Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | De-Cai Xiong | -- | 2983 | 2023-09-25 13:05:13 | | | |
| 2 | Rita Xu | -16 word(s) | 2967 | 2023-09-26 03:43:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wei, X.; Wang, P.; Liu, F.; Ye, X.; Xiong, D. Fluorine-Containing Glycomimetics. Encyclopedia. Available online: https://encyclopedia.pub/entry/49599 (accessed on 08 February 2026).
Wei X, Wang P, Liu F, Ye X, Xiong D. Fluorine-Containing Glycomimetics. Encyclopedia. Available at: https://encyclopedia.pub/entry/49599. Accessed February 08, 2026.
Wei, Xingxing, Pengyu Wang, Fen Liu, Xinshan Ye, Decai Xiong. "Fluorine-Containing Glycomimetics" Encyclopedia, https://encyclopedia.pub/entry/49599 (accessed February 08, 2026).
Wei, X., Wang, P., Liu, F., Ye, X., & Xiong, D. (2023, September 25). Fluorine-Containing Glycomimetics. In Encyclopedia. https://encyclopedia.pub/entry/49599
Wei, Xingxing, et al. "Fluorine-Containing Glycomimetics." Encyclopedia. Web. 25 September, 2023.
Copy Citation
Glycomimetics, which are synthetic molecules designed to mimic the structures and functions of natural carbohydrates, have been developed to overcome the limitations associated with natural carbohydrates. The fluorination of carbohydrates has emerged as a promising solution to dramatically enhance the metabolic stability, bioavailability, and protein-binding affinity of natural carbohydrates.
glycomimetics
fluorination
synthesis
1. Introduction
Carbohydrates play major structural, physical, and biological roles in organisms. They often form complex and diverse glycans on the cell surface by interacting with protein and lipid scaffolds. The glycan molecules bind to proteins and actively participate in various biological processes, such as embryogenesis, adhesion, immunity, inflammation, cancer metastasis, and host–pathogen interactions [1][2][3]. The composition of glycans changes during cell differentiation and tissue development. Disease states and the degree of inflammation also influence the compositional changes. These changes can be attributed to the altered degree of the expression of glycosidase and/or glycosyltransferase (GT) in cells. The generation of this dynamic expression is an integral part of the cell-to-cell communication process, and it can potentially provide new targets for disease treatment. Carbohydrate–protein binding is achieved by exploiting low-energy interactions (such as hydrogen bonding, salt bridges, and metal chelation), which cannot compensate for the high enthalpy cost of the desolvation of polar substrates and shallow protein-binding sites. The high polar surface area of natural carbohydrates hinders the passive penetration of the molecules into the intestinal membrane, making them non-bioavailable orally. It has also been observed that natural carbohydrates exhibit inherently poor pharmacokinetic properties, limiting their therapeutic potential [4][5].
Natural carbohydrates can be modified to improve their drug-like properties, and “carbohydrate-like compounds” can be designed to mimic the structure and function of natural carbohydrates to improve their affinity, pharmacokinetic properties, and bioavailability [6][7]. Fluorine, which exhibits unique properties, has been widely used in drug design and development. Its high electronegativity makes it a powerful tool for modulating the pKa and electron density of the groups present in its proximity, and it can serve as a considerable element to control molecular conformation [8][9][10]. Pharmaceuticals containing fluorine exhibit the property of tunable lipophilicity and increased metabolic stability. It is noteworthy that the oxidative metabolism of these molecules can be prevented. The strategy of the fluorination of carbohydrates has long been used to investigate protein-carbohydrate and carbohydrate-carbohydrate interactions. This method has been used to investigate the contributions of individual sugar alcohol groups [11][12][13] or design mechanism-based inhibitors [14][15]. The excellent NMR (nuclear magnetic resonance) properties of the 19F nucleus have been analyzed to study protein-carbohydrate binding at the molecular level [16][17][18][19][20][21][22][23][24]. The method of fluoridation is also used in drug discovery programs and to develop synthetic carbohydrate vaccines [25][26][27][28][29][30]. Fluorination could optimize the physicochemical properties, absorption ability, and distribution properties of natural sugars, and the introduction of fluorine can also help improve the binding affinity and pharmacokinetic properties of natural sugars [31][32].
2. Synthesis of Fluorine-Containing Glycomimetics
In essence, the fluorine-based carbohydrate modification method is followed to substitute various atoms on the molecular skeleton with fluorine atoms or fluorine-containing groups such as polyfluorene. The fluorinating reagents used for fluorination include deoxyfluorination agents: DAST (diethylaminosulfur trifluoride, 1) [33][34]; nucleophilic fluorination agents: TASF (tris(dimethylamino)sulfonium difluorotrimethylsilicate, 2) [35]; TBAF (tertbutylammonium fluoride, 3) [36]; MFn (Metal fluorides, 4) [37]; KHF2 (5) [38]; anhydrous hydrogen fluoride system (6) [39]; electrophilic fluorination agents: SelectFluor (1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate), also abbreviated as F-TEDA-BF4, 7) [40]; NFSI (N-fluorobenzenesulfonimide, 8) [41]; etc. (Figure 1). With the use of these fluorinating reagents above, the reactions for fluorination of sugars could be divided into nucleophilic reactions, electrophilic addition reactions, radical reactions, metal-catalyzed reactions, and de novo synthesis with fluorine-containing blocks.
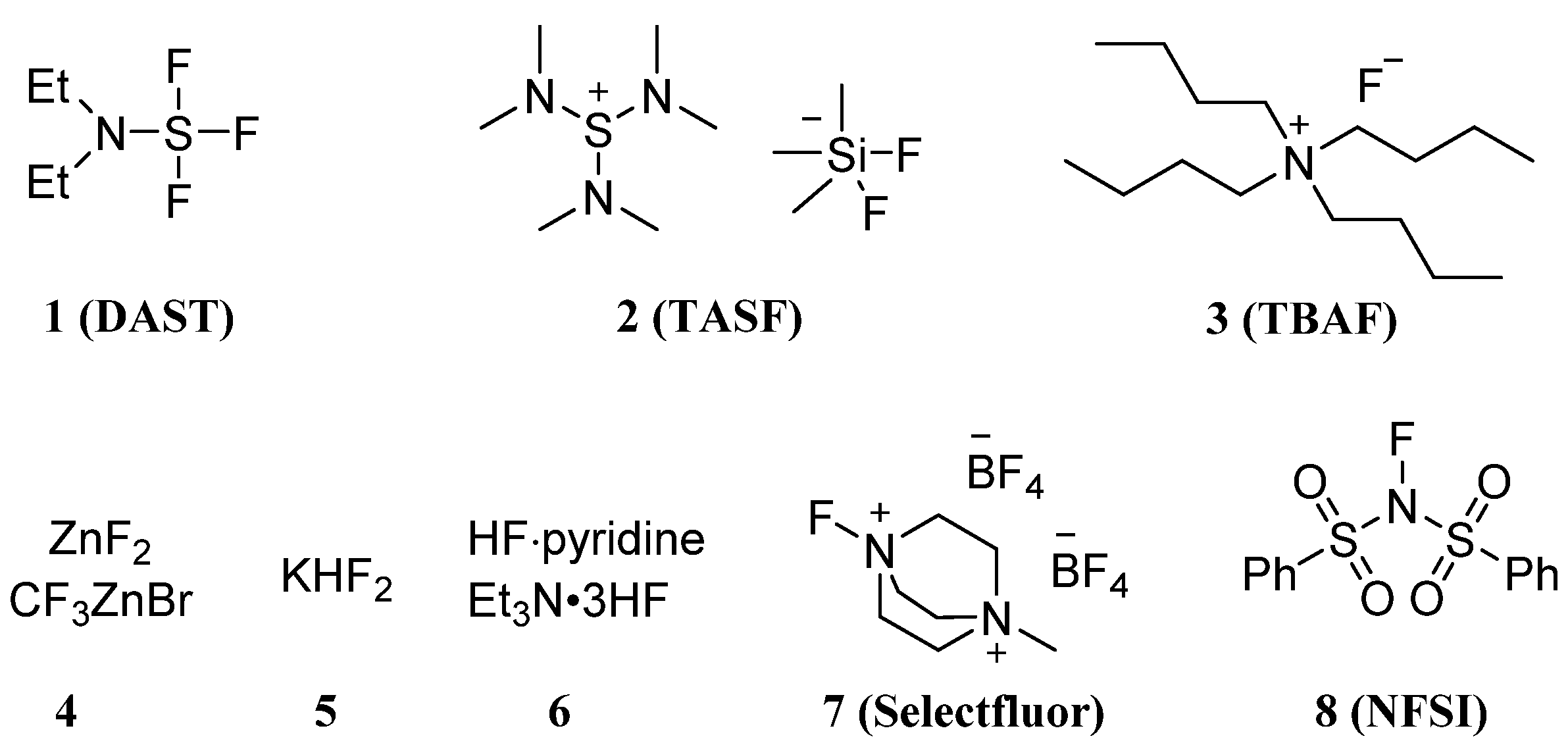
Figure 1. Typical fluorinating reagents: 1, DAST (diethylaminosulfur trifluoride); 2, TASF ((tris(dimethylamino)sulfonium difluorotrimethylsilicate), 3, TBAF (tertbutylammonium fluoride); 4, Metal fluorides; 5, KHF2; 6, anhydrous hydrogen fluoride system; 7, SelectFluor, (1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) and 8, NFSI (N-fluorobenzenesulfonimide).
2.1. Fluorination of Carbohydrates via Nucleophilic Fluorination
A large number of fluorine-containing carbohydrates are synthesized via nucleophilic reactions with fluoride ions. Depending on the reaction condition, fluoride ions can act as good nucleophilic reagents in polar non-protonic solvents [42]. Currently, fluorinated reagents such as DAST (diethylaminosulfur trifluoride), TASF (tris(dimethylamino)sulfonium difluorotrimethylsilicate), KHF2, etc., are mainly used to provide nucleophilic fluorine atoms, which subsequently replace the hydroxyl or sulfonate groups on the sugar ring to obtain fluorinated sugars. These are the most popular methods for the preparation of fluorinated carbohydrates [43][44][45][46] (Figure 2a–c). The fluorination of the equatorial hydroxyl group obtained 10 with fluorine nucleophilic substituted in the axial position, which undergoes an SN2 reaction mechanism. The stereo-configuration of the fluorination product is generally flipped from the starting material.
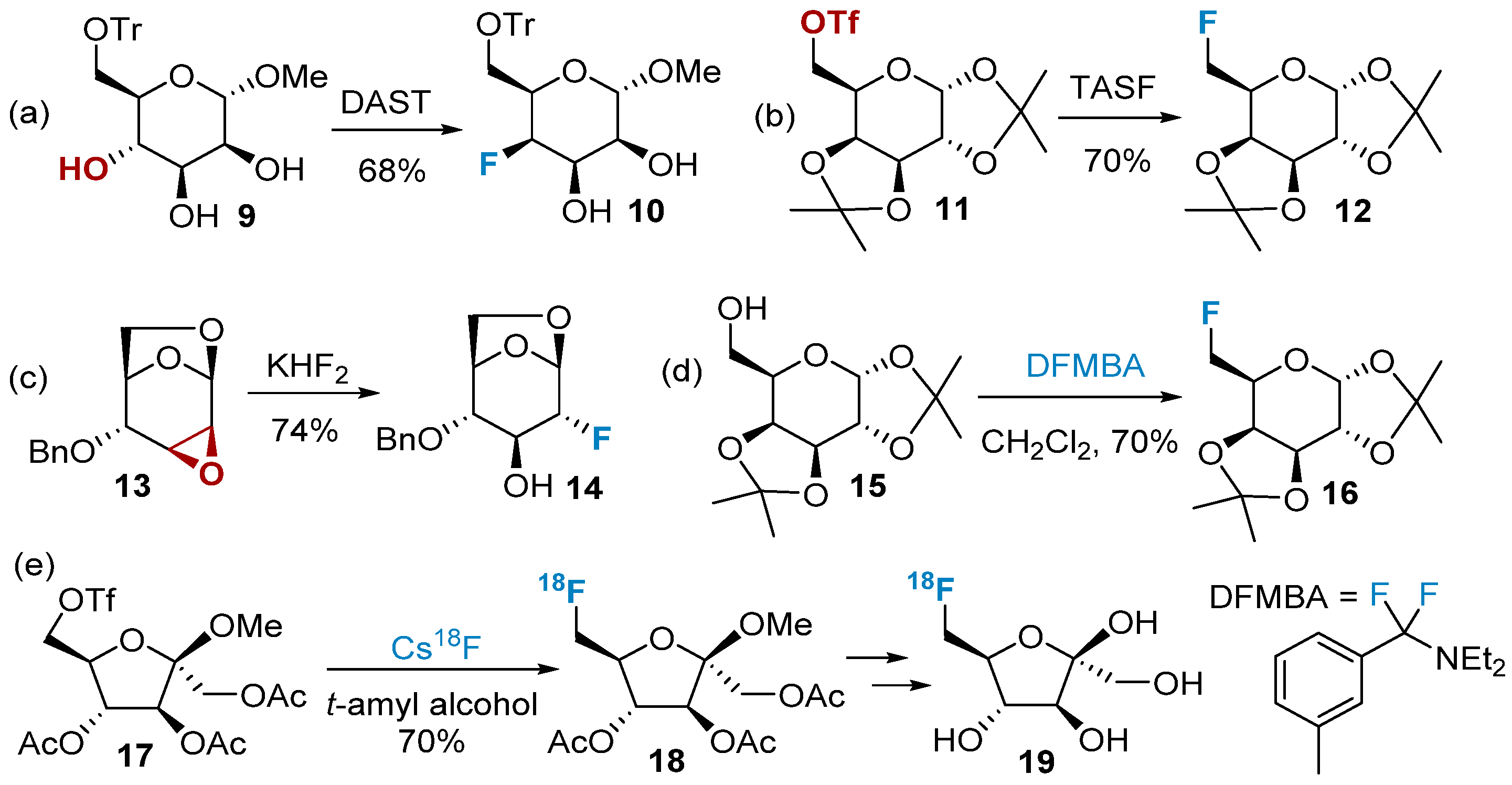
Figure 2. Nucleophilic fluorination of carbohydrates using different fluorinating reagents: (a) DAST as fluorinated reagents, (b) TASF as fluorinated reagents, (c) KHF2 as fluorinated reagents, (d) DFMBA as fluorinated reagents, (e) Cs18F as fluorinated reagents.
While reagents like DAST and TASF have been traditionally used in fluorination reactions, researchers have encountered certain drawbacks associated with their use. For example, protecting group migration can be a significant issue in some fluorination reactions, leading to reduced yields or even the absence of the desired target product [47]. DFMBA is considered milder compared to reagents like DAST and exhibits improved compatibility with various protecting groups [48][49], such as acetyl, methyl, silyl, benzyl, etc. (Figure 2d). In addition to the above-mentioned organic fluorinating agents, other inorganic fluorine-containing compounds, such as hydrogen fluoride, TBAF, CsF, AgF, iodine–fluorine compounds, etc., can also provide nucleophilic fluoride ions [45]. Cheeseman’s group successfully synthesized fluorinated furanose 18 using CsF as the fluorinating agent. This method allowed for the introduction of fluorine-18 into the substrate. After deprotection, the resulting product 19 was found to be suitable as a contrast agent for breast cancer (Figure 2e).
Polyfluorinated carbohydrates have gained attention due to their unique properties and potential applications in medicinal chemistry. The positional combinations for deoxy polyfluorination of pentoses and hexoses have been extensively studied in the context of nucleophilic fluorination reactions. Epoxide opening with fluoride sources is a versatile method for introducing fluorine in a regioselective manner. Thus, 2,3,4-trideoxy-2,3,4-trifluoro-D-glucopyranose (24) can be synthesized selectively, utilizing the regioselective opening of the epoxide, displacement of the triflate intermediate using Et3N·3HF, and subsequent nucleophilic fluorination with DAST (Figure 3). The synthesis of polyfluorinated carbohydrates, including ketosugars (such as sialic acids), aminosugars, and nucleosides, with controlled fluorine introduction at specific positions has garnered significant attention in recent years. This area of research has been recently reviewed [50].
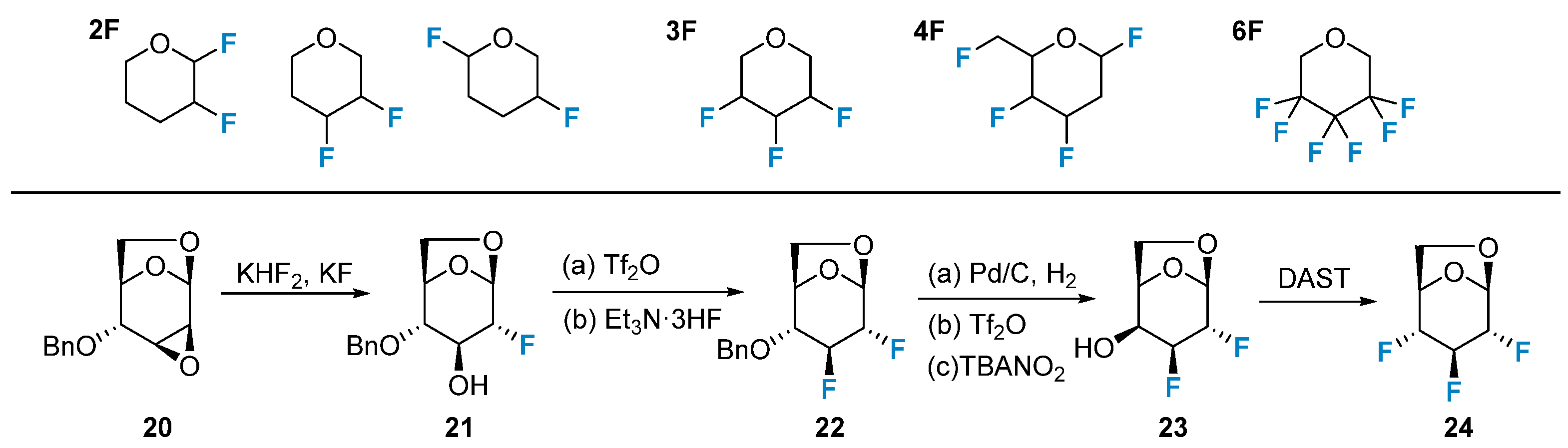
Figure 3. Polyfluorination of carbohydrates via iterative nucleophilic reactions and the synthesis of 2,3,4-trifluoro-glucopyranose.
The nucleophilic reaction of carbonyl-containing glycans with difluoroalkylation reagents produces difluorinated modification of glycans. This type of reaction was first reported by Bobek et al. in 1977 [51]. They utilized a DAST reagent to modify furanose 25 by introducing a difluoromethyl group, resulting in the formation of product 26 (Figure 4a). However, the yield of this reaction was low, and its applicability was limited, particularly for pyranose. In 2009, Jiménez-Barbero et al. [52] successfully synthesized difluorine-modified carboglycosides 28 using the Deoxo-Fluor reagent (Figure 4b). CBr2F2 is another commonly used reagent for difluorination, often in conjunction with additional phosphorus-containing small molecules to generate the active intermediate carbene. Slawin’s group [53] employed this method to successfully synthesize compound 30 (Figure 4c), which, upon reduction, produced the extracyclic difluoromethyl glycosides 31.
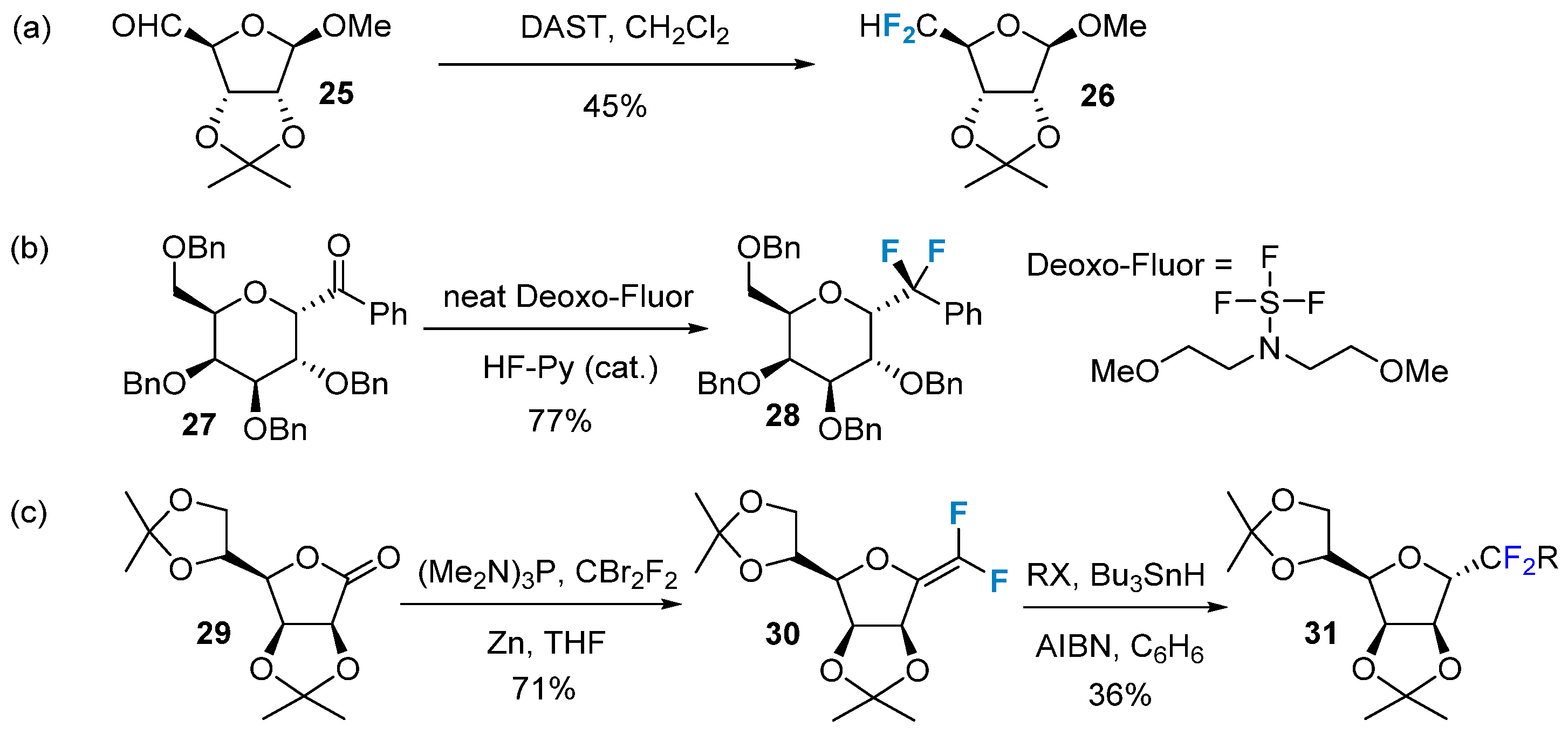
Figure 4. Difluorination of carbohydrates via nucleophilic reactions. (a) nucleophilic difluoroalkylation via DAST, (b) nucleophilic difluoroalkylation via Deoxo-Fluor, (c) nucleophilic difluoroalkylation via CBr2F2.
In recent years, newly developed difluoroalkylation reagents have emerged as effective tools for the difluorinated substitution modification of sugars. One commonly used and cost-effective difluoroalkylation reagent is BrCF2CO2Et [54]. Quirion’s group [55] utilized the Reformatsky reaction to efficiently and smoothly introduce the CF2CO2Et moiety into lactone 32, yielding the exocyclic difluorocarboside 33 (Figure 5a). This reaction exhibits high stereoselectivity and directly forms the β-mannose carboglycoside bond. Another reagent frequently employed for introducing 2-(ethoxycarbonyl)difluoromethyl is difluoroenol silyl ether. Quirion’s group [56], in 2009, employed this reagent in conjunction with boron trifluoride ether as a catalyst to couple with compound 34, resulting in the formation of exocyclic difluorocarboside 35 (Figure 5b).
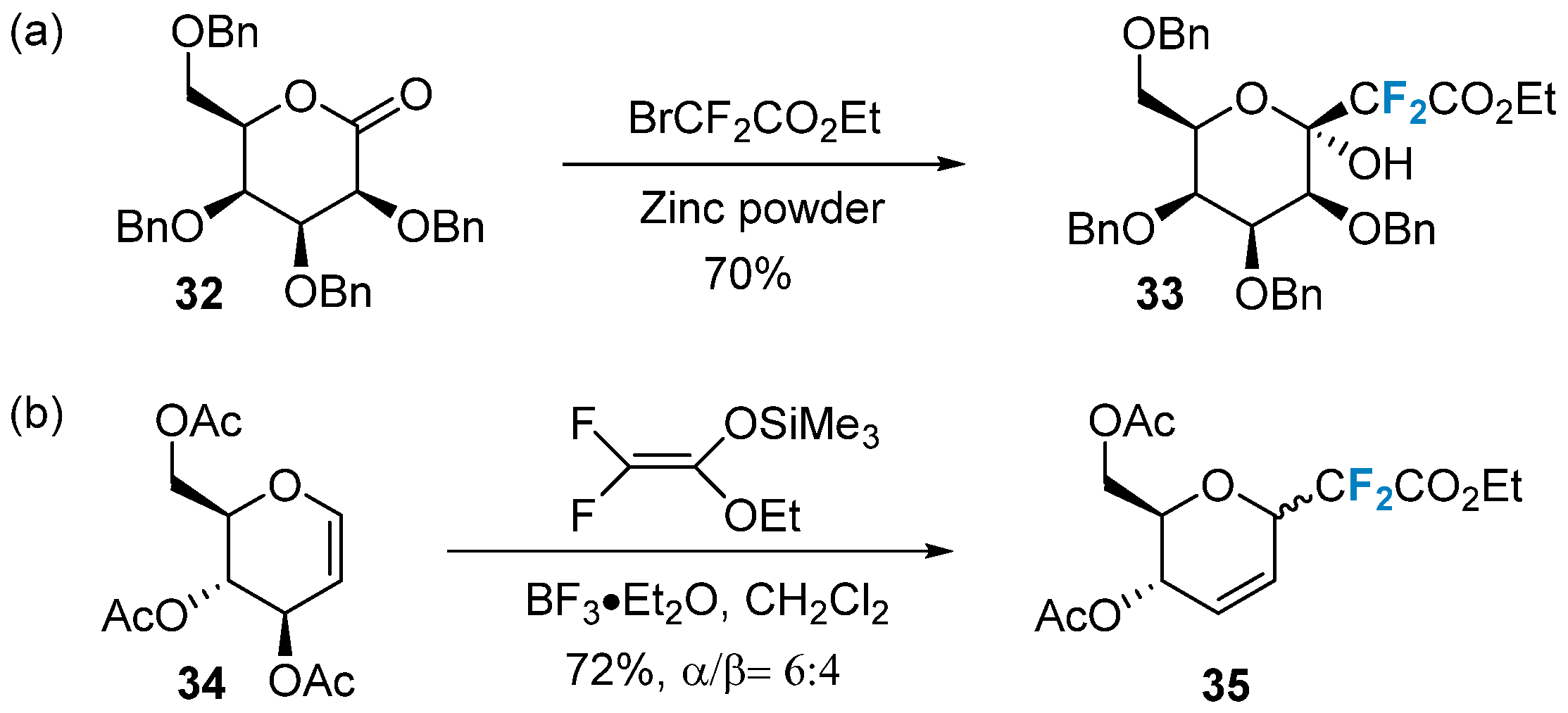
Figure 5. Difluoroalkylation of sugars via nucleophilic reactions. (a), difluoroalkylation via BrCF2CO2Et, (b) difluoroalkylation via CF2CO2Et.
The trifluoromethylation of carbohydrates can be achieved via the nucleophilic addition of trifluoromethyl groups to aldehydes or ketones. A commonly employed nucleophilic trifluoromethyl reagent is TMSCF3. This reagent enables the direct introduction of trifluoromethyl to the carbonyl carbon in the sugar structure. For instance, TMSCF3 was used to treat lactone 36, leading to the formation of compound 37 (Figure 6a) [57]. Moreover, TMSCF3 can also react with other carbonyl-containing sugars and subsequently undergo the Barton–McCombie dehydroxylation reaction to produce the corresponding trifluoromethyl-modified carbohydrates (Figure 6b,c) [58].
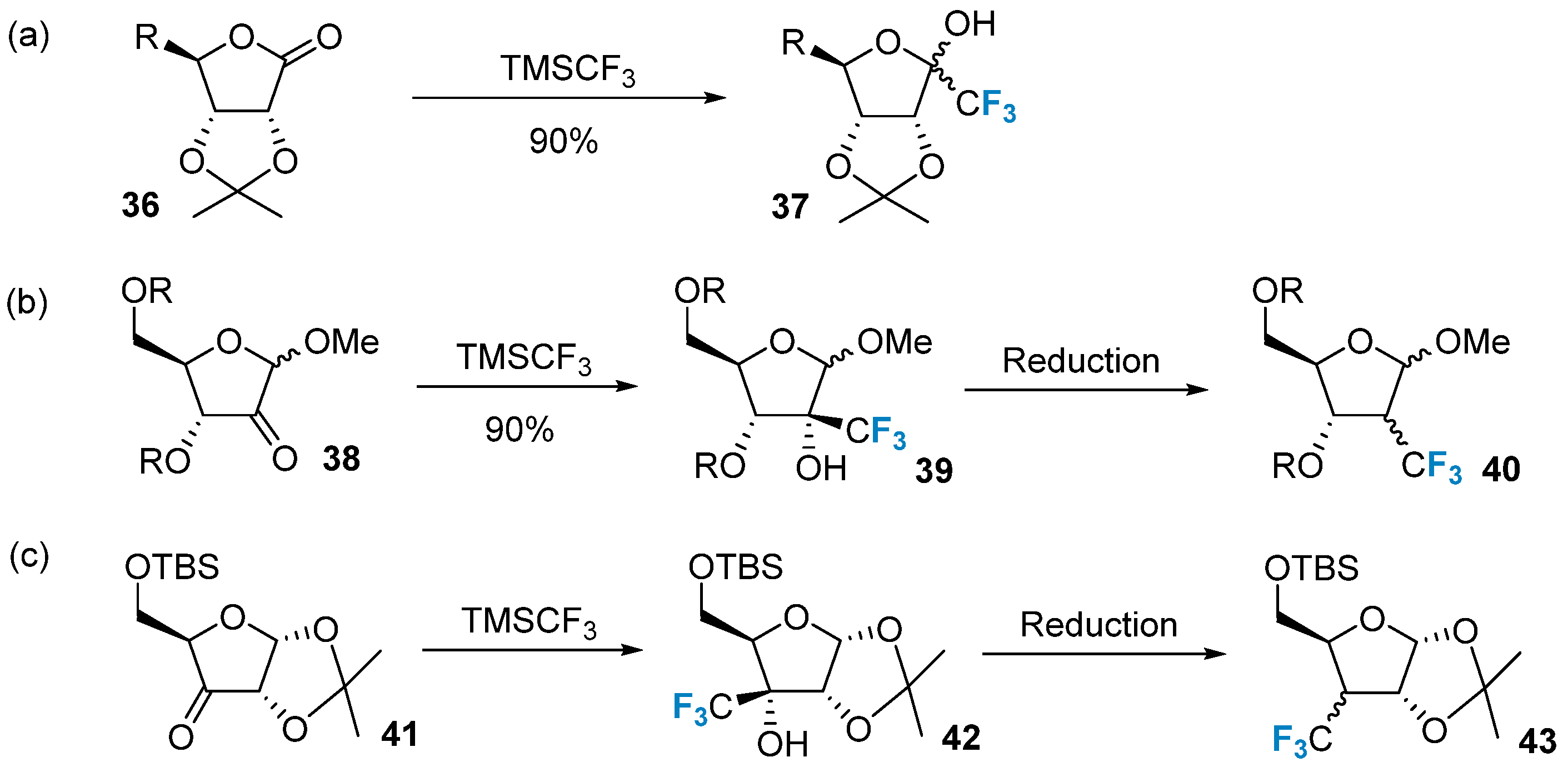
Figure 6. Trifluoromethylation of sugars via nucleophilic reactions. (a) trifluoromethylation via the nucleophilic addition of TMSCF3 to ketone on C-1, (b) trifluoromethylation of ketone on C-2, (c) trifluoromethylation of ketone on C-3.
2.2. Fluorination of Carbohydrates via Electrophilic Addition Reactions
In contrast to nucleophilic reactions, electrophilic addition reactions predominantly involve glycals. The commonly utilized reagents for these reactions are N-F reagents and F-O reagents. N-F reagents are well established, commercially available, and have proven their usefulness in numerous important reactions [59]. Established and readily available N-F reagents have demonstrated their utility in a variety of important reactions [59]. For instance, the Selectfluor reagent has proven to be a highly efficient and mild reagent for fluorination, surpassing the effectiveness of the DAST reagent. In 1997, Wong’s group [60] discovered that the Selectfluor reagent could be used for the one-pot synthesis of 2-fluoroglycosides (Figure 7a). The resulting product, 2-fluoro-2-deoxyglycoside 46, was utilized as a probe to investigate the catalytic mechanisms of glycosidases and glycosyltransferases. In that same year, Dax’s group [61] successfully achieved electrophilic addition to glycals using another N-F reagent known as N-fluorobenzenesulfonamide (Figure 7b).
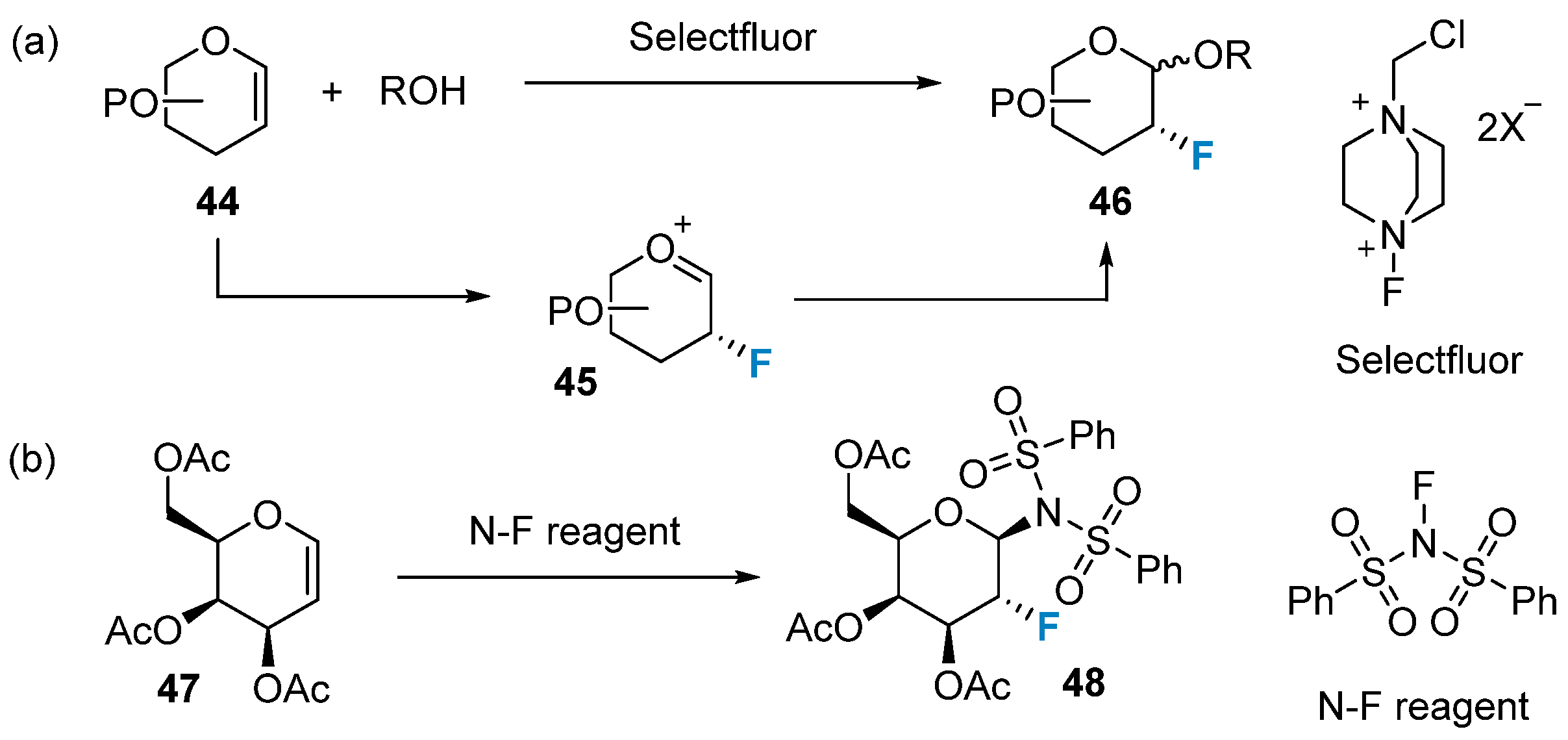
Figure 7. Monofluorination of glycals using N-F reagents. (a) monofluorination via Selectfluor, (b) monofluorination via N-fluorobenzenesulfonamide.
In addition to N-F-type fluorination reagents, certain O-F-type reagents can also be employed in the electrophilic addition reactions of glycals. In 1970, Adamson’s group [62] first synthesized 2-Fluoro-2-deoxy-D-glucose 49 using CF3OF (Figure 8). Since then, efforts have been made to improve this method. In 2006, Schrobilgen’s group [63] achieved the introduction of a fluorine atom into C-2 of glucal 34 using AcO18F (Figure 8). Interestingly, contrary to their expectations, they discovered that the resulting product was not 2-fluoro-2-deoxy-D-glucose but rather 2-fluoro-2-deoxy-D-allose. The researchers proposed that the acetyl groups at positions C-3 and C-4 were involved in the reaction, giving rise to an intermediate that caused a configuration change in the hydroxyl group at position C-3. Leveraging this method, they successfully synthesized 2-fluoro[18F]-2-deoxy-D-allose 50.
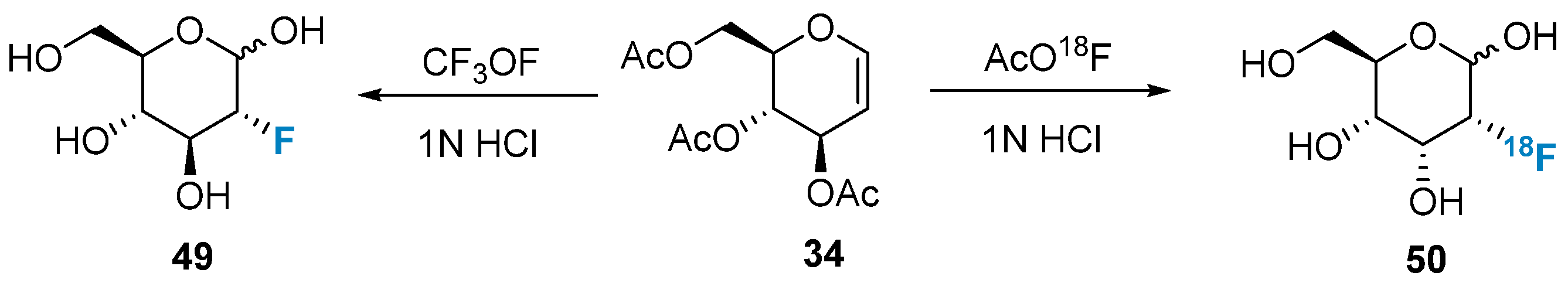
Figure 8. Monofluorination of glycals using O-F reagents.
2.3. Fluorination of Carbohydrates via Radical Reactions
In addition to nucleophilic and electrophilic reactions, radical reactions have also been employed in the fluorination of sugars. In 2020, Li’s group [64] successfully introduced a fluorine atom at the C-5 position of compound 56 using an Ag(II)-initiated radical reaction (Figure 9). The authors observed that the carbon atom at the C-6 position underwent an elimination reaction. They proposed a potential reaction mechanism as follows: Ag(II) initiates a radical reaction, leading to the transformation of the C-6 hydroxyl group of the sugar into the oxygen radical intermediate 57. Subsequently, intermediate 58 is formed via β-scission, followed by the fluorination of intermediate 58 to yield the final product 59. By utilizing this strategy, the group successfully synthesized a range of rare sugars and accomplished the total synthesis of Clostridium jejuni tetrasaccharides [65].
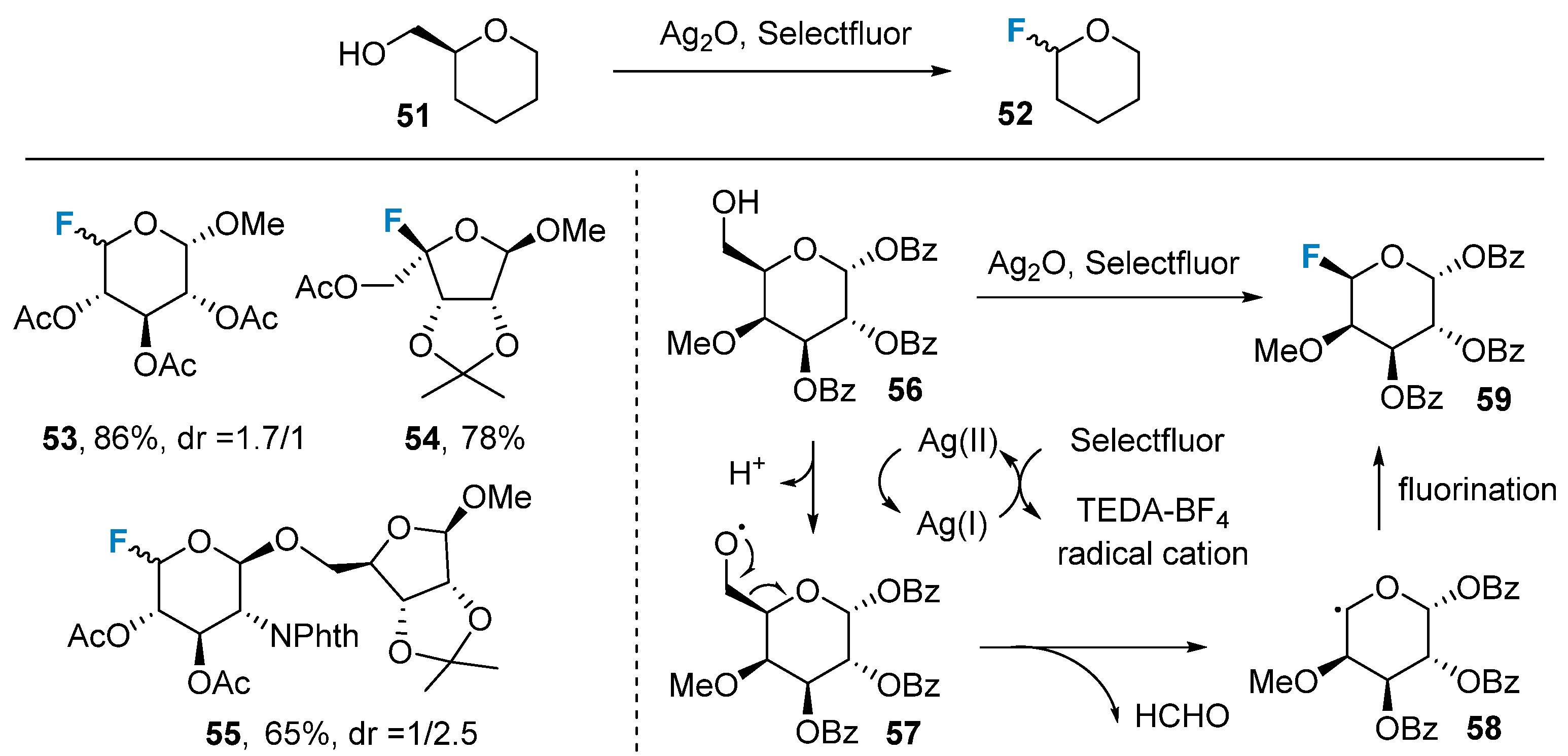
Figure 9. Dehydroxymethylative fluorination of sugars via Ag(II)-initiated radical reaction.
Based on the previous work, Li’s group further advanced the method for decarboxylative fluorination of uronic acids, employing a silver radical pathway [66] (Figure 10a). This reaction demonstrates superior substrate scope and compatibility with functional groups compared to dehydroxymethylative fluorination. Notably, it successfully transforms sugars containing benzyl groups and a hexp-(1-4)-hexp moiety. In a recent development, Li’s group established a protocol for the organophotocatalytic synthesis of glycosyl fluorides without the addition of silver species. This method relies on 9-mesityl-10-methyl-acridinium (Mes-Acr+)-mediated oxidative decarboxylative fluorination of uronic acids [67] (Figure 10b). The potential of this method is promising in the synthesis of fluorinated nucleosides.
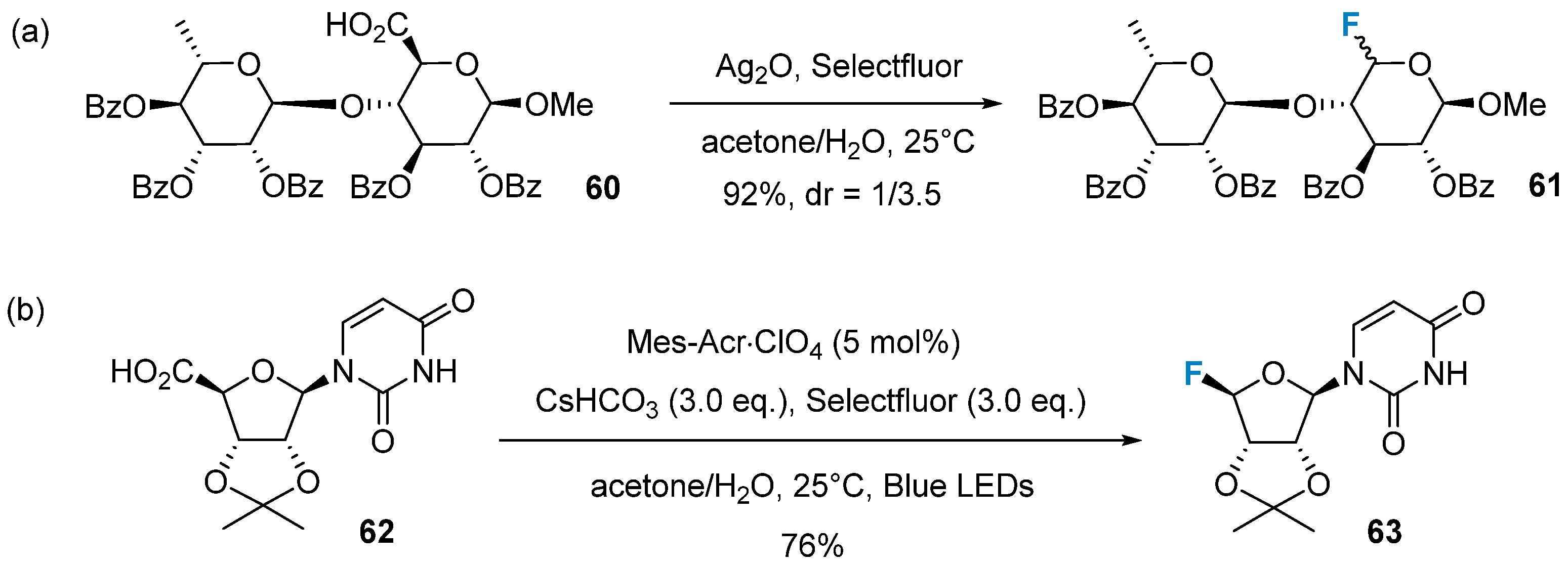
Figure 10. Decarboxylative fluorination of uronic acids via radical reaction. (a) decarboxylative fluorination via silver radical pathway, (b) Mes-Acr+-mediated oxidative decarboxylative fluorination.
Difluorinated sugars can also be synthesized via the radical addition to glycals. Commonly employed radical initiation conditions include BEt3 and metal catalysis, and others. Quirion’s group [68] achieved the successful introduction of 2-(ethoxycarbonyl)difluoromethyl into glycal 64 using BEt3 as a radical initiator, resulting in the formation of CF2-glycoside 65 (Figure 11).
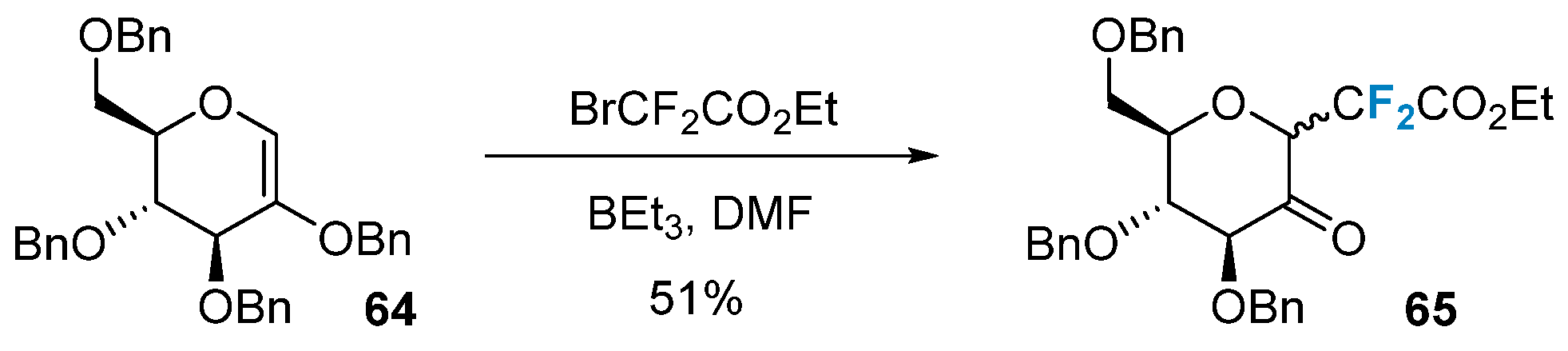
Figure 11. Difluoromethylation of glycals via radical reaction.
Radical addition reactions can also be employed in the preparation of trifluoromethyl-modified sugars. In 2015, Ye’s group [69] developed a photocatalytic C-H trifluoromethylation method using glycal 66 as substrates and Umemoto reagent as a trifluoromethyl source. This method exhibits mild reaction conditions, excellent compatibility with various protecting groups (such as acetyl, benzyl, and p-methoxybenzyl), and applicability to glycals featuring different types of sugars (such as glucose, galactose, rhamnose, arabinose, and lactose) (Figure 12).
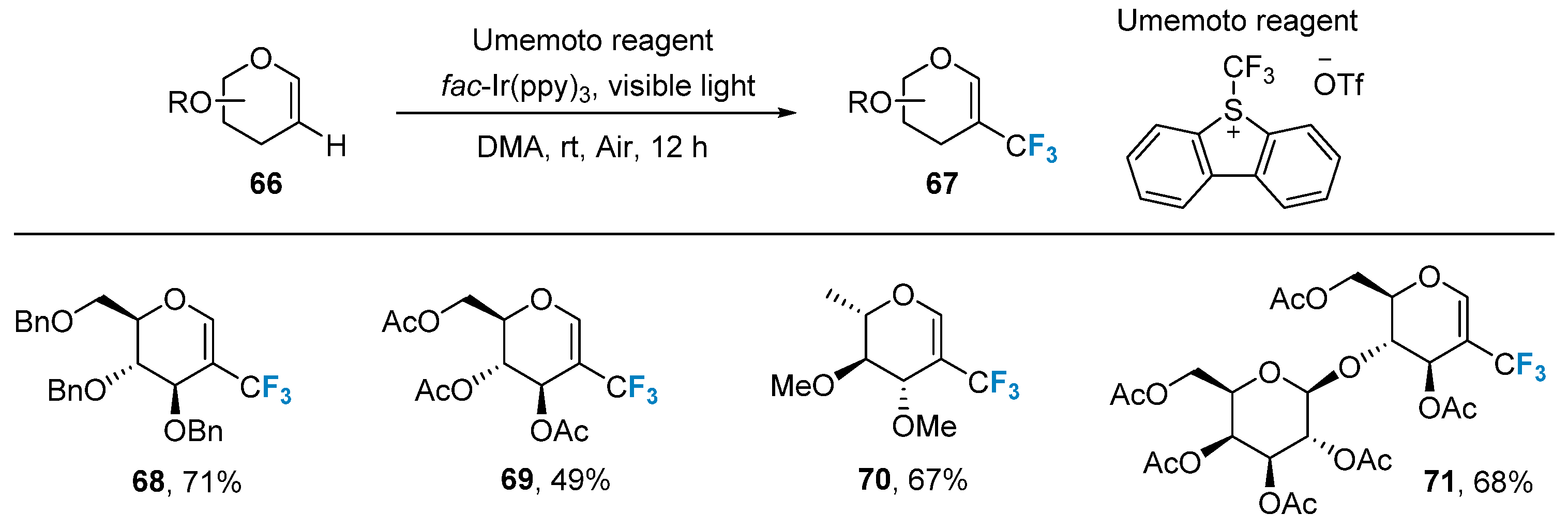
Figure 12. Trifluoromethylation of glycals using Umemoto reagent.
In 2018, Vincent’s group [70] successfully accomplished the C-H trifluoromethylation of exo-glycal 72 using photoredox and copper catalysis methods. Two different trifluoromethyl sources, A and B, were employed under distinct reaction conditions. The method displayed excellent selectivity in producing Z-configured trifluoromethylation product 73 (Figure 13) while maintaining good compatibility with various protecting groups, such as benzyl, silyl, and acetyl.
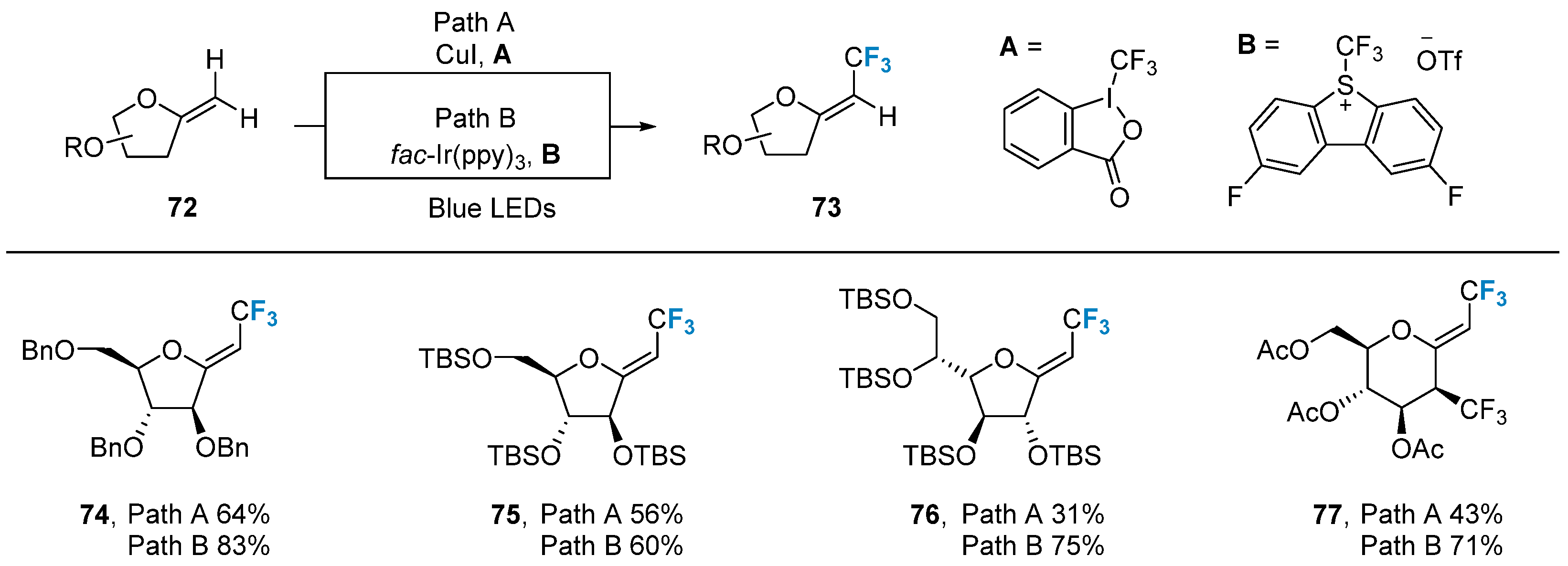
Figure 13. Trifluoromethylation of glycals via photoredox and copper catalysis.
In 2021, Ye’s group [71] used electrochemical methods for the first time in the trifluoromethyl modification of glycoconjugates. The authors used glycal 66 as the substrate, a cheap and readily available sodium trifluoromethanesulfonate reagent as the trifluoromethyl source, and added MnBr2 as the redox medium, and the reaction was carried out in acetonitrile solution. The method is mild and suitable for a wide range of glycoconjugate substrates protected by different protecting groups (Figure 14).
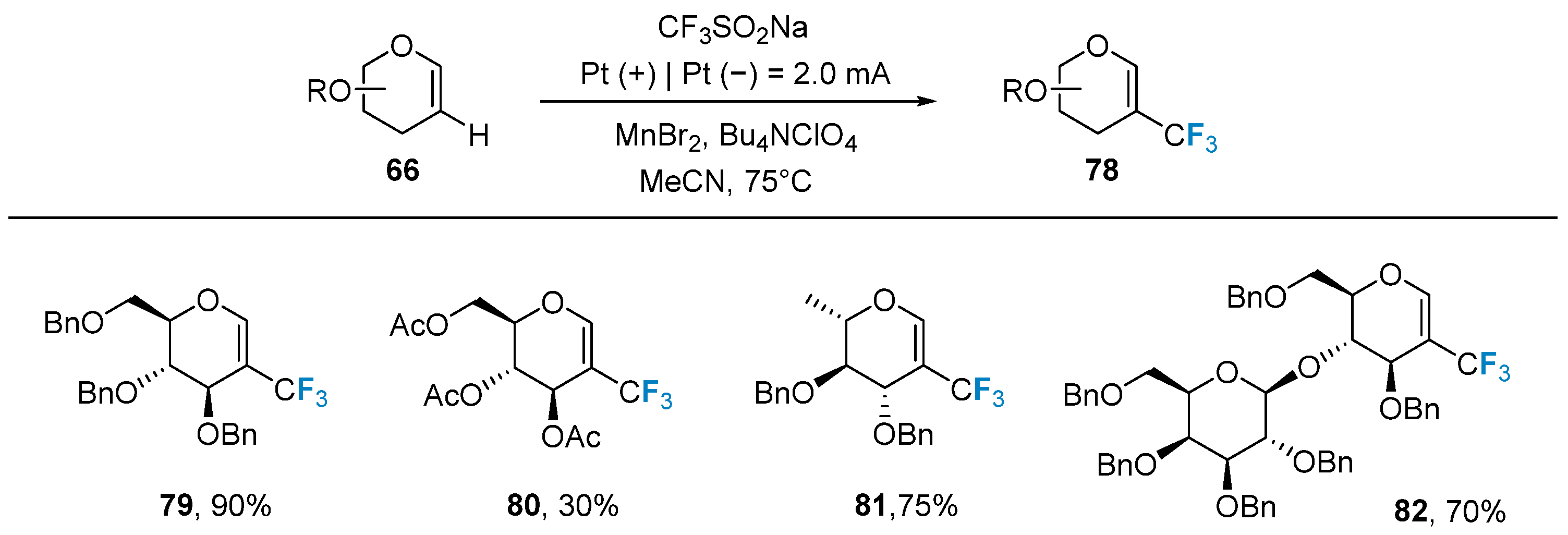
Figure 14. Trifluoromethylation of glycals via electrochemical method.
In 2020, Postigo’s group [72] utilized glycal 83 as a substrate, combined with iodinated perfluoroalkanes as a fluorine source, and employed Ir[dF(CF3)PPy]2(dtbPy)PF6 as a photocatalyst to achieve the synthesis of perfluoroalkyl-modified glycal 84 under blue light conditions (Figure 15).
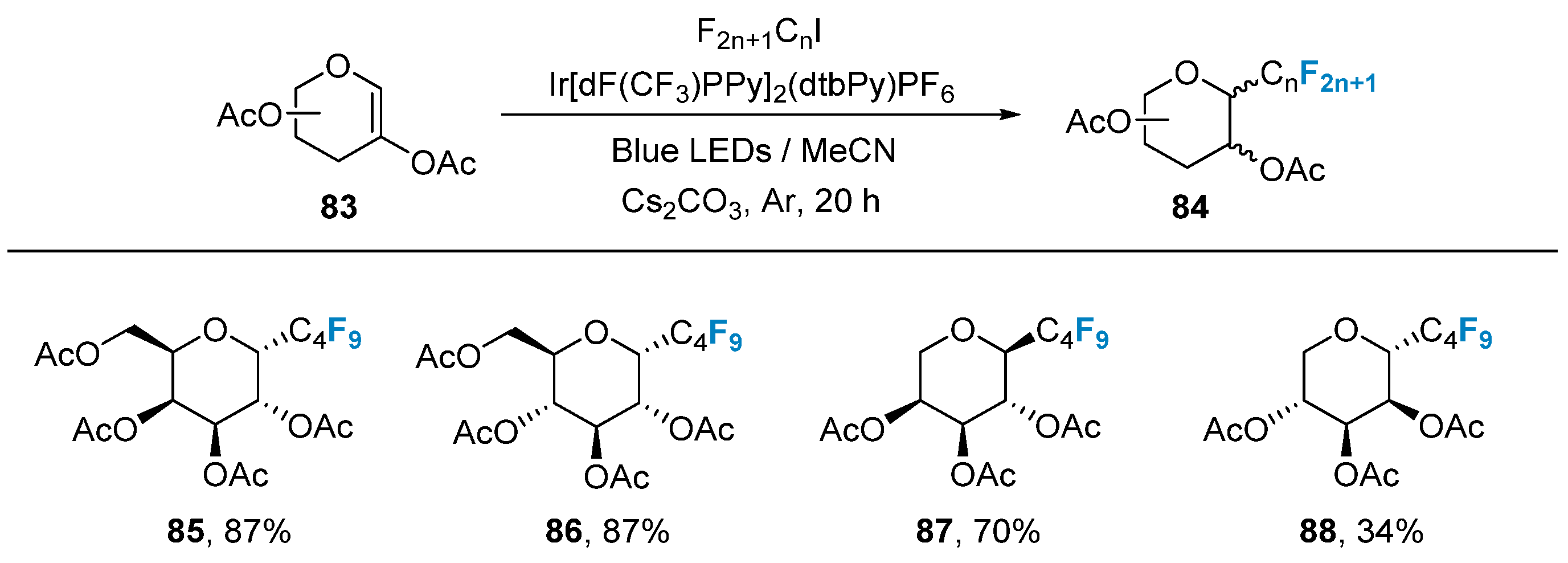
Figure 15. Photocatalyzed reductive fluoroalkylation of 2-acetoxyglycals.
2.4. Fluorination of Carbohydrates via Metal-Catalyzed Coupling Reactions
Pannecoucke’s group [73][74] developed a copper-catalyzed method for the synthesis of difluoroalkylated glycals. The researchers synthesized a series of difluoroalkylation-modified glycals using Cu(PF6)(MeCN)4 as the catalyst, BrCF2CO2Et as the difluoroalkylation reagent, and 1,10-phenanthroline as the ligand (Figure 16). This method exhibited good compatibility with various protecting groups (benzyl, acetyl, methyl, etc.) and could be applied to different types of sugars (glucose, galactose, rhamnose, arabinose, etc.). The proposed reaction mechanism suggests that, initially, copper(I) Cu(PF6)(MeCN)4 undergoes oxidative addition with BrCF2CO2Et to form compound 93, resulting in the trivalent copper species. Compound 93 then undergoes an addition reaction with glycal 66, selectively adding to the electron-rich C-2 position, generating the oxonium intermediate 94. Subsequently, the base removes a proton to form compound 95, which is, ultimately, reduced and eliminated to yield the desired product 89.
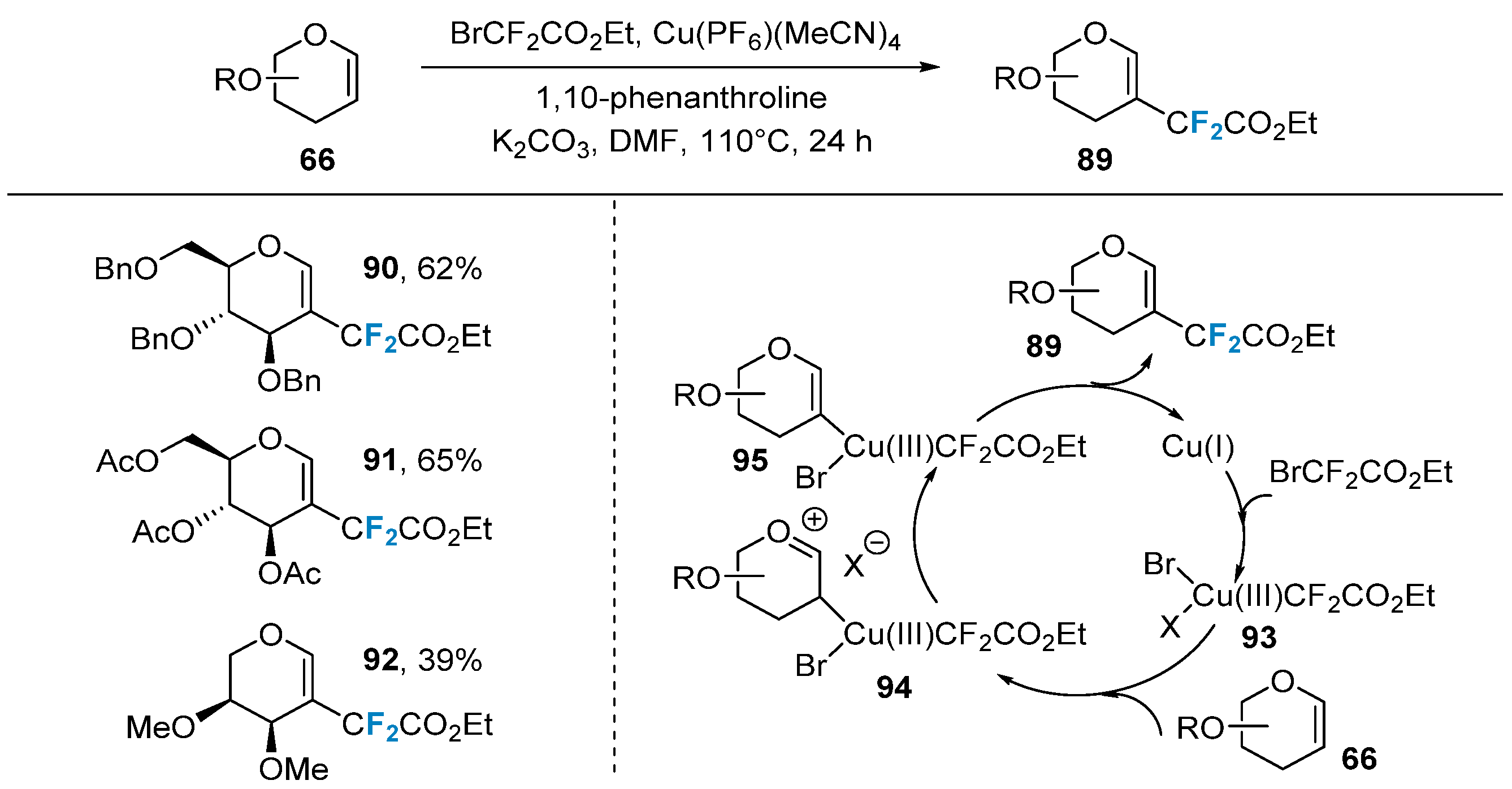
Figure 16. Copper-catalyzed β-difluoroacetylation of glycals via direct C–H functionalization.
The introduction of trifluoromethyl to the glycal was also achieved via metal-catalyzed coupling reactions. Boutureira’s group [75] utilized a 2-iodoglycal 96 as the substrate and CuCF3 as the trifluoromethyl source to successfully obtain the trifluoromethyl-modified glycal 97 in a satisfactory yield upon heating at 50 °C (Figure 17). However, this method required the pre-preparation of 2-iodoglycal, making it somewhat tedious and limited in scope. Alternatively, metal-catalyzed coupling reactions were employed to introduce perfluoroalkyl groups into glycal. Boutureira’s group [76] utilized 2-iodoglycal 96 as the substrate and CuC2F5 as the perfluoroalkyl source, achieving the synthesis of perfluoroalkyl-modified glycal 98 with higher yields under heating at 50 °C.
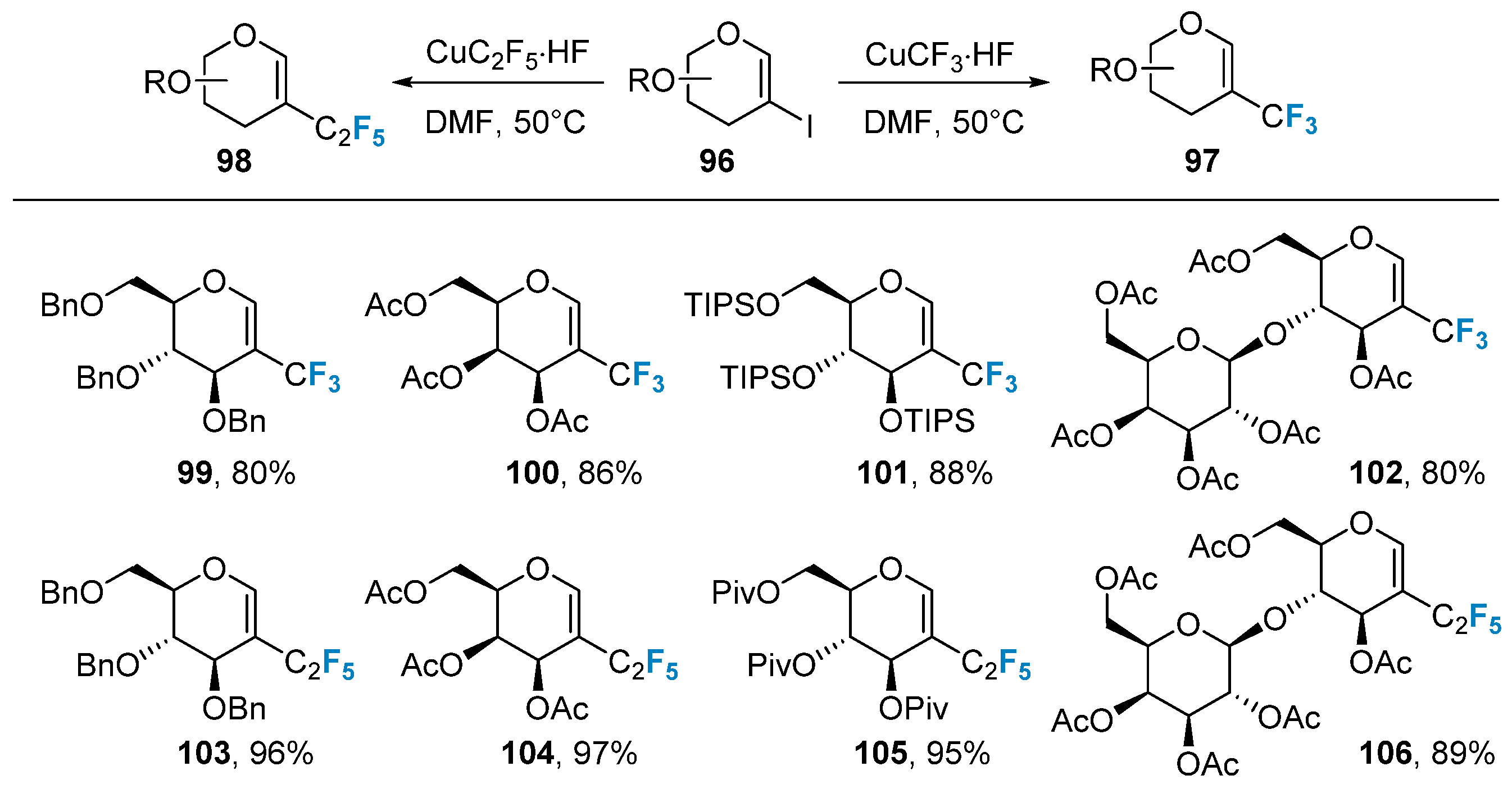
Figure 17. Copper-catalyzed trifluoromethylation of 2-iodoglycals.
2.5. Fluorination of Carbohydrates via Building Block Strategy
Fluorine-modified sugars can be synthesized using a fluorine-containing building block strategy. In 2007, Mootoo’s group [77] successfully synthesized monofluorinated glycosides using compounds 107 as the initial materials in a four-step tandem reaction, as shown in Figure 18: esterification, Takai reaction, cyclization reaction, and borohydride oxidation reaction. Through variation in the R group, a wide range of fluorinated carbohydrates with different substituents can be obtained.
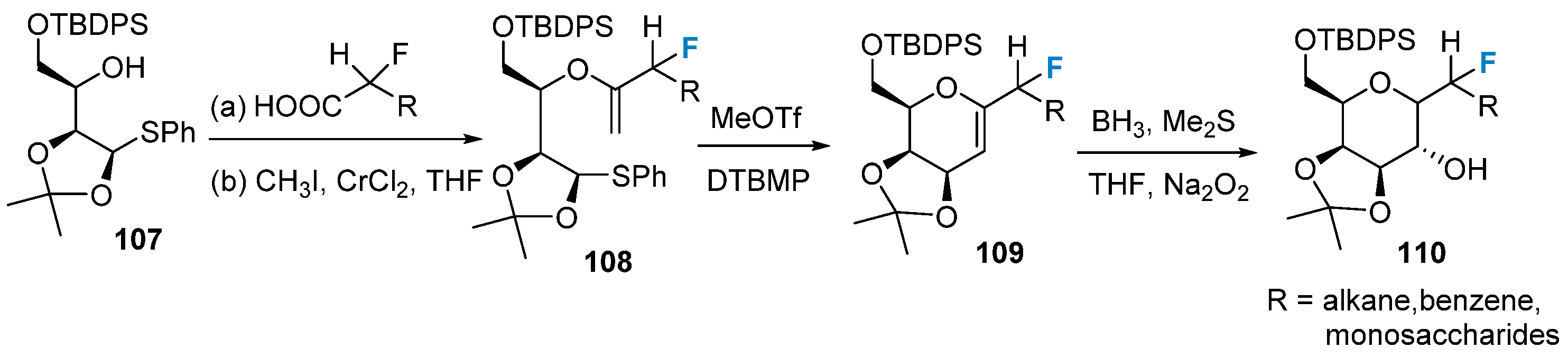
Figure 18. Monofluorination of sugars via building block strategy.
In addition to the methods mentioned above, fluorocarbohydrates can be obtained by modifying the backbone of the raw material using fluorinated reagents. Figure 19a shows the introduction of difluoroalkyl into the precursor 111 via the Reformatsky reaction, followed by ring closure to achieve difluoro-C-glycosides 113 [78]. Alternatively, the difluoroalkylated product 116 can be obtained by initially introducing two fluorine atoms at the C-2 position of the sugar ring, followed by a one-step ring closure (Figure 19b) [79].
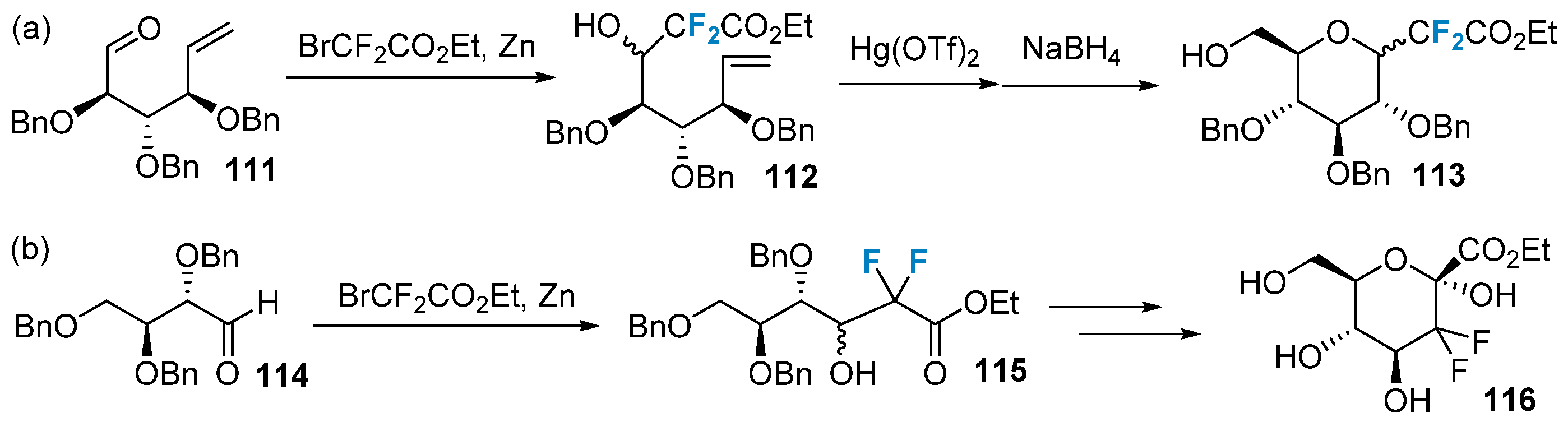
Figure 19. Difluorination of sugars via building block strategy: (a) synthesis of difluoro-C-glycosides 113 via building block strategy, (b) synthesis of difluoroalkylated product 116 via building block strategy.
The synthesis of saccharides modified with trifluoromethyl groups using non-sugar compounds as starting materials was initially achieved by the Kobayashi group [80]. They employed the trifluoromethyl-containing compound 117 as the starting material and generated the intermediate product 118 via a Lewis acid-catalyzed aldol condensation reaction. The carbonyl group in 118 was subsequently reduced, yielding the reduced compound. Furthermore, the double bond in the product was oxidized using a dilute potassium permanganate solution to produce the dihydroxy compound 120 (Figure 20a). In a related study, Burger’s group [81] utilized methyl trifluoropyruvate 122 as a source of trifluoromethyl groups. This compound was catalytically coupled with compound 121 using titanium trichloride, leading to the formation of compound 123. Subsequently, compound 123 underwent a ring-closing reaction under acidic conditions, resulting in the formation of compound 124. Finally, compound 125 was synthesized in a single vessel via a reductive reaction (Figure 20b).
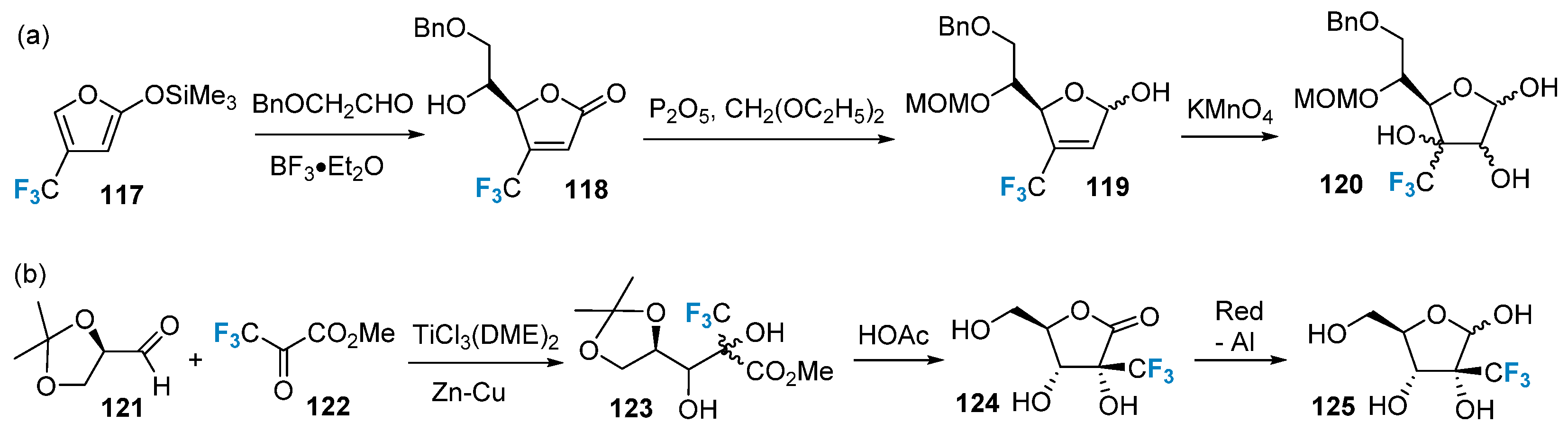
Figure 20. Trifluoromethylation of sugars via building block strategy: (a) synthesis of trifluoromethylated product 120 via building block strategy, (b) synthesis of trifluoromethylated product 125 via building block strategy.
The synthesis of sugars modified with polyfluorinated groups was achieved using non-sugar compounds that contained polyfluorinated modifications. In 2013, Vincent’s group [82] utilized compound 126, which featured a CF2CF2 structure, as the starting material. Through a ring-closing reaction, they successfully obtained the final product 128 (Figure 21).
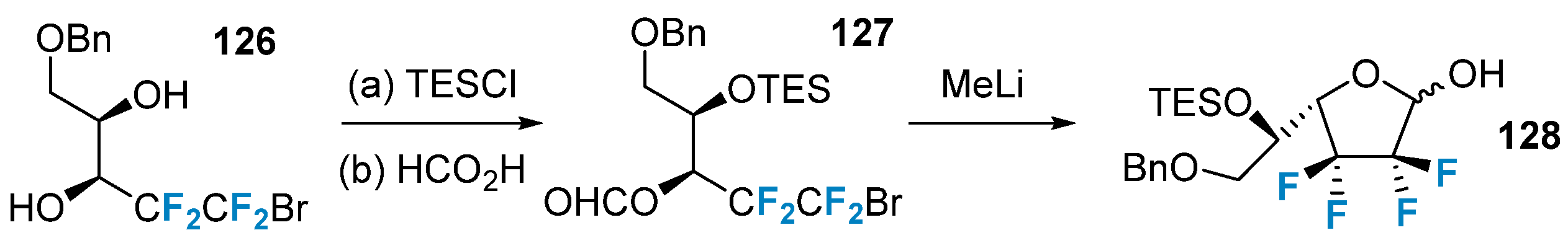
Figure 21. Polyfluorination of sugars via building block strategy.
References
- Dube, D.H.; Bertozzi, C.R. Glycans in cancer and inflammation--potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005, 4, 477–488.
- Taylor, S.L.; McGuckin, M.A.; Wesselingh, S.; Rogers, G.B. Infection’s sweet tooth: How glycans mediate infection and disease susceptibility. Trends Microbiol. 2018, 26, 92–101.
- Fuster, M.M.; Esko, J.D. The sweet and sour of cancer: Glycans as novel therapeutic targets. Nat. Rev. Cancer 2005, 5, 526–542.
- Ernst, B.; Magnani, J.L. From carbohydrate leads to glycomimetic drugs. Nat. Rev. Drug Discov. 2009, 8, 661–677.
- Magnani, J.L. Glycomimetic drugs—A new source of therapeutic opportunities. Discov. Med. 2009, 8, 247–252.
- Hevey, R. Bioisosteres of Carbohydrate Functional Groups in Glycomimetic Design. Biomimetics 2019, 4, 53.
- Hevey, R. Strategies for the Development of Glycomimetic Drug Candidates. Pharmaceuticals 2019, 12, 55.
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enzyme. Inhib. Med. Chem. 2007, 22, 527–540.
- Biffinger, J.C.; Kim, H.W.; DiMagno, S.G. The polar hydrophobicity of fluorinated compounds. ChemBioChem 2004, 5, 622–627.
- Alabugin. Stereoelectronic effects and general trends in hyperconjugative acceptor ability of σ bonds. J. Am. Chem. Soc. 2002, 124, 3175–3185.
- Glaudemans, C.P.; Kovac, P. Probing the combining site of monoclonal IgA J539 using deoxyfluoro- and other galactosides as ligands. Mol. Immunol. 1985, 22, 651–653.
- Street, I.P.; Armstrong, C.R.; Withers, S.G. Hydrogen bonding and specificity. Fluorodeoxy sugars as probes of hydrogen bonding in the glycogen phosphorylase-glucose complex. Bio Chem. Eur. J. 1986, 25, 6021–6027.
- Glaudemans, C.P.J. Mapping of subsites of monoclonal, anti-carbohydrate antibodies using deoxy and deoxyfluoro sugars. Chem. Rev. 2002, 91, 25–33.
- Tysoe, C.; Withers, S.G. Fluorinated mechanism-based inhibitors: Common themes and recent developments. Curr. Top. Med. Chem. 2014, 14, 865–874.
- Rempel, B.P.; Withers, S.G. Covalent inhibitors of glycosidases and their applications in biochemistry and biology. Glycobiology 2008, 18, 570–586.
- Arda, A.; Jimenez-Barbero, J. The recognition of glycans by protein receptors. Insights from NMR spectroscopy. Chem. Commun. 2018, 54, 4761–4769.
- Tengel, T.; Fex, T.; Emtenas, H.; Almqvist, F.; Sethson, I.; Kihlberg, J. Use of 19F NMR spectroscopy to screen chemical libraries for ligands that bind to proteins. Org. Biomol. Chem. 2004, 2, 725–731.
- Diercks, T.; Ribeiro, J.P.; Canada, F.J.; Andre, S.; Jimenez-Barbero, J.; Gabius, H.J. Fluorinated carbohydrates as lectin ligands: Versatile sensors in 19F-detected saturation transfer difference NMR spectroscopy. Chem. Eur. J. 2009, 15, 5666–5668.
- André, S.; Cañada, F.J.; Shiao, T.C.; Largartera, L.; Diercks, T.; Bergeron-Brlek, M.; Biari, K.; Papadopoulos, A.; Ribeiro, J.P.; Touaibia, M.; et al. Fluorinated carbohydrates as lectin ligands: Biorelevant sensors with capacity to monitor anomer affinity in 19F-NMR-based inhibitor screening. Eur. J. Org. Chem. 2012, 2012, 4354–4364.
- Matei, E.; Andre, S.; Glinschert, A.; Infantino, A.S.; Oscarson, S.; Gabius, H.J.; Gronenborn, A.M. Fluorinated carbohydrates as lectin ligands: Dissecting glycan-cyanovirin interactions by using 19F NMR spectroscopy. Chem. Eur. J. 2013, 19, 5364–5374.
- Ribeiro, J.P.; Diercks, T.; Jimenez-Barbero, J.; Andre, S.; Gabius, H.J.; Canada, F.J. Fluorinated carbohydrates as lectin ligands: 19F-based direct STD monitoring for detection of anomeric selectivity. Biomolecules 2015, 5, 3177–3192.
- Diercks, T.; Infantino, A.S.; Unione, L.; Jimenez-Barbero, J.; Oscarson, S.; Gabius, H.J. Fluorinated carbohydrates as lectin ligands: Synthesis of OH/F-Substituted N-Glycan core trimannoside and epitope mapping by 2D STD-TOCSY 19F-NMR spectroscopy. Chem. Eur. J. 2018, 24, 15761–15765.
- Valverde, P.; Quintana, J.I.; Santos, J.I.; Arda, A.; Jimenez-Barbero, J. Novel NMR avenues to explore the conformation and interactions of glycans. ACS Omega 2019, 4, 13618–13630.
- Martinez, J.D.; Manzano, A.I.; Calvino, E.; Diego, A.; Rodriguez de Francisco, B.; Romano, C.; Oscarson, S.; Millet, O.; Gabius, H.J.; Jimenez-Barbero, J.; et al. Fluorinated carbohydrates as lectin ligands: Simultaneous screening of a monosaccharide library and chemical mapping by 19F NMR spectroscopy. J. Org. Chem. 2020, 85, 16072–16081.
- Hoffmann-Roder, A.; Kaiser, A.; Wagner, S.; Gaidzik, N.; Kowalczyk, D.; Westerlind, U.; Gerlitzki, B.; Schmitt, E.; Kunz, H. Synthetic antitumor vaccines from tetanus toxoid conjugates of MUC1 glycopeptides with the Thomsen-Friedenreich antigen and a fluorine-substituted analogue. Angew. Chem. Int. Ed. 2010, 49, 8498–8503.
- Hoffmann-Roder, A.; Johannes, M. Synthesis of a MUC1-glycopeptide-BSA conjugate vaccine bearing the 3′-deoxy-3′-fluoro-Thomsen-Friedenreich antigen. Chem. Commun. 2011, 47, 9903–9905.
- Oberbillig, T.; Mersch, C.; Wagner, S.; Hoffmann-Roder, A. Antibody recognition of fluorinated MUC1 glycopeptide antigens. Chem. Commun. 2012, 48, 1487–1489.
- Wei, M.M.; Wang, Y.S.; Ye, X.S. Carbohydrate-based vaccines for oncotherapy. Med. Res. Rev. 2018, 38, 1003–1026.
- Anderluh, M.; Berti, F.; Bzducha-Wrobel, A.; Chiodo, F.; Colombo, C.; Compostella, F.; Durlik, K.; Ferhati, X.; Holmdahl, R.; Jovanovic, D.; et al. Recent advances on smart glycoconjugate vaccines in infections and cancer. FEBS J. 2022, 289, 4251–4303.
- Mettu, R.; Chen, C.Y.; Wu, C.Y. Synthetic carbohydrate-based vaccines: Challenges and opportunities. J. Biomed. Sci. 2020, 27, 9.
- Linclau, B.; Arda, A.; Reichardt, N.C.; Sollogoub, M.; Unione, L.; Vincent, S.P.; Jimenez-Barbero, J. Fluorinated carbohydrates as chemical probes for molecular recognition studies. Current status and perspectives. Chem. Soc. Rev. 2020, 49, 3863–3888.
- Hevey, R. The role of fluorine in glycomimetic drug design. Chem. Eur. J. 2021, 27, 2240–2253.
- Markovski, L.N.; Pashinnik, V.E. Applications of dialkylaminosulfur trifluorides for the syntheses of acid fluorides. Synthesis 1975, 12, 801–802.
- Middleton, W.J. New fluorinating reagents. Dialkylaminosulfur fluorides. J. Org. Chem. 1975, 40, 574–578.
- Doboszewski, B.; Hay, G.W.; Szarek, W.A. The rapid synthesis of deoxyfluoro sugars using tris(dimethylamino)sulfonium difluorotrimethylsilicate (TASF). Can. J. Chem. 1987, 65, 412–419.
- Card, P.J.; Hitz, W.D. Synthesis of 1′-deoxy-1′-fluorosucrose via sucrose synthetase mediated coupling of 1-deoxy-1-fluorofructose with uridine diphosphate glucose. J. Am. Chem. Soc. 1984, 106, 5348–5350.
- Masataka, Y. Methods of synthesis of glycosyl fluorides. Carbohydr. Res. 2000, 327, 5–14.
- Takahashi, Y.; Tsuneda, S.; Tsuchiya, T.; Koyama, Y.; Umezawa, S. Synthesis of 4’-deoxy-4’-fluorokanamycin A and B. Carbohydr. Res. 1992, 232, 89–105.
- Card, P.J. Synthesis of Fluorinated Carbohydrates. J. Carbohydr. Chem. 2007, 4, 451–487.
- Nyffeler, P.T.; Duron, S.G.; Burkart, M.D.; Vincent, S.P.; Wong, C.H. Selectfluor: Mechanistic insight and applications. Angew. Chem. Int. Ed. 2005, 44, 192–212.
- Cen, Y.; Sauve, A.A. Diastereocontrolled electrophilic fluorinations of 2-deoxyribonolactone: Syntheses of all corresponding 2-deoxy-2-fluorolactones and 2’-deoxy-2’-fluoro-NAD+s. J. Org. Chem. 2009, 74, 5779–5789.
- Liu, P.; Sharon, A.; Chu, C.K. Fluorinated nucleosides: Synthesis and biological implication. J. Fluorine Chem. 2008, 129, 743–766.
- Herdewijn, P.; Aerschot, A.V.; Kerremans, L. Synthesis of nucleosides fluorinated in the sugar moiety. The application of diethylaminosulfur trifluoride to the synthesis of fluorinated nucleosides. Nucleosides Nucleotides 1989, 8, 65–96.
- Uhrig, M.L.; Lantaño, B.; Postigo, A. Synthetic strategies for fluorination of carbohydrates. Org. Biomol. Chem. 2019, 17, 5173–5189.
- Dax, K.; Albert, M.; Ortner, J.; Paul, B.J. Synthesis of deoxyfluoro sugars from carbohydrate precursors. Carbohydr. Res. 2000, 327, 47–86.
- Miethchen, R. Modified natural substances—Fluorinated and fluoroalkylated monosaccharides and inositols. J. Fluorine Chem. 2004, 125, 895–901.
- Lloyd, A.; Coe, P.; Walker, R.; Howarth, O. Some intramolecular rearrangements when pentofuranoses are treated with diethylaminosulphur trifluoride (DAST). J. Fluorine Chem. 1993, 60, 239–250.
- Dmowski, W.; Kamiński, M. Dialkyl-α,α-difluorobenzylamines and dialkyl(trifluoromethyl)-amines-novel fluorinating reagents. J. Fluorine Chem. 1983, 23, 219–228.
- Kobayashi, S.; Yoneda, A.; Fukuhara, T.; Hara, S. Selective synthesis of fluorinated carbohydrates using N,N-diethyl-α,α-difluoro-(m-methylbenzyl) amine. Tetrahedron Lett. 2004, 45, 1287–1289.
- Huonnic, K.; Linclau, B. The synthesis and glycoside formation of polyfluorinated carbohydrates. Chem. Rev. 2022, 122, 15503–15602.
- Sharma, R.; Kavai, I.; Fu, Y.; Bobek, M. Synthesis of gem-difluorosaccharides. Tetrahedron Lett. 1977, 18, 3433–3436.
- Kolympadi, M.; Fontanella, M.; Venturi, C.; André, S.; Gabius, H.J.; Jiménez-Barbero, J.; Vogel, P. Synthesis and conformational analysis of (α-D-galactosyl) phenylmethane and α-, β-difluoromethane analogues: Interactions with the plant lectin viscumin. Chem. Eur. J. 2009, 15, 2861–2873.
- Houlton, J.S.; Motherwell, W.B.; Ross, B.C.; Tozer, M.J.; Williams, D.J.; Slawin, A.M. A convenient strategy for replacement of the anomeric hydroxyl group by difluoromethyl functionality in carbohydrate derivatives. Tetrahedron 1993, 49, 8087–8106.
- Leclerc, E.; Pannecoucke, X. Synthetic efforts towards the synthesis of fluorinated C-glycosidic analogues of α-galactosylceramides. C. R. Chim. 2012, 15, 57–67.
- Cuenca, A.B.; D’Hooge, F.; Gouge, V.; Castelot-Deliencourt, G.; Oulyadi, H.; Leclerc, E.; Jubault, P.; Pannecoucke, X.; Quirion, J.C. Addition of ethyl bromodifluoroacetate to lactones: Reactivity and stereoselectivity. Synlett 2005, 17, 2627–2630.
- Poulain, F.; Leclerc, E.; Quirion, J.C. Approaches to the synthesis of CF2-analogues of 2-deoxy-2-aminoglycosides. Tetrahedron Lett. 2009, 50, 1803–1805.
- Munier, P.; Giudicelli, M.B.; Picq, D.; Anker, D. Synthese des 5-Desoxy-5,5,5-trifluoro-D-et-LPentofuranoses. J. Carbohydr. Chem. 1996, 15, 739–762.
- Lavaire, S.; Plantier-Royon, R.; Portella, C. Synthesis of 3-deoxy-3-C-trifluoromethyl-D-ribose from D-xylose or D-glucose. J. Carbohydr. Chem. 1996, 15, 361–370.
- Lal, G.S.; Pez, G.P.; Syvret, R.G. Electrophilic NF fluorinating agents. Chem. Rev. 1996, 96, 1737–1756.
- Burkart, M.D.; Zhang, Z.Y.; Hung, S.C.; Wong, C.H. A new method for the synthesis of fluorocarbohydrates and glycosides using selectfluor. J. Am. Chem. Soc. 1997, 119, 11743–11746.
- Albert, M.; Dax, K.; Ortner, J. A novel direct route to 2-deoxy-2-fluoro-aldoses and their corresponding derivatives. Tetrahedron 1998, 54, 4839–4848.
- Adamson, J.; Foster, A.B.; Hall, L.D.; Johnson, R.N.; Hesse, R.H. Fluorinated carbohydrates: Part III. 2-deoxy-2-fluoro-D-glucose and 2-deoxy-2-fluoro-D-mannose. Carbohydr. Res. 1970, 15, 351–359.
- Ashique, R.; Chirakal, R.V.; Hughes, D.W.; Schrobilgen, G.J. Two-step regio-and stereoselective syntheses of 19F-and 18F-2-deoxy-2-(R)-fluoro-β-D-allose. Carbohydr. Res. 2006, 341, 457–466.
- Zhou, X.; Ding, H.; Chen, P.; Liu, L.; Sun, Q.; Wang, X.; Wang, P.; Lv, Z.; Li, M. Radical dehydroxymethylative fluorination of carbohydrates and divergent transformations of the resulting reverse glycosyl fluorides. Angew. Chem. Int. Ed. 2020, 59, 4138–4144.
- Li, T.; Wang, J.; Zhu, X.; Zhou, X.; Sun, S.; Wang, P.; Cao, H.; Yu, G.; Li, M. Synthesis of Rare 6-Deoxy-D-/L-heptopyranosyl fluorides: Assembly of a hexasaccharide corresponding to campylobacter jejuni strain CG8486 capsular polysaccharide. J. Am. Chem. Soc. 2021, 143, 11171–11179.
- Chen, P.; Wang, P.; Long, Q.; Ding, H.; Cheng, G.; Li, T.; Li, M. Synthesis of reverse glycosyl fluorides and rare glycosyl fluorides enabled by radical decarboxylative fluorination of uronic acids. Org. Lett. 2020, 22, 9325–9330.
- Ding, H.; Yan, N.; Wang, P.; Song, N.; Sun, Q.; Li, T.; Li, M. Synthesis of reverse glycosyl fluorides via organophotocatalytic decarboxylative fluorination of uronic acids. Org. Chem. Front. 2022, 9, 2808–2814.
- Moreno, B.; Quehen, C.; Rose-Helene, M.; Leclerc, E.; Quirion, J.C. Addition of difluoromethyl radicals to glycals: A new route to α-CF2-D-glycosides. Org. Lett. 2007, 9, 2477–2480.
- Wang, B.; Xiong, D.C.; Ye, X.S. Direct C-H trifluoromethylation of glycals by photoredox catalysis. Org. Lett. 2015, 17, 5698–5701.
- Frédéric, C.J.M.; Cornil, J.; Vandamme, M.; Dumitrescu, L.; Tikad, A.; Robiette, R.; Vincent, S.P. Highly (Z)-diastereoselective synthesis of trifluoromethylated exo-glycals via photoredox and copper catalysis. Org. Lett. 2018, 20, 6769–6773.
- Liu, M.; Luo, Z.X.; Li, T.; Xiong, D.C.; Ye, X.S. Electrochemical trifluoromethylation of glycals. J. Org. Chem. 2021, 86, 16187–16194.
- Flores, E.W.M.; Uhrig, M.L.; Postigo, A. Photocatalyzed reductive fluoroalkylation of 2-acetoxyglycals towards the stereoselective synthesis of α-1-fluoroalkyl-C-glycosyl derivatives. Org. Biomol. Chem. 2020, 18, 8724–8734.
- Belhomme, M.C.; Dru, D.; Xiong, H.Y.; Cahard, D.; Besset, T.; Poisson, T.; Pannecoucke, X. Coppermediated direct functionalization of unsaturated C–C bonds with ethyl bromo (difluoro) acetate: A straightforward access to highly valuable difluoromethylated alkenes. Synthesis 2014, 46, 1859–1870.
- Belhomme, M.C.; Poisson, T.; Pannecoucke, X. Copper catalyzed β-difluoroacetylation of dihydropyrans and glycals by means of direct C-H functionalization. Org. Lett. 2013, 15, 3428–3431.
- Mestre, J.; Lishchynskyi, A.; Castillon, S.; Boutureira, O. Trifluoromethylation of electron-rich alkenyl iodides with fluoroform-derived “ligandless” CuCF3. J. Org. Chem. 2018, 83, 8150–8160.
- Mestre, J.; Castillón, S.; Boutureira, O. “Ligandless” pentafluoroethylation of unactivated (hetero) aryl and alkenyl halides enabled by the controlled self-condensation of TMSCF3-derived CuCF3. J. Org. Chem. 2019, 84, 15087–15097.
- Tony, K.A.; Denton, R.W.; Dilhas, A.; Jiménez-Barbero, J.; Mootoo, D.R. Synthesis of β-C-galactopyranosides with fluorine on the pseudoanomeric substituent. Org. Lett. 2007, 9, 1441–1444.
- Stéphane, M.; François, D.; Sathunuru, R.; Christian, F.; Xavier, P.; Jean-Charles, Q. Synthesis of new α- and β-gem-difluoromethylene C-glycosides in the galactose and glucose series. Tetrahedron Lett. 2001, 42, 5879–5882.
- Li, Y.; Drew, M.G.; Welchman, E.V.; Shirvastava, R.K.; Jiang, S.; Valentine, R.; Singh, G. Syntheses of ethyl 3-deoxy-3, 3-difluoro-D-arabino-heptulosonate and analogues. Tetrahedron 2004, 60, 6523–6531.
- Kawada, K.; Kitagawa, O.; Taguchi, T.; Hanzawa, Y.; Kobayashi, Y.; Iitaka, Y. Studies on organic fluorine compounds. XLVII. synthesis of trifluoromethylated sugars through the Aldol Reaction of 2-trimethylsilyloxy-4-trifluoromethylfuran. Chem. Pharm. Bull. 1986, 17, 4216–4222.
- Eilitz, U.; Bottcher, C.; Sieler, J.; Gockel, S.; Haas, A.; Burger, K. Synthesis of 2-C-trifluoromethyl substituted D-ribose. Tetrahedron 2001, 57, 3921–3925.
- N’Go, I.; Golten, S.; Arda, A.; Canada, J.; Jimenez-Barbero, J.; Linclau, B.; Vincent, S.P. Tetrafluorination of sugars as strategy for enhancing protein-carbohydrate affinity: Application to UDP-Galp mutase inhibition. Chem. Eur. J. 2014, 20, 106–112.
More
Information
Subjects:
Chemistry, Medicinal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
26 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




