You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pasquale Longo | -- | 2342 | 2023-09-22 10:25:18 | | | |
| 2 | Sirius Huang | -1 word(s) | 2341 | 2023-09-25 02:50:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Catalano, A.; Mariconda, A.; Sinicropi, M.S.; Ceramella, J.; Iacopetta, D.; Saturnino, C.; Longo, P. Biological Activities of Ruthenium NHC Complexes. Encyclopedia. Available online: https://encyclopedia.pub/entry/49510 (accessed on 23 December 2025).
Catalano A, Mariconda A, Sinicropi MS, Ceramella J, Iacopetta D, Saturnino C, et al. Biological Activities of Ruthenium NHC Complexes. Encyclopedia. Available at: https://encyclopedia.pub/entry/49510. Accessed December 23, 2025.
Catalano, Alessia, Annaluisa Mariconda, Maria Stefania Sinicropi, Jessica Ceramella, Domenico Iacopetta, Carmela Saturnino, Pasquale Longo. "Biological Activities of Ruthenium NHC Complexes" Encyclopedia, https://encyclopedia.pub/entry/49510 (accessed December 23, 2025).
Catalano, A., Mariconda, A., Sinicropi, M.S., Ceramella, J., Iacopetta, D., Saturnino, C., & Longo, P. (2023, September 22). Biological Activities of Ruthenium NHC Complexes. In Encyclopedia. https://encyclopedia.pub/entry/49510
Catalano, Alessia, et al. "Biological Activities of Ruthenium NHC Complexes." Encyclopedia. Web. 22 September, 2023.
Copy Citation
Ruthenium N-heterocyclic carbene (NHC) complexes have unique physico-chemical properties as catalysts and a huge potential in medicinal chemistry and pharmacology, exhibiting a variety of notable biological activities.
Ru-NHC complexes
N-heterocyclic carbenes
antitumor agents
antiproliferative activity
antibacterials
antimicrobials
antifungals
1. N-Heterocyclic Carbenes (NHCs)
N-heterocyclic carbenes (NHCs) are a category of electron donor ligands able to form dative metal–ligand bonds, leading to a universal class of compounds in organometallic and coordination chemistry. NHCs typically mimic the chemical properties of phosphines [1]. They belong to five different families: imidazolinylidenes, imidazolylidenes, triazolinylidenes, thiazolilylidenes and pyrazolinylidenes (Figure 1) [2].
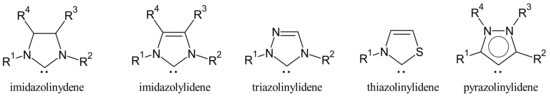
Figure 1. Structures of NHCs.
NHCs are suitable for efficient design because they can be synthesized within a few steps, offering the possibility of the N and C functionalization for structure modification. They act as excellent σ-donors, generating a structural variety ranging from linear, square pyramidal, trigonal bipyramidal, tetrahedral and octahedral geometries of metal-NHC complexes. The essential role of NHCs is related to their ability to form complexes with metals. Metal-NHCs are widely used for organic processes, such as the formation of amide linkage, hydrogenation, isomerization, cycloisomerization, cyclopropanation, hydrosilylation, allylation and deallylation, enol-ester synthesis, heterocycle synthesis and C-C alkyne coupling [2]. Most importantly, different organometallic-NHC complexes, such as silver, gold, platinum, copper, palladium and selenium demonstrated interesting biological properties [3][4][5][6], including antimicrobial [7][8][9], anticancer [10][11][12][13][14][15][16], antiparasitic [17], hemolytic and thrombolytic activities [18][19]. Recent studies for gold and silver-NHC compounds are addressed to breast [20][21], ovarian [22] and cervical human cancer [23]. Research has been carried out in order to understand the mechanism of action of gold and silver-NHC complexes as anticancer agents, finding activity against human topoisomerases I and II [24][25], actin [26] and tubulin [27], or triggering the reactive oxygen species-dependent intrinsic apoptotic pathway [28]. Recently, gold and silver compounds with NHC have been indicated as promising compounds for the treatment of COVID-19 [29], as they demonstrated a strong inhibition of the S/ACE2 interaction and particularly of the PLpro enzymatic activity [30]. Moreover, an Ag-N-heterocyclic carbene complex bearing the hydroxyethyl ligand determined the inhibition of human carbonic anhydrase I (hCA I) and hCA II isoenzymes, α-glycosidase and AChE and BChE enzymes, thus suggesting the selection of NHC complexes for further studies in glaucoma, epilepsy and other diseases related to metabolic enzymes [31].
2. Ruthenium-NHC Complexes
Besides the importance of ruthenium-NHC complexes in organometallic catalysis [32][33] and bioinorganic chemistry, they have been described as effective anticancer and antimicrobial agents [2][5][9]. Patil et al., 2020, published the advances in the design, synthesis, characterization and biomedical applications, particularly the antimicrobial and anticancer activities, of ruthenium NHC–metal complexes and other metals (silver, gold, palladium, rhodium, iridium, and platinum), covering works published from 2015 to 2020 [1]. Several studies regard only the activity of Ru-NHC complexes as antiproliferative agents, whereas the antimicrobial activity is almost always studied along with other activities, including antiproliferative and antioxidant. The two paragraphs below summarized these studies.
2.1. Ru(II)-NHC Complexes with Antiproliferative Activity
Recent studies regarding the antiproliferative activity of Ru(II)-NHC complexes are summarized and the half-maximal (50%) inhibitory concentration (IC50) values are given, when reported in the literature.
Lam et al., 2018, [34] investigated several halide-substituted benzimidazolium-derived NHC of RuII/OsII complexes, using NHCs that were symmetrically and non-symmetrically methyl- and benzyl-substituted, and reported their inhibition of the selenoenzyme thioredoxin reductase (TrxR) and antiproliferative activities. The anticancer activity was studied against human colon (HCT-116), human cervical (SiHa) and human breast cancer (NCI-H460) cells. The diiodido(1,3-dibenzylbenzimidazol-2-ylidene)(η6-p-cymene)ruthenium(II) complex 1 is a potent TrxR inhibitor and an antiproliferative agent. The authors found out that there was no clear correlation between the two activities, thus, it was suggested that TrxR inhibition was unlikely to be the main mode of action.
Tabrizi et al., 2019, [35] studied the in vitro antiproliferative potential and cyclooxygenase-2 (COX-2) inhibitory activity of a cyclometalated Ru(II) complex (2) containing ibuprofen (Ibu), 1,3,5-triaza-7-phosphaadamantane (PTA) and a CCC-pincer containing naproxen moiety (CCC-Nap). Antiproliferative studies were carried out on breast cancer (MCF-7 and MDA-MB-231), colon cancer (HT-29) cell lines, healthy breast cell lines (MCF-10A), and human embryonic kidney normal cells (HEK293) by means of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, after 72 h exposure. The complex 2 was quite potent, about twice as active as cisplatin against breast cancer cells, and around 14 times less active against HT-29 cell lines than cisplatin. Interestingly, the complex 2 inhibition studies against COX-2 revealed that it displayed approximately about 16- and 5-times stronger interactions than the free Ibu and CCC-Nap ligands, respectively. Moreover, it improved the production of reactive oxygen species (ROS) by 10.7-fold compared to H2O2, when used as a positive control in MCF-7 cells.
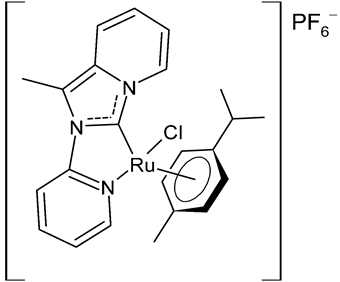
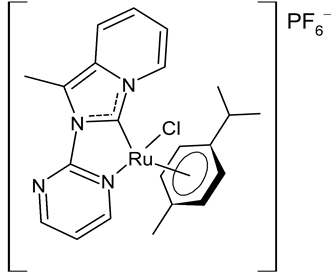
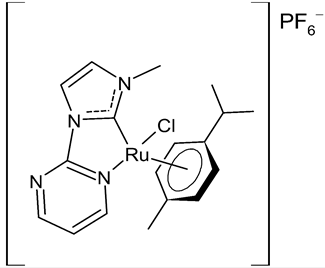
Rana et al., 2021, [36] described the synthesis and study of two pyridine and pyrimidine Ru(II)-NHC complexes, functionalized annulated NHC 3 and 4 with a half-sandwich geometry, and their in vitro cytotoxicity studies against lung (A549), colon (HCT-116) and breast (MCF-7) cancer cell lines and non-cancerous 3T3 cells (embryonic fibroblast isolated from a mouse), using cisplatin as the reference (IC50 were 64; 23.2; 14 and 64 µM, respectively) via the MTT assay. Both compounds were more active than the reference, with the exception of 3 against HCT-116 cancer cells, and, overall, compound 4 was more active than compound 3. Moreover, in silico studies, predicting the binding-sites and atomic interactions of lead molecules with B cell CLL/lymphoma (BCL-2) (PDB entry: 4lvt) and DNA dodecamer (PDB entry: 1bna), suggested that the order of reactivity of the molecules is 4 > 3. Subsequently, the same group [37] investigated a pyrimidine functionalized non-annulated half-sandwich Ru(II)-NHC complex, namely chloro(p-cymene)-1-methyl-3-pyrimidylimidazolideneruthenium(II)-hexafluorophosphate 5, derived from the non-annulated NHC precursor 1-methyl-3-pyrimidylimidazolium-hexafluorophosphate. The half-sandwich geometry of the molecule was established with single crystal X-ray diffraction. As well, this compound was more active than cisplatin against the cell lines used [36]. Particularly, 5 and 4 were 3- to 4-fold more active than 3 and cisplatin. Predicting binding affinities for 5 and 4 were −7.1 and −7.0 kcal/mol–1, respectively, for BCL-2 and −7.3 and −8.2 kcal/mol–1, respectively, for DNA. DNA cleavage activity of complex 5 confirmed the ability of ruthenium to perform a direct double-strand breaking. Moreover, molecular docking analysis suggested that complex 5 binds, with the highest binding affinity, a hydrophobic pocket in BLC-2, different from that of complexes 3 and 4. The opposite was found in the contact simulation of the complexes with a DNA strand: complexes 4 and 5 are superimposed, while complex 3 binds the other side of the DNA strand. However, the binding affinity predicted for 4 was higher than 5.
Rodriguez-Prieto et al., 2021, [38] reported the synthesis of three spherical carbosilane metallodendrimers of different generations holding Ru-NHC complexes. Compounds 6 and 7, which belong to the first- and second-generation dendrimers, respectively, are shown in Table 1, while the third-generation dendrimer is not shown. These compounds showed cytotoxic activity similar, or even better, than cisplatin against four cancer cell lines, namely advanced prostate (PC3), breast (HCC1806), cervix (HeLa), human liver (HEPG2) and the non-tumoral fibroblast (HFF-1) cell line, as determined by MTT assay. IC50 for cisplatin was referred to data of the literature: IC50 = 30.18 ± 2.58 µM (MCF-7), 11.75 ± 1.23 µM (HeLa), 17.29 ± 1.05 µM (HFF-1) [39]. The complexes have been proposed as possible candidates for cancer treatment, due to their combined double action, i.e., antitumoral and carrier for anticancer siRNA. These compounds were capable of forming dendriplexes by promoting the entrance of Mcl-1-FITC (myeloid cell leukemia-1 fluorescein labelled) small interfering RNA (siRNA) to HEPG2 cancer cells, protecting the siRNA from RNAse. Moreover, the cellular uptake of the three complexes was studied by confocal microscopy with Mcl-1-FITC siRNA. Particularly, the second-generation dendrimer 7 was more active than first-generation dendrimer 6. Compound 7 displayed promising antitumoral properties, being selective, even more than cisplatin, against cancer cell lines, with respect to the normal ones. The different activity was related to the inability of first-generation dendrimer 6 to interact with the siRNA. The compounds were internalized into the cells by endocytosis and internalization increased by generation of the dendritic system.
Table 1. Antiproliferative activity of Ru(II)-NHC complexes.
| Structure | Compd. | Biological Activities | Ref |
|---|---|---|---|
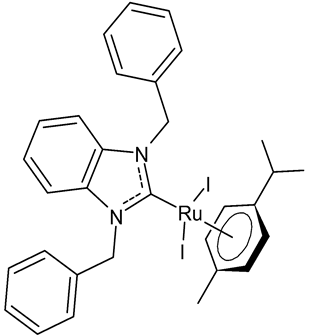 |
1 | Trx inhibition (%): 71 ± 8 IC50 = 6.2 ± 0.4 µM (HCT-116) IC50 = 8.4 ± 0.2 µM (SiHa) IC50 = 7.8 ± 1.0 µM (NCI-H460) |
Lam et al., 2018 [34] |
 |
2 | IC50 = 0.91 ± 0.02 µM (MCF-7) IC50 = 1.32 ± 0.05 µM (MDA-MB-231) IC50 = 35.82 ± 0.52 µM (HT-29) IC50 = 4.71 ± 0.05 µM (MCF-10A) IC50 = 108.20 ± 0.03 µM (HEK-293) |
Tabrizi et al., 2019 [35] |
 |
3 | IC50 = 28.7 ± 2.3 µM (A549) IC50 = > 100 µM (HCT-116) IC50 = 14.8 ± 2.3 µM (MCF-7) IC50 = 44.64 ± 2.6 µM (3T3) |
Rana et al., 2021 [36] |
 |
4 | IC50 = 2.1 ± 0.7 µM (A549) IC50 = 8.6 ± 1.8 µM (HCT-116) IC50 = 3.3 ± 0.4 µM (MCF-7) IC50 = 9.36 ± 1.16 µM (3T3) |
Rana et al., 2021 [36] |
 |
5 | IC50 = 2.8 ± 0.4 µM (A549) IC50 = 2.3 ± 0.3 µM (HCT-116) IC50 = 4.7 ± 0.7 µM (MCF-7) IC50 = 8.56 ± 1.6 µM (3T3) |
Rana et al., 2020 [37] |
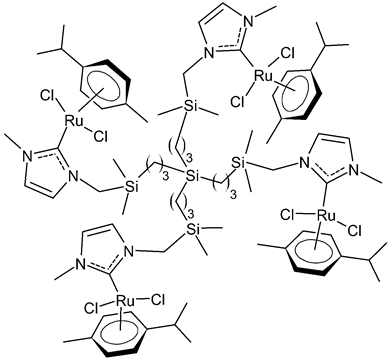 |
6 | IC50 = 37.2 ± 3.6 µM (PC3) IC50 = 25.3 ± 7.6 µM (HCC1806) IC50 = 71.6 ± 15.4 µM (HeLa) IC50 = 10.3 ± 1.7 µM (HEPG2) IC50 = 21.2 ± 1.8 µM (HFF-1) |
Rodriguez-Prieto et al., 2021 [38] |
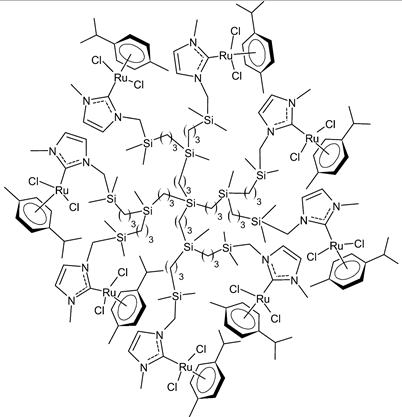 |
7 | IC50 = 21.4 ± 0.9 µM (PC3) IC50 = 20.6 ± 1.9 µM (HCC1806) IC50 = 8.3 ± 1.1 µM (HeLa) IC50 = 6.6 ± 0.5 µM (HEPG2) IC50 = 69.3 ± 1.2 µM (HFF-1) |
Rodriguez-Prieto et al., 2021 [38] |
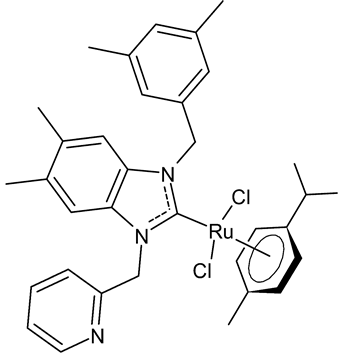 |
8 | IC50 = 14.2 ± 0.5 mM (C6) IC50 = 11.1 ± 0.5 mM (HeLa) |
Paşahan et al., 2022 [40] |
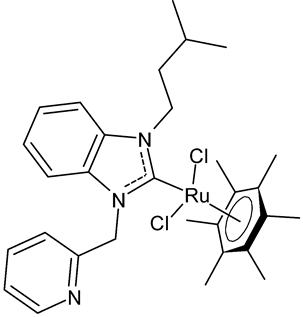 |
9 | IC50 = 16.2 ± 0.4 mM (C6) IC50 = 13.7 ± 0.3 mM (HeLa) |
Paşahan et al., 2022 [40] |
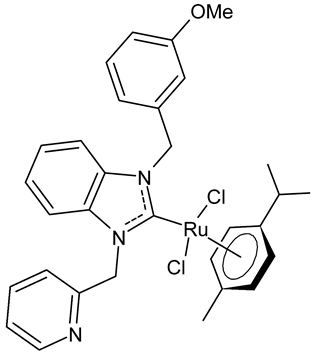 |
10 | IC50 = 24.2 ± 0.7 mM (C6) IC50 = 22.8 ± 0.8 mM (HeLa) |
Paşahan et al., 2022 [40] |
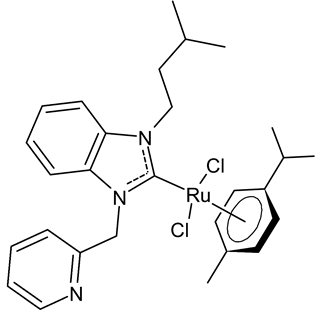 |
11 | IC50 = 37.3 ± 0.9 mM (C6) IC50 = 17.3 ± 0.8 mM (HeLa) |
Paşahan et al., 2022 [40] |
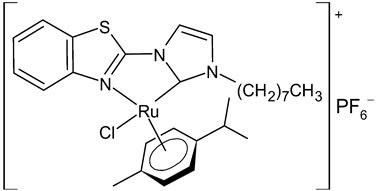 |
12 | IC50 = 2.74 ± 0.15 mM (A2780) | Chen et al., 2020 [41] |
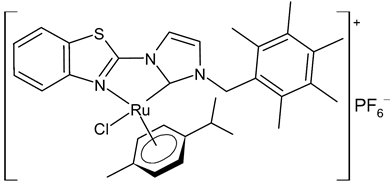 |
13 | IC50 = 1.98 ± 0.10 mM (A2780) | Chen et al., 2020 [41] |
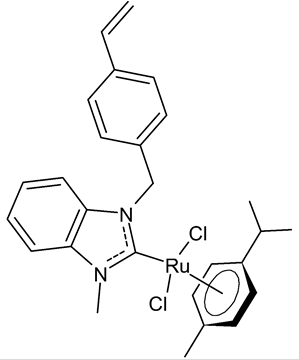 |
14 | IC50 of 3.61 µM (MCF-7) | Sari et al., 2020 [42] |
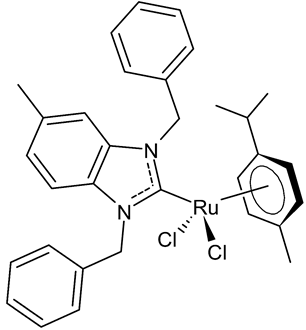 |
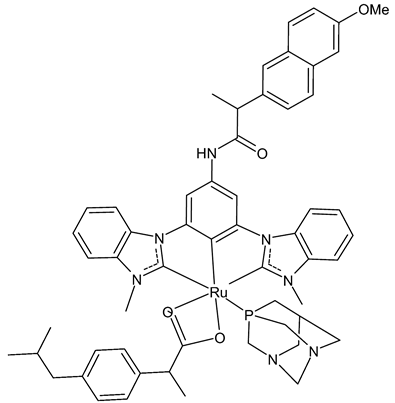
15 (HB324) | G1 Arrest * = ≥1 µM AC50 ** = ~4 µM |
Wilke et al., 2023 [43] |
* G1 arrest, which means a proliferation inhibition equal to 100% or above, indicating occurring cell death; ** AC50 is the concentration necessary to induce apoptosis in half of the cell population.
Paşahan et al., 2022, [40] recently reported a study on Ru-NHC complexes as potential anticancer agents, examining their antiproliferative activity against rat glioblastoma (C6) and human cervix adenocarcinoma (HeLa) cell lines by ELISA assay. Four complexes, namely 8–11, were more active than cisplatin, used as the standard (IC50 = 136 ± 0.7 mM (C6) and 126 ± 0.6 mM (HeLa)).
Chen et al., 2020, [41] reported the synthesis and in vitro antiproliferative activity evaluation of a small panel of NHC-coordinated ruthenium(II) arene complexes. The compounds showed cytotoxic activities against the human ovarian A2780 cancer cells. The highest cytotoxic activities were found for 12 and 13, which were about 2-fold more potent than cisplatin. Furthermore, these compounds induced apoptosis in a caspase-dependent manner, primarily through intracellular ROS overproduction and cell cycle arrest at the G1 phase. Moreover, in a preclinical metastatic model of A2780 tumor xenograft, administration of 12 and 13 resulted in a marked inhibition of tumor progression and metastasis. A significant alleviated systemic toxicity was observed in animals for both complexes in comparison with cisplatin.
Sari et al., [42] designed four (NHC)Ru(II)(η6-p-cymene) complexes, bearing 2-morpholinoethyl and 4-vinylbenzyl substituents to the benzimidazole core and different alkyl/aryl groups to the second nitrogen atom, and studied their cytotoxic activity against MCF-7 breast cancer cells and their DNA-binding properties. The authors individuated the compound 14 as lead, showing an IC50 of 3.61 µM, and that it can bind the plasmidic DNA without exerting genotoxic effects.
A recent interesting article by Wilke et al., [43] described the study of the ruthenium complex HB324 (15), which showed promising potential as a novel anticancer agent in vitro. This complex showed good effects, even in low micromolar concentrations, especially regarding proliferation inhibition and apoptosis induction via the mitochondrial pathway on human B-cell precursor leukemia Nalm-6 cells. Moreover, of particular interest is the upregulation of the Harakiri resistance protein, which inhibits the anti-apoptotic and death repressor proteins BCL-2 and BCL-xL. Finally, compound 15 showed synergistic activity with various established anticancer drugs, including vincristine, and overcame the resistance in several cell lines, such as neuroblastoma cells.
2.2. Antimicrobial Ru(II)-NHC Complexes Possessing Other Additional Abilities (Antiproliferative, Antioxidant and Anticholinesterase)
Antimicrobic resistance is a cause of great concern worldwide and significantly affects humanity’s capacity to prevent and treat a growing number of bacterial and fungal infections [44]. In the past decade, the antimicrobial activity of ruthenium complexes has been reviewed [45] and studied as antimicrobial agents and alternatives or adjuvants to the more traditional antibiotics [46]. The recent antimicrobial studies regarding complexes of NHC-ruthenium are summarized in Table 2, and the minimal inhibitory concentrations (MICs) are reported.
Table 2. Antimicrobial and other activities (antiproliferative, antioxidant and anticholinesterase) of Ru(II)-NHC complexes.
| Structure | Biological Activities | Ref |
|---|---|---|
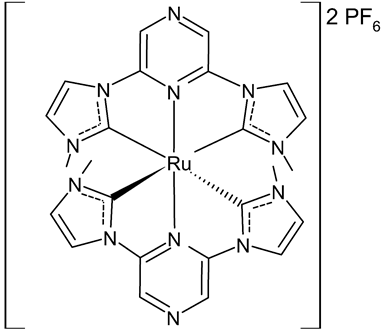 |
MIC = 8 µM (S. epidermidis NCIM 2493) MIC = 8 µM (P. aeruginosa ATCC 27853) MIC = 16 µM (C. albicans SJ11, unicellular fungus) IC50 = 22.70 ± 1.3 µM (HCT15) 50 = 18.46 ± 2.3 µM (Hep2) |
Roymahapatra et al., 2015 [47] |
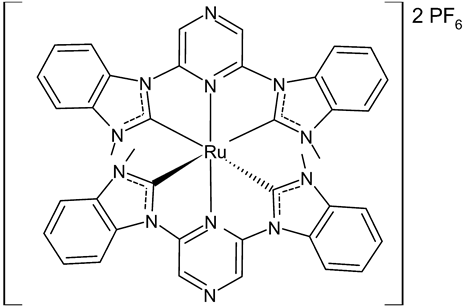 |
MIC = 64 µM (S. epidermidis NCIM 2493) MIC = 64 µM (P. aeruginosa ATCC 27853) MIC = 256 µM (C. albicans SJ11, unicellular fungus) IC50 = 82.2 ± 4.6 µM (HCT15) IC50 = 61.8 ± 3.3 µM (Hep2) |
Roymahapatra et al., 2015 [47] |
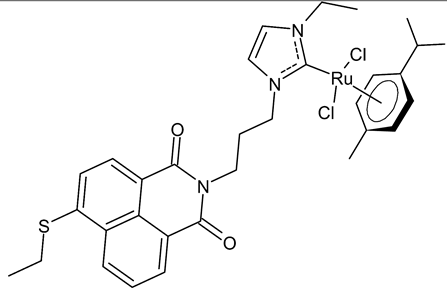 |
MIC = 16 µg/mL; 22.9 µM (B. subtilis 168 DSM402) MIC = 16 µg/mL; 22.9 µM (S. aureus DSM 20231) MIC = 16 µg/mL; 22.9 µM (S. aureus ATCC 43300) IC50 = 11.6 ± 1.0 µM (MCF-7) IC50 = 26.4 ± 1.1 µM (HT-29) |
Streciwilk et al., 2018 [48] |
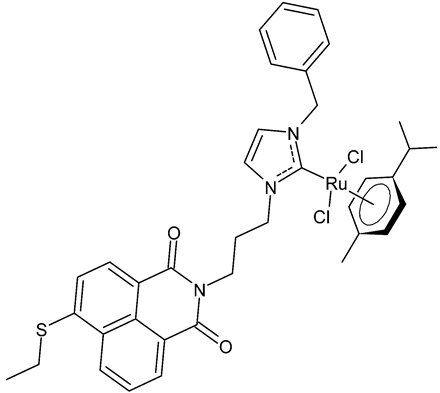 |
MIC = 8 µg/mL; 10.5 µM (B. subtilis 168 DSM402) MIC = 8 µg/mL; 10.5 µM (S. aureus DSM 20231) MIC = 8 µg/mL; 10.5 µM (S. aureus ATCC 43300) IC50 = 4.8 ± 0.1 µM (MCF-7) IC50 = 4.9 ± 0.02 µM (HT-29) |
Streciwilk et al., 2018 [48] |
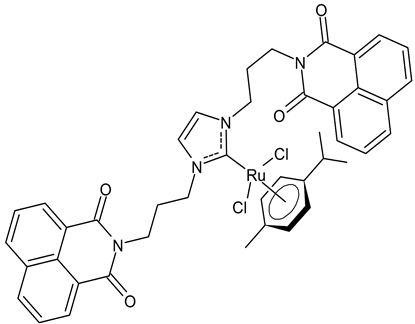 |
MIC = 16 µg/mL; 18.8 µM (B. subtilis 168 DSM402) MIC = 16 µg/mL; 18.8 µM (S. aureus DSM 20231) MIC = 8 µg/mL; 9.4 µM (S. aureus ATCC 43300) IC50 = 26.0 ± 1.1 µM (MCF-7) IC50 > 100 µM (HT-29) |
Streciwilk et al., 2018 [49] |
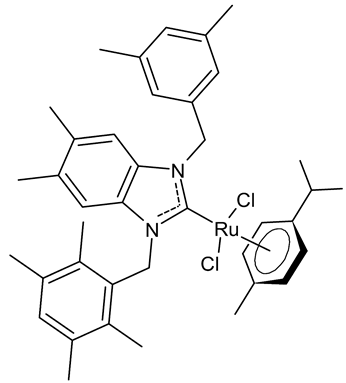 |
MIC = 0.0195 mg/mL (M. luteus LB14110) MIC = 15.6 µg/mL (L. monocytogenes ATCC 19117) MIC = 3.9 µg/mL (S. aureus ATCC 6538) MIC = 3.9 µg/mL (S. typhimurium ATCC 14028) MIC = 1.25 µg/mL (C. albicans ATCC 10231) IC50 = 0.67 ± 0.2 µg/mL (MCF-7) IC50 = 0.8 ± 0.2 µg/mL (MDA-MB-231) IC50 = 2.52 µg/mL (AChE) IC50 = 19.88 µg/mL (TyrE) |
Boubakri et al., 2019 [50][51] |
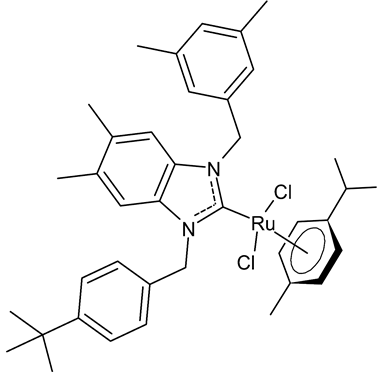 |
MIC = 0.0195 mg/mL (M. luteus LB14110) MIC = 0.0781 mg/mL (L. monocytogenes ATCC 19117) MIC = 1.25 mg/mL (S. typhimurium ATCC 14028) Inhibition zone = 15 ± 0.2 mm (E. coli) IC50 = 0.68 ± 3.2 µg/mL (MCF-7) IC50 = 1.93 ± 2.6 µg/mL (MDA-MB-231) |
Boubakri et al., 2019 [50] |
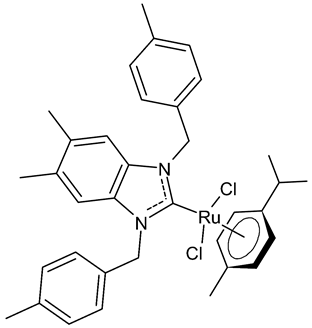 |
MIC = 3.9 µg/mL (L. monocytogenes ATCC 19117) MIC = 1.95 µg/mL (S. aureus ATCC 6538) MIC = 1.95 µg/mL (S. typhimurium ATCC 14028) MIC = 1.25 µg/mL (C. albicans ATCC 10231) IC50 = 0.68 ± 0.2 µg/mL (MCF-7) IC50 = 0.8 ± 0.1 µg/mL (MDA-MB-231) IC50 = 5.06 µg/mL (AChE) IC50 = 24.95 µg/mL (TyrE) EC50 = 32.18 µg/mL (DPPH) EC50 = 18.17 µg/mL (ABTS) EC50 = 92.25 µg/mL (β-carotene) |
Boubakri et al., 2022 [51] |
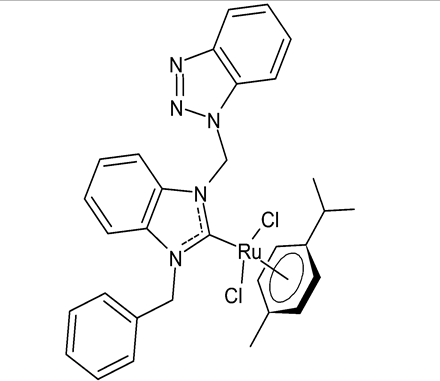 |
MIC = 200 µg/mL (B. subtilis ATCC 21332) MIC = 100 µg/mL (E. coli ATCC 25922) MIC = 200 µg/mL (C. albicans ATCC 60193) IC50 = 100 ± 7 µM (Caco-2) IC50 = 137 ± 2 µM (MCF-7) |
Onar et al., 2019 [52] |
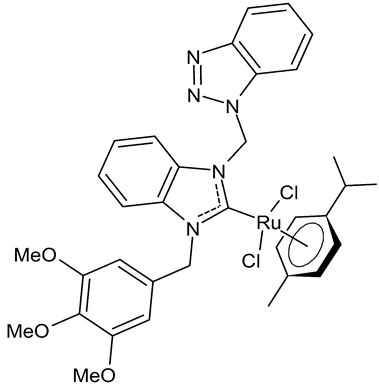 |
MIC = 100 µg/mL (B. subtilis ATCC 21332) MIC = 200 µg/mL (E. coli ATCC 25922) MIC = 200 µg/mL (C. albicans ATCC 60193) IC50 = 90 ± 1 µM (Caco-2) IC50 = 270 ± 12 µM (MCF-7) |
Onar et al., 2019 [52] |
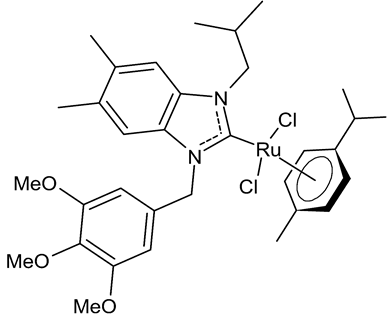 |
MIC = 0.0195 mg/mL (M. luteus LB 14110) MIC = 0.1562 mg/mL (L. monocytogenes ATCC 19117) MIC = 0.0781 mg/mL (S. typhimurium ATCC 14028) IC50 = 0.6 ± 1.1 µg/mL (MCF-7) IC50 = 1.1 ± 0.3 µg/mL (MDA-MB-231) |
Slimani et al., 2020 [53] |
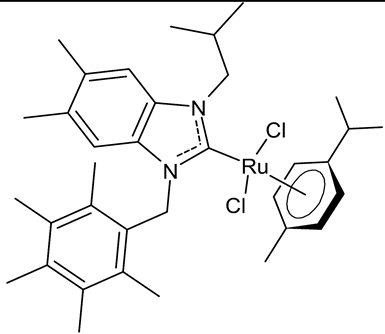 |
MIC = 0.0195 mg/mL (M. luteus LB 14110) MIC = 0.0781 mg/mL (L. monocytogenes ATCC 19117) MIC = 1.25 mg/mL (S. typhimurium ATCC 14028) IC50 = 0.68 ± 1.2 µg/mL (MCF-7) IC50 = 1.7 ± 0.6 µg/mL (MDA-MB-231) |
Slimani et al., 2020 [53] |
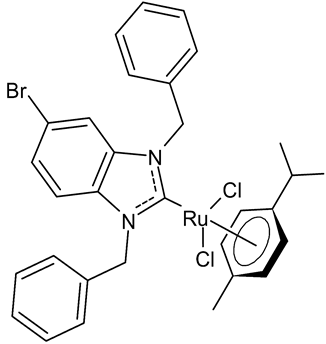 |
MIC = 11.7 µM (B. subtilis) MIC = 23.4 µM (S. aureus DSM 20231) MIC = 11.7 µM (S. aureus ATCC 43300) |
Burmeister et al., 2021 [54] |
References
- Patil, S.A.; Hoagland, A.P.; Patil, S.A.; Bugarin, A. N-heterocyclic carbene-metal complexes as bio-organometallic antimicrobial and anticancer drugs, an update (2015–2020). Future Med. Chem. 2020, 12, 2239–2275.
- Jalal, M.; Hammouti, B.; Touzani, R.; Aouniti, A.; Ozdemir, I. Metal-NHC heterocycle complexes in catalysis and biological applications: Systematic review. Mater. Today Proc. 2020, 31, S122–S129.
- Roos, L.; Malan, F.P.; Landman, M. Naphthalimide-NHC complexes: Synthesis and properties in catalytic, biological and photophysical applications. Coord. Chem. Rev. 2021, 449, 214201.
- Oehninger, L.; Rubbiani, R.; Ott, I. N-Heterocyclic carbene metal complexes in medicinal chemistry. Dalton Trans. 2013, 42, 3269–3284.
- Ott, I. Metal N-heterocyclic carbene complexes in medicinal chemistry. In Advances in Inorganic Chemistry; Sadler, P.J., Van Eldik, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 75, pp. 121–148.
- Hussaini, S.Y.; Haque, R.A.; Razali, M.R. Recent progress in silver(I)-, gold(I)/(III)- and palladium(II)-N-heterocyclic carbene complexes: A review towards biological perspectives. J.Organomet. Chem. 2019, 882, 96–111.
- Nomiya, K.; Morozumi, S.; Yanagawa, Y.; Hasegawa, M.; Kurose, K.; Taguchi, K.; Sakamoto, R.; Mihara, K.; Kasuga, N.C. Syntheses, structures, and antimicrobial activities of gold(I)– and copper(I)–N-heterocyclic carbene (NHC) complexes derived from basket-shaped dinuclear Ag(I)–NHC complex. Inorg. Chem. 2018, 57, 11322–11332.
- Prencipe, F.; Zanfardino, A.; Di Napoli, M.; Rossi, F.; D’Errico, S.; Piccialli, G.; Mangiatordi, G.F.; Saviano, M.; Ronga, L.; Varcamonti, M.; et al. Silver (I) N-Heterocyclic carbene complexes: A winning and broad spectrum of antimicrobial properties. Int. J. Mol. Sci. 2021, 22, 2497.
- Liang, J.; Sun, D.; Yang, Y.; Li, M.; Li, H.; Chen, L. Discovery of metal-based complexes as promising antimicrobial agents. Eur. J. Med. Chem. 2021, 224, 113696.
- Karaca, Ö.; Meier-Menches, S.M.; Casini, A.; Kühn, F.E. On the binding modes of metal NHC complexes with DNA secondary structures: Implications for therapy and imaging. Chem. Commun. 2017, 53, 8249–8260.
- Jakob, C.H.G.; Muñoz, A.W.; Schlagintweit, J.F.; Weiß, V.; Reich, R.M.; Sieber, S.A.; Correia, J.D.G.; Kühn, F.E. Anticancer and antibacterial properties of trinuclear Cu(I), Ag(I) and Au(I) macrocyclic NHC/urea complexes. J. Organomet. Chem. 2021, 932, 121643.
- Rufino-Felipe, E.; Colorado-Peralta, R.; Reyes-Márquez, V.; Valdés, H.; Morales-Morales, D. Fluorinated-NHC transition metal complexes: Leading characters as potential anticancer metallodrugs. Anti-Cancer Agents Med. Chem. 2021, 21, 938–948.
- Zou, T.; Lok, C.-N.; Wan, P.-K.; Zhang, Z.-F.; Fung, S.-K.; Che, C.-M. Anticancer metal-N-heterocyclic carbene complexes of gold, platinum and palladium. Curr. Opin. Chem. Biol. 2018, 43, 30–36.
- Mora, M.; Gimeno, M.C.; Visbal, R. Recent advances in gold–NHC complexes with biological properties. Chem. Soc. Rev. 2019, 48, 447–462.
- Porchia, M.; Pellei, M.; Marinelli, M.; Tisato, F.; Del Bello, F.; Santini, C. New insights in Au-NHCs complexes as anticancer agents. Eur. J. Med. Chem. 2018, 146, 709–746.
- Tialiou, A.; Chin, J.; Keppler, B.K.; Reithofer, M.R. Current developments of N-heterocyclic carbene Au(I)/Au(III) complexes toward cancer treatment. Biomedicines 2022, 10, 1417.
- Touj, N.; Nasr, I.S.A.; Koko, W.S.; Khan, T.A.; Özdemir, I.; Yasar, S.; Mansour, L.; Alresheedi, F.; Hamdi, N. Anticancer, antimicrobial and antiparasitical activities of copper(I) complexes based on N-heterocyclic carbene (NHC) ligands bearing aryl substituents. J. Coord. Chem. 2020, 73, 2889–2905.
- Habib, A.; Iqbal, M.A.; Bhatti, H.N.; Kamal, A.; Kamal, S. Synthesis of alkyl/aryl linked binuclear silver(I)-N-heterocyclic carbene complexes and evaluation of their antimicrobial, hemolytic and thrombolytic potential. Inorg. Chem. Commun. 2020, 111, 107670.
- Kamal, A.; Iqbal, M.A.; Bhatti, H.N.; Ghaffar, A. Selenium-N-heterocyclic carbene (Se-NHC) complexes with higher aromaticity inhibit microbes: Synthesis, structure, and biological potential. J. Coord. Chem. 2022, 75, 1915–1928.
- Ielo, I.; Iacopetta, D.; Saturnino, C.; Longo, P.; Galletta, M.; Drommi, D.; Rosace, G.; Sinicropi, M.S.; Plutino, M.R. Gold derivatives development as prospective anticancer drugs for breast cancer treatment. Appl. Sci. 2021, 11, 2089.
- Ceramella, J.; Mariconda, A.; Sirignano, M.; Iacopetta, D.; Rosano, C.; Catalano, A.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Novel Au carbene complexes as promising multi-target agents in breast cancer treatment. Pharmaceuticals 2022, 15, 507.
- Massai, L.; Messori, L.; Carpentieri, A.; Amoresano, A.; Melchiorre, C.; Fiaschi, T.; Modesti, A.; Gamberi, T.; Magherini, F. The effects of two gold-N-heterocyclic carbene (NHC) complexes in ovarian cancer cells: A redox proteomic study. Cancer Chemother. Pharmacol. 2022, 89, 809–823.
- Md Zin, N.F.H.; Ooi, S.Y.S.; Khor, B.-K.; Chear, N.J.-Y.; Tang, W.K.; Siu, C.-K.; Razali, M.R.; Haque, R.A.; Yam, W. Cytotoxicity of asymmetric mononuclear silver(I)-N-heterocyclic carbene complexes against human cervical cancer: Synthesis, crystal structure, DFT calculations and effect of substituents. J. Organomet. Chem. 2022, 976, 122439.
- Mariconda, A.; Iacopetta, D.; Sirignano, M.; Ceramella, J.; Costabile, C.; Pellegrino, M.; Rosano, C.; Catalano, A.; Saturnino, C.; El-Kashef, H.; et al. N-Heterocyclic carbene (NHC) silver complexes as versatile chemotherapeutic agents targeting human topoisomerases and actin. ChemMedChem 2022, 17, e202200345.
- Johnson, N.A.; Southerland, M.R.; Youngs, W.J. Recent developments in the medicinal applications of silver-NHC complexes and imidazolium salts. Molecules 2017, 22, 1263.
- Iacopetta, D.; Ceramella, J.; Rosano, C.; Mariconda, A.; Pellegrino, M.; Sirignano, M.; Saturnino, C.; Catalano, A.; Aquaro, S.; Longo, P.; et al. N-Heterocyclic Carbene-Gold(I) Complexes Targeting Actin Polymerization. Appl. Sci. 2021, 11, 5626.
- Iacopetta, D.; Rosano, C.; Sirignano, M.; Mariconda, A.; Ceramella, J.; Ponassi, M.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Is the way to fight cancer paved with gold? Metal-based carbene complexes with multiple and fascinating biological features. Pharmaceuticals 2020, 13, 91.
- Iacopetta, D.; Mariconda, A.; Saturnino, C.; Caruso, A.; Palma, G.; Ceramella, J.; Muià, N.; Perri, M.; Sinicropi, M.S.; Caroleo, M.C.; et al. Novel gold and silver carbene complexes exert antitumor effects triggering the reactive oxygen species dependent intrinsic apoptotic pathway. ChemMedChem 2017, 12, 2054–2065.
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Pellegrino, M.; Mariconda, A.; Longo, P.; Sinicropi, M.S.; Aquaro, S. COVID-19 at a glance: An up-to-date overview on variants, drug design and therapies. Viruses 2022, 14, 573.
- Gil-Moles, M.; Türck, S.; Basu, U.; Pettenuzzo, A.; Bhattacharya, S.; Rajan, A.; Ma, X.; Büssing, R.; Wölker, J.; Burmeister, H.; et al. Metallodrug profiling against SARS-CoV-2 target proteins identifies highly potent inhibitors of the S/ACE2 interaction and the Papain-like Protease PLpro. Chem.—Eur. J. 2021, 27, 17928–17940.
- Aktas, A.; Barut Celepci, D.; Gok, Y.; Taslimi, P.; Akincioglu, H.; Gulcin, İ. A novel Ag-N-heterocyclic carbene complex bearing the hydroxyethyl ligand: Synthesis, characterization, crystal and spectral structures and bioactivity properties. Crystals 2020, 10, 171.
- Grisi, F.; Costabile, C.; Gallo, E.; Mariconda, A.; Tedesco, C.; Longo, P. Ruthenium-based complexes bearing saturated chiral N-heterocyclic carbene ligands: Dynamic behavior and catalysis. Organometallics 2008, 27, 4649–4656.
- Perfetto, A.; Costabile, C.; Longo, P.; Grisi, F. Ruthenium olefin metathesis catalysts with frozen NHC ligand conformations. Organometallics 2014, 33, 2747–2759.
- Lam, N.Y.S.; Truong, D.; Burmeister, H.; Babak, M.V.; Holtkamp, H.U.; Movassaghi, S.; Ayine-Tora, D.M.; Zafar, A.; Kubanik, M.; Oehninger, L.; et al. From catalysis to cancer: Toward structure–activity relationships for benzimidazol-2-ylidene-derived N-heterocyclic-carbene complexes as anticancer agents. Inorg. Chem. 2018, 57, 14427–14434.
- Tabrizi, L.; Olasunkanmi, L.O.; Fadare, O.A. Experimental and theoretical investigations of cyclometalated ruthenium(ii) complex containing CCC-pincer and anti-inflammatory drugs as ligands: Synthesis, characterization, inhibition of cyclooxygenase and in vitro cytotoxicity activities in various cancer cell lines. Dalton Trans. 2019, 48, 728–740.
- Rana, B.K.; Roymahapatra, G.; Das, H.S.; Giri, S.; Cardoso, M.H.; Franco, O.L.; Kiran, N.K.; Santra, M.K.; Bag, P.P.; Bertolasi, V.; et al. Pyridine and pyrimidine functionalized half-sandwich Ru(II)-N heterocyclic carbene complexes: Synthesis, structures, spectra, electrochemistry and biological studies. J. Mol. Struct. 2021, 1231, 129822.
- Rana, B.K.; Das, H.S.; Giri, S.; Roymahapatra, G.; Mahapatra, P.K.; Dinda, J.; Cardoso, M.H.; Franco, O.L.; Bag, P.P. A comparative analysis of Ru(II) complexes containing non-annulated and annulated n-heterocyclic carbene ligand towards structure, spectra, electrochemistry and biological activity. J. Indian Chem. Soc. 2020, 97, 2699–2712.
- Rodríguez-Prieto, T.; Michlewska, S.; Hołota, M.; Ionov, M.; de la Mata, F.J.; Cano, J.; Bryszewska, M.; Gómez, R. Organometallic dendrimers based on ruthenium(II) N-heterocyclic carbenes and their implication as delivery systems of anticancer small interfering RNA. J. Inorg. Biochem. 2021, 223, 111540.
- Marzano, C.; Sbovata, S.M.; Gandin, V.; Michelin, R.A.; Venzo, A.; Bertani, R.; Seraglia, R. Cytotoxicity of cis-platinum(II) cycloaliphatic amidine complexes: Ring size and solvent effects on the biological activity. J. Inorg. Biochem. 2009, 103, 1113–1119.
- Paşahan, R.; Akkoç, M.; Yaşar, Ş.; Köprülü, T.; Tekin, Ş.; Yaşar, S.; Özdemir, I. Synthesis and investigation of antiproliferative activity of Ru-NHC complexes against C6 and HeLa cancer cells. Turk. J. Chem. 2022, 46, 1097–1109.
- Chen, C.; Xu, C.; Li, T.; Lu, S.; Luo, F.; Wang, H. Novel NHC-coordinated ruthenium (II) arene complexes achieve synergistic efficacy as safe and effective anticancer therapeutics. Eur. J. Med. Chem. 2020, 203, 112605.
- Sarı, Y.; Gürses, C.; Celepci, D.B.; Keleştemur, Ü.; Aktaş, A.; Yüksel, Ş.; Ateş, B.; Gök, Y. 4-Vinylbenzyl and 2-morpholinoethyl substituted ruthenium(II) complexes: Design, synthesis, and biological evaluation. J. Mol. Struct. 2020, 1202, 127355.
- Wilke, N.L.; Burmeister, H.; Frias, C.; Ott, I.; Prokop, A. Ruthenium complex HB324 induces apoptosis via mitochondrial pathway with an upregulation of Harakiri and overcomes cisplatin resistance in neuroblastoma cells in vitro. Int. J. Mol. Sci. 2023, 24, 952.
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug resistance (MDR): A widespread phenomenon in pharmacological therapies. Molecules 2022, 27, 616.
- Li, F.; Collins, J.G.; Keene, F.R. Ruthenium complexes as antimicrobial agents. Chem. Soc. Rev. 2015, 44, 2529–2542.
- Southam, H.M.; Butler, J.A.; Chapman, J.A.; Poole, R.K. Chapter One—The Microbiology of Ruthenium Complexes. In Advances in Microbial Physiology; Poole, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 71, pp. 1–96.
- Roymahapatra, G.; Dinda, J.; Mishra, A.; Mahapatra, A.; Hwang, W.S.; Mandal, S.M. Cytotoxic potency of self-assembled Ruthenium(II)-NHC complexes with pincer type 2,6-bis(N-methylimidazolylidene/benzimidazolylidene)pyrazine ligands. J. Cancer Res. Ther. 2015, 11, 105–113.
- Streciwilk, W.; Terenzi, A.; Cheng, X.; Hager, L.; Dabiri, Y.; Prochnow, P.; Bandow, J.E.; Wölfl, S.; Keppler, B.K.; Ott, I. Fluorescent organometallic rhodium(I) and ruthenium(II) metallodrugs with 4-ethylthio-1,8-naphthalimide ligands: Antiproliferative effects, cellular uptake and DNA-interaction. Eur. J. Med. Chem. 2018, 156, 148–161.
- Streciwilk, W.; Terenzi, A.; Lo Nardo, F.; Prochnow, P.; Bandow, J.E.; Keppler, B.K.; Ott, I. Synthesis and biological evaluation of organometallic complexes bearing bis-1,8-naphthalimide ligands. Eur.J. Inorg. Chem. 2018, 2018, 3104–3112.
- Boubakri, L.; Chakchouk-Mtibaa, A.; Al-Ayed, A.S.; Mansour, L.; Abutaha, N.; Harrath, A.H.; Mellouli, L.; Özdemir, I.; Yasar, S.; Hamdi, N. Ru(II)–N-heterocyclic carbene complexes: Synthesis, characterization, transfer hydrogenation reactions and biological determination. RSC Adv. 2019, 9, 34406–34420.
- Boubakri, L.; Chakchouk-Mtiba, A.; Naouali, O.; Mellouli, L.; Mansour, L.; Özdemir, I.; Yaser, S.; Sauthier, M.; Hamdi, N. Ruthenium(II) complexes bearing benzimidazole-based N-heterocyclic carbene (NHC) ligands as potential antimicrobial, antioxidant, enzyme inhibition, and antiproliferative agents. J. Coord. Chem. 2022, 75, 645–667.
- Onar, G.; Gürses, C.; Karataş, M.O.; Balcıoğlu, S.; Akbay, N.; Özdemir, N.; Ateş, B.; Alıcı, B. Palladium(II) and ruthenium(II) complexes of benzotriazole functionalized N-heterocyclic carbenes: Cytotoxicity, antimicrobial, and DNA interaction studies. J. Organomet. Chem. 2019, 886, 48–56.
- Slimani, I.; Chakchouk-Mtibaa, A.; Mansour, L.; Mellouli, L.; Özdemir, I.; Gürbüzd, N.; Hamdi, N. Synthesis, characterization, biological determination and catalytic evaluation of ruthenium(II) complexes bearing benzimidazole-based NHC ligands in transfer hydrogenation catalysis. New J. Chem. 2020, 44, 5309–5323.
- Burmeister, H.; Dietze, P.; Preu, L.; Bandow, J.E.; Ott, I. Evaluation of ruthenium(II) N-heterocyclic carbene complexes as antibacterial agents and inhibitors of bacterial thioredoxin reductase. Molecules 2021, 26, 4282.
More
Information
Subjects:
Chemistry, Medicinal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
758
Revisions:
2 times
(View History)
Update Date:
25 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




