-Healing Hydrogels for Central Nervous System Repair. ACS Appl. Mater. Interfaces 2020, 12, 40108–40120.
- Li, Y.; Wang, X.; Fu, Y.-n.; Wei, Y.; Zhao, L.; Tao, L. Self-Adapting Hydrogel to Improve the Therapeutic Effect in Wound-Healing. ACS Appl. Mater. Interfaces 2018, 10, 26046–26055.
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: The Golden Pigment from Golden Spice. Cancer Res. Treat. 2014, 46, 2–18.
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199.
- Deng, W.; Boey, J.; Chen, B.; Byun, S.; Lew, E.; Liang, Z.; Armstrong, D.G. Platelet-rich plasma, bilayered acellular matrix grafting and negative pressure wound therapy in diabetic foot infection. J. Wound Care 2016, 25, 393–397.
- Qian, Z.; Wang, H.; Bai, Y.; Wang, Y.; Tao, L.; Wei, Y.; Fan, Y.; Guo, X.; Liu, H. Improving Chronic Diabetic Wound Healing through an Injectable and Self-Healing Hydrogel with Platelet-Rich Plasma Release. ACS Appl. Mater. Interfaces 2020, 12, 55659–55674.
- Huang, Y.; Mu, L.; Zhao, X.; Han, Y.; Guo, B. Bacterial Growth-Induced Tobramycin Smart Release Self-Healing Hydrogel for Pseudomonas aeruginosa-Infected Burn Wound Healing. ACS Nano 2022, 16, 13022–13036.
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760.
- Norouzi, M.; Nazari, B.; Miller, D.W. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discovery Today 2016, 21, 1835–1849.
- Sofias, A.M.; Dunne, M.; Storm, G.; Allen, C. The battle of “nano” paclitaxel. Adv. Drug Del. Rev. 2017, 122, 20–30.
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681.
- Yang, L.; Li, Y.; Gou, Y.; Wang, X.; Zhao, X.; Tao, L. Improving tumor chemotherapy effect using an injectable self-healing hydrogel as drug carrier. Polym. Chem. 2017, 8, 5071–5076.
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Naga Prasad, S.V.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630.
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168–180.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
956
Revisions:
2 times
(View History)
Update Date:
26 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




